Abstract
Background: Patients with hypertrophic cardiomyopathy (HCM) have a high incidence of atrial fibrillation. They also have a longer P‐wave duration than healthy controls, indicating conduction alterations. Previous studies have demonstrated orthogonal P‐wave morphology alterations in patients with paroxysmal atrial fibrillation. In the present study, the P‐wave morphology of patients with HCM was compared with that of matched controls in order to explore the nature of the atrial conduction alterations.
Methods and Results: A total of 65 patients (45 men, mean age 49 ± 15) with HCM were included. The control population (n = 65) was age and gender matched (45 men, mean age 49 ± 15). Five minutes of 12‐lead ECG was recorded. The data were subsequently transformed to orthogonal lead data, and unfiltered signal‐averaged P‐wave analysis was performed.
The P‐wave duration was longer in the HCM patients compared to the controls (149 ± 22 vs 130 ± 16 ms, P < 0.0001). Examination of the P‐wave morphology demonstrated changes in conduction patterns compatible with interatrial conduction block of varying severity in both groups, but a higher degree of interatrial block seen in the HCM population. These changes were most prominent in the Leads Y and Z.
Conclusion: The present study suggests that the longer P‐wave duration observed in HCM patients may be explained by a higher prevalence of block in one or more of the interatrial conduction routes.
Keywords: hypertrophic cardiomyopathy, atrial fibrillation, noninvasive, signal‐averaged ECG analysis
Atrial fibrillation (AF) is more common in patients with hypertrophic cardiomyopathy (HCM) than in the general population, 1 , 2 , 3 with a prevalence approximately four times higher (25%). 2 , 3 The exact mechanism of the increased prevalence is not known, but left atrial enlargement and dysfunction (decreased left atrial fractional shortening) have been shown to be independently linked to AF in HCM patients. 3 , 4 , 5 In addition, specific gene mutations have been shown to be associated with a particularly high AF prevalence, possibly indicating a role for an intrinsic atrial myopathy causing conduction disturbances. 6
The atrial activation impulse is conducted from the right to the left atrium via discrete pathways located superiorly (Bachmann's bundle), posteriorly and inferiorly in the interatrial septum. 7 , 8 , 9 The presence 7 , 8 , 9 and function 10 , 11 of the different pathways have been shown to vary. Evidence of atrial conduction disturbance (i.e., lengthening of the P wave on surface ECG) is seen more frequently in HCM patients compared with normal subjects. 12 , 13 Recently, interatrial conduction defects have been suggested to play a role in the development of AF. 14 , 15 , 16 Our group has in a limited material using signal‐averaged P‐wave ECG previously reported altered orthogonal P‐wave morphology, with only marginal concomitant P‐wave lengthening, in patients with paroxysmal AF compared with controls. 17
In the present study, we investigated patients with HCM, using a signal‐averaging technique of unfiltered P waves from derived vectorcardiograms (VCG) to gain insight of atrial conduction beyond P‐wave duration alone. 17 , 18
METHODS
Study Population
Patients with HCM were consecutively included on an outpatient basis at a tertiary referral center (The Heart Hospital, London, U.K.). Diagnosis of HCM was based on echocardiographic identification of a hypertrophied, nondilated left ventricle in the absence of other cardiac or systemic diseases capable of producing the magnitude of hypertrophy evident. 2 Exclusion criteria were chronic AF or atrial pacing.
Age‐ and gender‐matched control subjects were identified in a 1:1 case–control comparison. The control subjects were recruited from a database of healthy volunteers. The study was approved by the local Ethics committee. The study complied with the Declaration of Helsinki, and all subjects gave informed consent to participation.
Data Acquisition
Five minutes of 12‐lead ECG was acquired using a custom made optically isolated PC card (Siemens Elema AB, Solna, Sweden). Data (1 kHz sampling, 16‐bit analog‐to‐digital conversion, 0.625 μV resolution) were transferred to a computer and stored for subsequent off‐line processing.
Signal‐Averaging P Waves
Data analysis was performed using custom‐made software running on matlab R14 for Linux (MathWorks, Inc., Natick, MA; http://www.mathworks.com). To enable the analysis of orthogonal P‐wave morphology, VCG was derived from the 12‐lead ECG using the inverse Dower transform. 18 , 19 The analysis was carried out on the entire 5‐minute recording.
The VCG was high‐pass filtered to exclude slow baseline drift due to respiratory movement of the thorax. Power line interference was reduced using a 50‐Hz bandstop filter. QRS complexes were identified automatically and included according to similarity (a cross‐correlation coefficient of more than 0.9 was applied to exclude artifacts and abnormal events such as ventricular extra beats). P waves were extracted using 250 ms long signal windows preceding each QRS complex. If cases with unusually long PQ‐time or P‐wave duration were found, the window could be shifted manually, in order to fully cover the P wave. Subsequently, the signal windows were time shifted to estimate the maximal correlation in each lead. P waves with a cross‐correlation coefficient of more than 0.9 (in all leads analyzed separately) were grouped together and averaged. The actual P waves were defined by manual setting of the onset and end. The method used is described in detail elsewhere. 20
P‐Wave Morphology Characterization and Classification
The morphologies of Leads X and Y were defined by the location (i.e., time from P‐wave onset) and amplitude of the maximum peak in respective lead (Xmax and Ymax). Lead Z was characterized by the minimum peak (Zmin) location and amplitude, the location of the first zero crossing (Zzero) following Zmin, and the location and amplitude of the maximum peak (Zmax) following Zzero. Finally, the calculated spatial magnitude (SM) (X2+ Y2+ Z2)1/2 was characterized by the location of the maximum peak (SM1). If two peaks were present, the location of the second (SM2) and the distance between the two peaks (SMdiff) were calculated. In SM, the location of the local minima just before the manually set P‐wave end (Nadir) was recorded (it has been suggested that Nadir is a more reproducible way to define the end of the P wave 21 ). The derived parameters are schematically illustrated in Figure 1.
Figure 1.
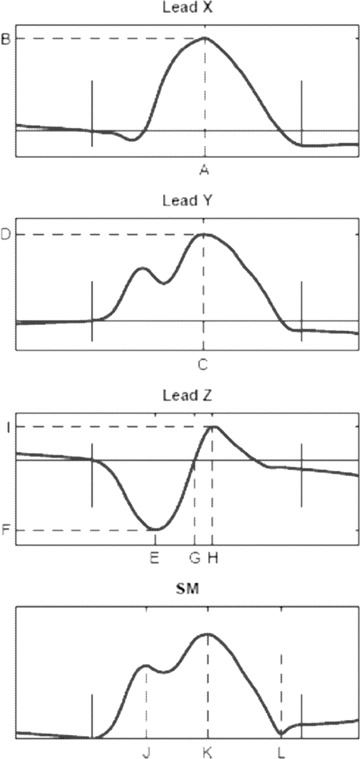
Schematic illustration of the signal‐averaged P‐wave morphologies. The derived parameters are indicated with dashed lines. Lead X: Xmax location (A) and amplitude (B); Lead Y: Ymax location (C) and amplitude (D); Lead Z: Zmin location (E) and amplitude (F), Zzero location (G), Zmax location (H) and amplitude (I); Spatial magnitude (SM); location of the maximum peak (SM1) (J) and the negative extreme (Nadir) (L). If a second peak was identified (SM2) (K), its location and distance to SM1 were calculated.
In previous studies, two distinct P‐wave morphologies have been defined: Type 1 (predominately positive Leads X and Y and predominantly negative Lead Z) and Type 2 (predominately positive Leads X and Y and biphasic Lead Z [negative, positive]). Typical examples of each type are illustrated in Figure 2A and B, respectively. 17 , 18 , 22 Type 2 morphology has been shown to be more abundant in patients with paroxysmal AF. 17 After preliminary analysis, the P‐wave morphologies were manually classified. The classification was made in a blinded, randomized fashion by a single operator (PGP). Each ECG was analyzed twice in order to evaluate the reproducibility of classification. In cases where there was a discrepancy between the first and second classification, a third inspection was made to finalize the classification.
Figure 2.
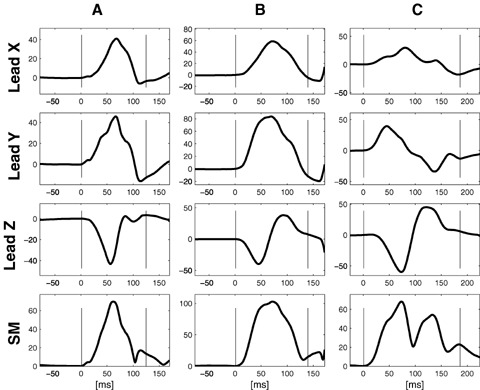
Typical examples of Type 1 P‐wave morphology (A), and Type 2 P‐wave morphology (B). Type 3 P‐wave morphology (C) was identified in 11 patients (10 HCM patients and 1 control subject). The pattern is compatible with Bachmann's bundle block. 23 Two patients from the HCM population were excluded from subsequent analysis because of P‐wave morphology noncompatible with sinus rhythm (i.e., predominantly negative Lead Y).
To further strengthen the hypothesis of the presence of discrete interatrial routes (i.e., Type 1 and Type 2 morphologies), Zzero in relation to P‐wave duration (Zzero/Pdur) was calculated. The different Zzero/Pdur values were plotted in a histogram and tested for normality.
To optimize morphology analysis, only recordings probable to have been generated from sinus rhythm were included. Hence, atypical morphologies (e.g., predominantly negative Lead Y) were excluded from subsequent analysis.
Echocardiography
A standard transthoracic echocardiographic examination was performed in association with the inclusion of the HCM patients. Dimensions of the cardiac chambers as well as left ventricular septal and posterior wall thickness and regional and global systolic function (left ventricular ejection fraction [LVEF]) were measured according to standard criteria.
Statistical Analysis
Data are presented as mean ± standard deviation. Student's t‐test was used for comparison between groups. The chi‐square test was used to evaluate the relationship between two dichotomous variables. The Shapiro‐Wilk W test was used in testing for normality. Statistical significance was defined as P < 0.05. All statistical analyses were performed using STATISTICA for Windows version 6.1 (StatSoft, Inc., Tulsa, OK).
RESULTS
Study Population
A total of 65 patients (45 men, mean age 49 ± 15, range 19–81 years) with HCM were included. The comorbidity, history of arrhythmia, ongoing medication, and current clinical status (i.e., NYHA‐class, blood pressure, and routine echocardiographic parameters) are summarized in Table 1.
Table 1.
Clinical Characteristics in Study Population
| HCM Patients (n = 65) | Control Subjects (n = 65) | P Value | |
|---|---|---|---|
| Age (years) | 49 ± 15 | 49 ± 15 | NS |
| Male/female | 45/20 | 45/20 | NS |
| Comorbidity | |||
| None | 50 (77%) | 65 (100%) | |
| Ischemic heart disease | 7 (11%) | ||
| Valvular heart disease | 8 (12%) | ||
| COPD | 4 (6%) | ||
| Diabetes mellitus | 2 (3%) | ||
| History of arrhythmia | |||
| None | 49 (75%) | 65 (100%) | |
| Atrial fibrillation | 7 (11%) | ||
| Nonsustained VT | 12 (18%) | ||
| VT/VF | 3 (5%) | ||
| Cardioactive drugs | |||
| None | 9 (14%) | 65 (100%) | |
| β‐blocker | 28 (43%) | ||
| Ca2+ channel blocker | 12 (18%) | ||
| Amiodarone | 13 (22%) | ||
| Disopyramide | 9 (14%) | ||
| ACEi/ARB | 4 (6%) | ||
| Diuretics | 9 (14%) | ||
| Blood pressure | |||
| Systolic | 123 ± 18 | 125 ± 13 | NS |
| Diastolic | 73 ± 12 | 78 ± 8 | <0.01 |
| MAP | 90 ± 13 | 94 ± 9 | NS |
| NYHA class (I /II /III/IV) | 83/11/6 /0% | N/A | |
| Echocardiography | |||
| LA diameter (mm) | 45 ± 8 | N/A | |
| LVEF (%) | 65 ± 7 | N/A | |
| IVS D (mm) | 17 ± 6 | N/A | |
| Posterior LV wall (mm) | 10 ± 3 | N/A | |
| Heart rate (bpm) | 63 ± 11 | 70 ± 9 | <0.0001 |
ACEi = angiotensin converting enzyme inhibitor; ARB = angiotensin receptor blocker; bpm = beats per minute; COPD = chronic obstructive pulmonary disease; HCM = hypertrophic cardiomyopathy; IVS D = interventricular septum diastole; LA = left atrial; LV = left ventricular; LVEF = left ventricular ejection fraction; MAP = mean arterial blood pressure; VF = ventricular fibrillation; VT = ventricular tachycardia.
The 65 control subjects (45 men, mean age 49 ± 15, range 20–80 years) were healthy, normotensive volunteers without history of cardiac disease (Table 1). The populations were well matched, with the difference between the average age of the two groups being 0.002 years and the median absolute age difference between patients and controls being 0.6 years. The only significant differences between the two populations were slightly lower heart rate (63 ± 11 vs 70 ± 9 bpm, P < 0.0001) and diastolic blood pressure (73 ± 12 vs 78 ± 8 mmHg, P = 0.01) in HCM patients.
Signal‐Averaged P‐Wave Analysis
P‐wave duration was significantly longer in HCM patients compared with controls, 149 ± 22 vs 130 ± 16 ms (P < 0.0001).
Analysis of individual leads revealed that Xmax was located later and exhibited higher amplitude in HCM patients. Ymax amplitude was also higher in HCM patients, but the location of Ymax was similar to that seen in controls. Finally, Zmin and Zmax were located later and their amplitudes greater in HCM patients, but the location of Zzero was similar in the two populations, which was due the difference in P‐wave morphology in the Z lead (see below).
SM1 was located at the same position in HCM patients and in controls, but a second peak (SM2) was more commonly seen in HCM patients and when it was seen, its location was later. Consequently, SMdiff was greater in HCM patients compared with controls. In three cases more than two peaks were present (these cases were excluded from the SM analyses). Nadir was located later in HCM patients compared with controls.
The results of the complete comparison are listed in Table 2.
Table 2.
P‐Wave Morphology Parameters
| HCM Patients (n = 63) | Control Subjects (n = 65) | P Value | ||
|---|---|---|---|---|
| P‐wave duration | (ms) | 149 ± 22 | 130 ± 16 | <0.0001 |
| Lead X | ||||
| Xmax location | (ms) | 83 ± 16 | 70 ± 9 | <0.0001 |
| Xmax amplitude | (μV) | 84 ± 33 | 53 ± 14 | <0.0001 |
| Lead Y | ||||
| Ymax location | (ms) | 64 ± 19 | 64 ± 11 | NS |
| Ymax amplitude | (μV) | 66 ± 33 | 79 ± 26 | <0.05 |
| Lead Z | ||||
| Zmin location | (ms) | 50 ± 11 | 45 ± 8 | <0.01 |
| Zmin amplitude | (μV) | −43 ± 26 | −29 ± 12 | <0.001 |
| Zzero location | (ms) | 72 ± 17 | 68 ± 13 | NS |
| Zmax location | (ms) | 98 ± 19 | 91 ± 19 | <0.05 |
| Zmax amplitude | (μV) | 49 ± 31 | 22 ± 11 | <0.0001 |
| Spatial magnitude | ||||
| SM1 location | (ms) | 66 ± 17 | 61 ± 12 | NS |
| Second peak present | 51% | 25% | <0.01 | |
| SM2 location | (ms) | 104 ± 22 | 78 ± 15 | <0.0001 |
| SMdiff | (ms) | 46 ± 18 | 30 ± 13 | <0.01 |
| Nadir | (ms) | 127 ± 20 | 110 ± 11 | <0.0001 |
HCM = hypertrophic cardiomyopathy.
Classification of P‐Wave Morphologies
The intraindividual reproducibility in the classification process was high, with only four (3%) of the cases needing a third inspection for final classification.
Ten (16%) HCM patients and 29 (45%) of the controls were classified as Type 1 (Fig. 2A), while 44 (70%) of the HCM patients and 35 (54%) of the controls were classified as Type 2 (Fig. 2B).
Nine (14%) HCM patients and one (2%) control subject exhibited a pattern of advanced interatrial block, compatible with sinus rhythm and Bachmann's bundle block (positive Lead X, biphasic Lead Y (negative mode > 40 ms) and biphasic Lead Z; illustrated in Figure 2C. 23 These subjects were included in the subsequent analyses and the morphology pattern was labeled Type 3. Two HCM patients were excluded from subsequent analysis owing to a conduction pattern noncompatible with sinus rhythm (i.e., negative Lead Y indicating an inferior–superior activation). The relative proportion of individuals classified as respective type was significantly different in HCM patients and controls (10 / 44 / 9 vs 29 / 35 / 1, P < 0.0001).
Zzero/Pdur was 0.50 ± 0.11. As expected, Zzero/Pdur was different between Type 1 and Type 2 or Type 3 (0.60 ± 0.08 vs 0.45 ± 0.08, P < 0.0001). The histogram of Zzero/Pdur clearly exhibits two peaks, thus implying a nonnormal distribution (P < 0.01) (Fig. 3).
Figure 3.
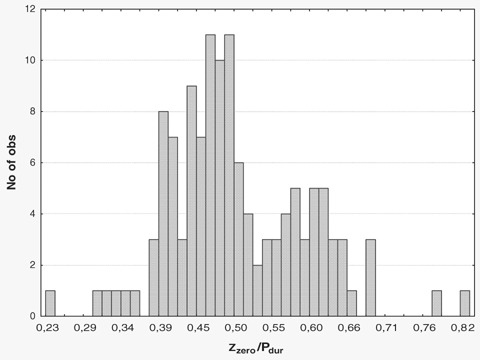
The presence of discrete pathways was further explored using the relative position of the zero crossing in Lead Z (Zzero/Pdur). As seen in the histogram, the parameter is not normally distributed (P < 0.01, Shapiro‐Wilk W test) with two different peaks visible. The two peaks represent Type 2 or 3 and Type 1 morphologies, respectively.
Baseline characteristics and echocardiographic parameters differed to a large extent between the different classes, with differences seen in clinical characteristics as well as echocardiographic parameters and P‐wave duration (Table 3).
Table 3.
Clinical Characteristics in Relation to Classification
| Type 1 (n = 39) | Type 2 (n = 79) | Type 3 (n = 10) | |
|---|---|---|---|
| Age (years) | 43 ± 16 | 53 ± 13 | 45 ± 15 |
| NYHA class (I/II/III/IV) | 100/0 /0 /0% | 84/7/9 /0%* | 67/33/0/0% |
| Echocardiography | |||
| LA diameter (mm) | 42 ± 7 | 46 ± 8 | 50 ± 7† |
| LVEF (%) | 70 ± 4‡ | 64±7 | 64 ± 5 |
| IVS D (mm) | 13 ± 3 | 17 ± 6 | 20 ± 5† |
| Posterior LV wall (mm) | 9 ± 3 | 10 ± 3 | 11 ± 2 |
| P‐wave duration (ms) | 135 ± 21 | 140 ± 21 | 152 ± 18† |
| Heart rate (bpm) | 68 ± 10 | 67 ± 10 | 57 ± 7§ |
IVS D = interventricular septum diastole; LA = left atrial; LV = left ventricular; LVEF = left ventricular ejection fraction; MAP = mean arterial blood pressure; VF = ventricular fibrillation; VT = ventricular tachycardia. For the NYHA class analyses and the echocardiographic parameters only data from the HCM patients were available.
*P < 0.05 Type 1 vs Type 2 and Type 2 vs Type 3 †P = 0.01 Type 1 vs Type 3; ‡P < 0.05 Type 1 vs Type 2 and Type 1 vs Type 3; §P < 0.01 Type 3 vs Type 1 and Type 3 vs Type 2.
DISCUSSION
The present study demonstrates that the longer P‐wave duration previously reported in HCM patients 12 , 13 is associated with morphological differences in the signal‐averaged VCG P wave. The morphological changes of the P wave may be explained by a higher prevalence of block in one or more of the interatrial conduction routes.
Methodological Issues
Signal‐averaged ECG with orthogonal lead configuration has been used in several publications for analyzing atrial activity, using the orthogonal lead configuration. 12 , 17 , 22 , 24 However, VCG is rarely recorded in everyday clinical routine; instead 12‐lead ECG is recorded in a vast majority of patients. Recently, it was shown that the information held by the 12‐lead ECG could readily be transformed to a VCG, using the inverse Dower transform. 18 In the present study, 12‐lead ECGs were recorded as part of a clinical routine in the included patients. Hence, 12‐lead ECGs were used and subsequently transformed to derived VCG. The ECGs from both HCM patients and control subjects were recorded, transformed, and analyzed in the same fashion.
The manual identification of the end of the P wave may on some occasions be problematic. However, in the present study the differences in mean P‐wave duration and mean Nadir location between HCM patients and controls were virtually identical.
The classification process was carried out in a blinded fashion. The intraindividual reproducibility was high. However, as of today, automatic classification algorithms are not available.
Study Population
The studied HCM population was included consecutively and the clinical characteristics are similar to previously published series. 12 , 13 The age‐ and gender‐matched control population was comparable with the HCM population with two exceptions, diastolic blood pressure and heart rate. Although a reduction in P‐wave duration has been shown to occur when hypertensive patients are adequately treated, 25 the difference in diastolic blood pressure in the present study was very small and probably neglectable. The heart rate was lower in the HCM population. This is most likely due to the cardioactive drug treatment. The literature is conflicting regarding the impact of heart rate on P‐wave duration. 26 , 27 Moreover, when the HCM patients in the present study were compared with the control subjects exhibiting a heart rate equal to or lower than the population median (69 bpm), the findings in the P‐wave morphology parameters were similar. All things considered, it seems reasonable to conclude that the difference in heart rate was not a major confounding factor in the present study.
Approximately, one‐third of the HCM patients used antiarrhythmic drugs; the possible impact of this is discussed later.
To conclude, the HCM population and the control population were well matched and no bias, likely to influence the results significantly, was inferred.
Differences in P‐Wave Morphology
In the present study, patients with HCM exhibited a longer P‐wave duration than the age‐ and gender‐matched healthy control subjects. This is well in keeping with previous studies. 12 , 13 , 28 Moreover, the P‐wave morphology was vastly different with the changes seen in Leads X and Z being the most prominent (Table 2).
Lead X is predominantly positive in both HCM patients and in controls, indicating a right to left activation. Lead X in the HCM population is characterized by a delay and increase of the maximum amplitude. Lead Y was also predominantly positive in both investigated groups, indicating a superior–inferior activation of the atria. No major differences were observed in this lead, besides from a modest increase in maximum amplitude. Changes in Lead Z were characterized by a prolongation and amplification of the latter part of the P wave (i.e., after Zzero).
The left atrial dimensions were enlarged in the HCM patients compared with standard reference values (<40 mm). All the above findings could be representing a larger atrial mass being activated during an extended time frame during the later part of the activation. Alternatively a conduction delay in the left atrium or in the conduction from the right to the left atrium could produce the observed phenomena. Yet another explanation would be that the left atrium like the left ventricle is affected by a progressive disease. One study showing that a specific gene‐mutation increases the likelihood of AF 6 may indicate that HCM, at least in some forms, is not only a disease of the ventricles but also the atria. A pathological study investigating the macroscopic and histological features of HCM, including the left atria, did not find any evidence of atrial myocyte disarray. 29 However, atrial hypertrophy, dilatation, and fibrosis were found in subsets of the studied hearts. 29 Thus, data are sparse and to some extent conflicting, but if HCM does affect the atria, this may explain the findings in the present study.
Interatrial Block in Relation to P‐Wave Morphologies
There is still a lack of knowledge regarding the exact intra‐ and interatrial spreading of activation and the corresponding orthogonal ECG lead data. The interatrial connections are multiple and exhibit large interindividual variability. 7 , 8 , 9 Two studies investigated the interatrial activation in humans during sinus rhythm, using a noncontact mapping technique. 10 , 11 The results were to a certain degree conflicting, with the proportion of subjects activating the left atrium via Bachmann's bundle being lower in one study (37%) 11 than the other (100%). 10 Wherever activation was initiated, separate wave front activations were observed in the right and left atrium without separate interatrial conduction via the true septum. 10 Consequently, the interpretation of our findings is, to some extent, based on theoretical assumptions regarding the atrial activation sequence.
Type 1 morphology is generated by a right–left (positive Lead X), superior–inferior (positive Lead Y), and posterior–anterior activation pattern (negative Lead Z). With the sinus node located posteriorly, superiorly in the right atrium, the observed vector (i.e., predominately negative Lead Z) is most likely generated by an activation of the left atrium with participation of connections located posteriorly. 7 , 8 Although concomitant conduction via Bachmann's bundle cannot be excluded, it is unlikely that the main activation sequence of the left atrium (posterior–anterior) would be the result of conduction exclusively via Bachmann's bundle.
Consequently, Type 2 morphologies are generated by a right–left, superior–inferior, and posterior–anterior–posterior activation pattern. In parallel with the logic discussed earlier, this would mean that the activation wave front, is most likely to spread to the left atrium via connections located anteriorly and superiorly, that is, the Bachmann's bundle area without noticeable contribution from posterior or inferior connections. 7 , 8
It has previously been suggested that biphasic P waves in inferior leads (12‐lead ECG) or in Lead Y (orthogonal lead configuration) are most likely due to Bachmann's bundle block producing an advanced intraatrial block. 23 , 30 Unexpectedly, 14% of the HCM patients in the present study were found to have biphasic P waves in Lead Y, compatible with Bachmann's bundle block (Type 3). This should be compared with 2% of the control subjects and a prevalence in the general population of 1%, according to previous studies. 30 As shown in Figure 2C, the presence of a biphasic Lead Y was always accompanied by a biphasic Lead Z; hence the activation direction was not only characterized by the superior–inferior–superior route but also a posterior–anterior–posterior propagation, indicating not only a Bachmann's bundle block 23 but also a block or a delayed conduction in the posterior route. 7 , 8
In other words, both Type 2 and Type 3 morphologies may well be a consequence of block or delayed conduction in one or more of the different interatrial routes. In contrast, Type 1 morphology can, theoretically, only be generated via a functioning posterior route.
Interestingly, several of the baseline characteristics differed between individuals belonging to the different groups (Table 3). Overall, a worsening of symptoms, a greater left ventricular hypertrophy and more marked left atrial enlargement was observed from Type 1 to Type 2 to Type 3. Moreover, the presence of Type 2 and Type 3 morphologies were more common in the HCM population compared with the controls. This is in agreement with the hypothesis of Type 3 being the most pronounced form, with block or delayed conduction both in the posterior and superior routes. Moreover, all the echocardiographic parameters exhibited progressively escalating pathology from Type 1 to Type 2 to Type 3, possibly offering a mechanistic explanation to the increased prevalence of interatrial conduction block. Finally, advanced interatrial block (Type 3 in the present study) has previously been shown to be associated with a high incidence of atrial arrhythmias (e.g., AF). 31 The high prevalence of Type 3 morphologies in HCM patients may be a clue to the high propensity for AF seen in HCM patients.
To further investigate the hypothesis of the presence of several separate conduction routes Zzero/Pdur was estimated. The histogram in Figure 3 clearly illustrates two different zero crossings (i.e., Type 1 and Type 2 or Type 3), indicating the two discrete routes.
To conclude, the findings in the present study are compatible with the presence and variable influence of different interatrial routes. Indeed, most of the changes observed between the HCM population and the control subjects may be driven by changes in the properties of the interatrial conduction.
Antiarrhythmic Drugs and P‐Wave Morphology
Approximately one‐third of the HCM patients used antiarrhythmic drugs, the drugs used being amiodarone (n = 13) and disopyramide (n = 9). These patients were not excluded to keep the HCM population clinically representative. Amiodarone has been shown to decrease P‐wave duration in patients having effect of the treatment (no AF recurrence), but in the population as a whole the amiodarone effect on P‐wave duration was neutral. 32 In one study, disopyramide did increase P‐wave duration significantly, although the increase was modest (approximately 4–8% compared to a mean of 14% P‐wave prolongation in HCM subjects in our study). 24 Thus, it seems unlikely that the marked difference observed in the present study is due to the small minority of patients using disopyramide. The use of antiarrhythmic drugs was entirely clinically driven in the present study. Thus, it is likely that exclusion or separate analysis of this subset of HCM patients may introduce significant bias (e.g., worse state of the disease or a propensity for arrhythmia). Nonetheless, when the HCM patients without antiarrhythmic drugs were compared with the control population, all the observed differences were conserved. This indicates that the observed differences are not a result of differences in antiarrhythmic drug usage.
CONCLUSIONS
The present study confirms earlier‐reported findings of longer P‐wave duration in HCM patients. 12 , 13 In addition, the increase in P‐wave duration is associated with morphological differences of the signal‐averaged VCG P wave, to our knowledge never before observed. The degree of deviation from normal P‐wave morphology correlates to clinical features of disease status. The morphological changes in the P wave appear best explained by a higher prevalence of block in one or more of the interatrial conduction routes.
Acknowledgments
Acknowledgments: The study was supported by travel grants from the Faculty of Medicine at Lund University. The authors thank Mrs. Bibi Smideberg, at the Department of Clinical Sciences, Lund University, for recording the ECGs of the control population.
REFERENCES
- 1. McKenna WJ, England D, Doi YL, et al Arrhythmia in hypertrophic cardiomyopathy. I: Influence on prognosis. Br Heart J 1981;46:168–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Maron BJ. Hypertrophic cardiomyopathy. Lancet 1997;350:127–133. [DOI] [PubMed] [Google Scholar]
- 3. Olivotto I, Maron BJ, Cecchi F. Clinical significance of atrial fibrillation in hypertrophic cardiomyopathy. Curr Cardiol Rep 2001;3:141–146. [DOI] [PubMed] [Google Scholar]
- 4. Maron BJ, McKenna WJ, Danielson GK, et al American College of Cardiology/European Society of Cardiology clinical expert consensus document on hypertrophic cardiomyopathy. A report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents and the European Society of Cardiology Committee for Practice Guidelines. J Am Coll Cardiol 2003;42:1687–1713. [DOI] [PubMed] [Google Scholar]
- 5. Losi MA, Betocchi S, Aversa M, et al Determinants of atrial fibrillation development in patients with hypertrophic cardiomyopathy. Am J Cardiol 2004;94:895–900. [DOI] [PubMed] [Google Scholar]
- 6. Gruver EJ, Fatkin D, Dodds GA, et al Familial hypertrophic cardiomyopathy and atrial fibrillation caused by Arg663His beta‐cardiac myosin heavy chain mutation. Am J Cardiol 1999;83:13H–18H. [DOI] [PubMed] [Google Scholar]
- 7. Ho SY, Sanchez‐Quintana D, Cabrera JA, et al Anatomy of the left atrium: Implications for radiofrequency ablation of atrial fibrillation. J Cardiovasc Electrophysiol 1999;10:1525–1533. [DOI] [PubMed] [Google Scholar]
- 8. Kozlowski D, Kaminski R, Piwko G, et al Preliminary study of external interatrial muscle fascicles. Folia Morphol (Warsz) 2002;61:97–101. [PubMed] [Google Scholar]
- 9. Mitrofanova L, Ivanov V, Platonov PG. Anatomy of the inferior interatrial route in humans. Europace 2005;7(Suppl. 2):49–55. [DOI] [PubMed] [Google Scholar]
- 10. Lemery R, Soucie L, Martin B, et al Human study of biatrial electrical coupling: Determinants of endocardial septal activation and conduction over interatrial connections. Circulation 2004;110:2083–2089. [DOI] [PubMed] [Google Scholar]
- 11. Markides V, Schilling RJ, Ho SY, et al Characterization of left atrial activation in the intact human heart. Circulation 2003;107:733–739. [DOI] [PubMed] [Google Scholar]
- 12. Cecchi F, Montereggi A, Olivotto I, et al Risk for atrial fibrillation in patients with hypertrophic cardiomyopathy assessed by signal averaged P wave duration. Heart 1997;78:44–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ozdemir O, Soylu M, Demir AD, et al P‐wave durations as a predictor for atrial fibrillation development in patients with hypertrophic cardiomyopathy. Int J Cardiol 2004;94:163–166. [DOI] [PubMed] [Google Scholar]
- 14. O'Donnell D, Bourke JP, Furniss SS. Interatrial transseptal electrical conduction: Comparison of patients with atrial fibrillation and normal controls. J Cardiovasc Electrophysiol 2002;13:1111–1117. [DOI] [PubMed] [Google Scholar]
- 15. Papageorgiou P, Anselme F, Kirchhof CJ, et al Coronary sinus pacing prevents induction of atrial fibrillation. Circulation 1997;96:1893–1898. [DOI] [PubMed] [Google Scholar]
- 16. Agarwal YK, Aronow WS, Levy JA, et al Association of interatrial block with development of atrial fibrillation. Am J Cardiol 2003;91:882. [DOI] [PubMed] [Google Scholar]
- 17. Platonov PG, Carlson J, Ingemansson MP, et al Detection of inter‐atrial conduction defects with unfiltered signal‐averaged P‐wave ECG in patients with lone atrial fibrillation. Europace 2000;2:32–41. [DOI] [PubMed] [Google Scholar]
- 18. Carlson J, Havmoller R, Herreros A, et al Can orthogonal lead indicators of propensity to atrial fibrillation be accurately assessed from the 12‐lead ECG? Europace 2005;7(Suppl. 2):39–48. [DOI] [PubMed] [Google Scholar]
- 19. Edenbrandt L, Pahlm O. Vectorcardiogram synthesized from a 12‐lead ECG: Superiority of the inverse Dower matrix. J Electrocardiol 1988;21:361–367. [DOI] [PubMed] [Google Scholar]
- 20. Carlson J. Exploration of supraventricular conduction with respect to atrial fibrillation. Methodological aspects on selected techniques. Doctoral Dissertation, Lund University, Lund, Sweden. 2005. Available at: http://theses.lub.lu.se/scripta-archive/2005/11/08/med_1202/1190_Inlaga.pdf [Accessed December 9, 2005.
- 21. Raitt MH, Ingram KD. A new method for measuring signal averaged P‐wave duration. (abstract). Pacing Clin Electrophysiol 1996;19:586. [DOI] [PubMed] [Google Scholar]
- 22. Carlson J, Johansson R, Olsson SB. Classification of electrocardiographic P‐wave morphology. IEEE Trans Biomed Eng 2001;48:401–405. [DOI] [PubMed] [Google Scholar]
- 23. Ariyarajah V, Spodick DH. Advanced interatrial block: A classic electrocardiogram. Cardiology 2005;104:33–34. [DOI] [PubMed] [Google Scholar]
- 24. Kubara I, Ikeda H, Hiraki T, et al Dispersion of filtered P wave duration by P wave signal‐averaged ECG mapping system: Its usefulness for determining efficacy of disopyramide on paroxysmal atrial fibrillation. J Cardiovasc Electrophysiol 1999;10:670–679. [DOI] [PubMed] [Google Scholar]
- 25. Fuenmayor AJ, Moreno G, Landaeta A, et al Inter‐atrial conduction time shortens after blood pressure control in hypertensive patients with left ventricular hypertrophy. Int J Cardiol 2005;102:443–446. [DOI] [PubMed] [Google Scholar]
- 26. Watanabe Y, Kohgame Y, Nakano H, et al P wave changes in body surface potential maps due to increasing heart rate during exercise in normals. Jpn Circ J 1988;52:349–356. [DOI] [PubMed] [Google Scholar]
- 27. Karakaya O, Saglam M, Barutcu I, et al Comparison of the predictors for atrial rhythm disturbances between trained athletes and control subjects. Tohoku J Exp Med 2005;207:165–170. [DOI] [PubMed] [Google Scholar]
- 28. Kose S, Aytemir K, Sade E, et al Detection of patients with hypertrophic cardiomyopathy at risk for paroxysmal atrial fibrillation during sinus rhythm by P‐wave dispersion. Clin Cardiol 2003;26:431–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Varnava AM, Elliott PM, Sharma S, et al Hypertrophic cardiomyopathy: The interrelation of disarray, fibrosis, and small vessel disease. Heart 2000;84:476–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bayes de Luna A, Fort de Ribot R, Trilla E, et al Electrocardiographic and vectorcardiographic study of interatrial conduction disturbances with left atrial retrograde activation. J Electrocardiol 1985;18:1–13. [DOI] [PubMed] [Google Scholar]
- 31. Bayes de Luna A, Guindo J, Vinolas X, et al Third‐degree inter‐atrial block and supraventricular tachyarrhythmias. Europace 1999;1:43–46. [DOI] [PubMed] [Google Scholar]
- 32. Banasiak W, Telichowski A, Anker SD, et al Effects of amiodarone on the P‐wave triggered signal‐averaged electrocardiogram in patients with paroxysmal atrial fibrillation and coronary artery disease. Am J Cardiol 1999;83:112–114. [DOI] [PubMed] [Google Scholar]


