Abstract
Background: Microvolt T‐wave alternans (MTWA) has been proposed as a predictor of the risk of ventricular tachyarrhythmias (VT) and sudden cardiac death (SCD). Aim of this study was to perform a systematic review of the literature and a meta‐analysis of MTWA in primary prevention patients with ischemic and nonischemic cardiomyopathy.
Methods: The positive predictive value (PPV), negative predictive value (NPV), and relative risk (RR) of MTWA in predicting death, cardiac death, and SCD during follow‐up were reported.
Results: Fifteen studies involving 5681 patients (mean age 62 years, mean ejection fraction 32%) were included. The summary PPV during the average 26‐month follow‐up was 14% (95% CI: 13–15); NPV was 95% (95% CI: 94–96), and the univariate RR was 2.35 (95% CI: 1.68–3.28). The predictive value of MTWA was similar in patients with ischemic and nonischemic cardiomyopathy. The average RR for SCD or VT events of an abnormal MTWA was 2.40, similar to that for cardiac death. When we grouped the studies together depending upon whether beta‐blockers were withheld prior to MTWA screening, the beta‐blockers group showed an RR of 5.88. By contrast, the group in which beta‐blocker therapy was withheld had an RR of 1.63.
Conclusion: A positive MTWA determined an approximately 2.5‐fold higher risk of cardiac death and life‐threatening arrhythmia and showed a very high NPV both in ischemic and nonischemic patients. An abnormal MTWA test was associated with a 5‐fold increased risk for cardiac mortality in the low‐indeterminate group and about a 6‐fold increased risk in beta‐blockers group.
Ann Noninvasive Electrocardiol 2011;16(4):388–402
Keywords: T‐wave alternans, cardiomyopathy, sudden death
Sudden cardiac death (SCD) due to ventricular tachyarrhythmias is a major public health problem. In recent years, there have been impressive advances in therapy for the prevention of SCD due to ventricular tachyarrhythmias. Specifically, the development of the implantable cardioverter/defibrillator (ICD) has provided effective and specific preventive treatment for patients known to be at high risk of SCD. 1 , 2 , 3 , 4 However, ICD therapy is expensive and not without complications for the patient. Until recently, advances in therapeutic modalities have not been paralleled by advances in noninvasive diagnostic technologies to identify high‐risk patients. Ideally, effective noninvasive diagnostic methods would identify those patients at increased risk of SCD, and then be used to guide prophylactic treatment. Current strategies for the identification of high‐risk patients mainly involve the detection of reduced ejection fraction (EF). 1 , 2 , 3 , 4 In recent years, the measurement of microvolt T‐wave alternans (MTWA) has been proposed as a noninvasive predictor of the risk of ventricular tachyarrhythmias and SCD in a number of different patient populations. 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 Some studies 8 , 11 , 12 , 13 , 14 , 15 , 16 , 19 have shown that patients with a negative MTWA test have an extremely low risk of SCD; however, conflicting results have been reported. 10 , 20 , 21
The aim of the present study was to perform a systematic review of the literature and a meta‐analysis of MTWA as a means of risk stratification for cardiac death in primary prevention patients with ischemic and nonischemic cardiomyopathy. We examined whether the diagnostic accuracy of MTWA is influenced by the etiology of cardiomyopathy (ischemic or nonischemic), and by the primary end point (MTWA studies in which most primary events were appropriate ICD shocks versus studies in which few or no patients had implanted ICD). Furthermore, to determine the role of MTWA in the identification of SCD with respect of cardiac death we have performed a subanalysis of MTWA studies in which SCD and ventricular tachyarrhythmic events were reported. Finally, we have analyzed the diagnostic capability of MTWA in relationship with the proportion of events in indeterminate patients.
METHODS
Literature Search
We performed a literature search by using the PubMed and Medline databases to identify articles published between January 1994 and February 2009 that included the word “alternans” in the title. The search was restricted to English language literature and human subjects. Two reviewers (F.N. and T.D.) then independently evaluated the titles identified, and manuscripts were retrieved for any publication that either reviewer felt was potentially relevant.
Inclusion Criteria for Studies
Studies were included if they met the following criteria: (1) they were prospective and included >40 patients with ischemic and nonischemic cardiomyopathy who underwent exercise‐induced MTWA testing to predict SCD or ventricular arrhythmias; (2) they examined MTWA in primary prevention (studies that included patients with a history of resuscitated SCD or malignant arrhythmia were excluded); (3) average follow‐up was ≥ 1 year; (4) they provided primary data on the results of MTWA testing and clinical outcomes, including SCD, cardiac death, total mortality, ventricular arrhythmias, and appropriate ICD interventions; (5) they adopted a similar definition of abnormal MTWA, i.e., positive and indeterminate; (6) they analyzed MTWA by means of the spectral analytic method.
When they reviewed the articles, the two investigators (F.N. and T.D.) were blinded to the authors, journals, and institutions. Disagreements were resolved by consensus. From each article, the following data were extracted: selection criteria (inclusion/exclusion criteria), study design, patient characteristics (number, mean age, percentage of men, type of patient population, details of MTWA assessment (including definition of abnormal test), end points of the study, average follow‐up, reported findings, including positive predictive value (PPV) and negative predictive value (NPV), relative risk (RR), or hazard ratios of future cardiac events. With regard to the end point of the studies, we identified two ICD groups: a “low‐ICD” group and a “high‐ICD” group. Studies in which no patients or few patients had implanted ICDs and appropriate ICD therapies accounted for a small fraction (≤ 15%) or none of the reported ventricular tachyarrhythmic events were defined as “low‐ICD”; studies in which many patients had implanted ICDs and > 15% of the reported ventricular tachyarrhythmic events were appropriate ICD therapies were defined as “high‐ICD.” 25 Moreover, we selected the studies in which the proportion of events occurred in indeterminate patients was known. We classified the studies as “low‐indeterminate” when ≤ 20% of the end points was observed in patients with indeterminate MTWA. If > 20% of the end points were observed in the indeterminate patients, studies were classified as “high‐indeterminate.” Furthermore, we performed a meta‐analysis of MTWA studies in which SCD and arrhythmic events were reported to determine the diagnostic accuracy of MTWA in the identification of SCD in comparison with cardiac death. Finally, we have performed a separated analysis in the group of studies in which beta‐blockers were withheld prior to MTWA screening (no beta‐blockers group) or not withheld (beta‐blockers group).
Statistical Analysis
The PPV, NPV, and univariate RR with confidence intervals (CI) of MTWA for the prediction of death during follow‐up were reported for each study selected. The Higgins heterogeneity test was performed to check the heterogeneity of the studies analyzed. On the basis of the results of the Higgins heterogeneity test, summary estimates of the predictive value with regard to mortality were made by using the DerSimonian and Laird method, which is based on the random‐effects model. Publication bias was assessed by using a funnel plot, and the Egger method was applied. The contribution of each study to the meta‐analysis, called weight, was calculated according to the amount of information it contains, in function of the number of patients and their variability. A subanalysis was performed in which studies including ischemic and nonischemic, low‐ and high‐ICD, low‐ and high‐indeterminate and beta‐blockers and without beta‐blockers groups were considered. All analyses were performed by means of the statistical software StataSE 9.0 (StataCorp, College Station, TX, USA).
RESULTS
After eliminating clearly ineligible studies, 24 articles were identified for full review. Nine of these 24 were eliminated from the meta‐analysis for various reasons: number of events not available (33%), patients with indeterminate MTWA excluded (44%), and population already analyzed in other studies (22%). The remaining 15 prospective studies involved 5681 patients. Their mean age was 62 years (range 48–67), the mean percentage of men was 79% (range 71–89), the mean left ventricular EF was 32% (range 28–55%), and the average follow‐up was 26 months (range 14–52). Table 1 details the characteristics of the studies included. The event rates in these studies are presented. In some studies 9 , 12 , 15 , 19 , 22 the event rate was relatively low.
Table 1.
Characteristics of 15 Prospective Studies of Microvolt T‐Wave Alternans Included in the Meta‐Analysis
| Author | Total (n) | Mean Age (y) | Men (%) | Mean EF (%) | ICD (%) | Primary End Points | Etiology | Event Rate | Average FU (m) |
|---|---|---|---|---|---|---|---|---|---|
| Klingenheben et al. 8 | 107 | 56 | 80 | 28 | – | SCD, VT/VF | Isc + Non−Isc | 12% | 15 |
| Tapanainen et al. 9 | 246 | 62 | 72 | 45 | – | Mortality | Ischemic | 1% | 14 |
| Grimm et al. 10 | 263 | 48 | 73 | 30 | 13 | SCD, VT/VF | Nonischemic | 14% | 52 |
| Hohnloser et al. 11 | 137 | 55 | 77 | 29 | 27 | SCD, VT/VF | Nonischemic | 13% | 14 |
| Hohnloser et al. 12 | 129 | 63 | 87 | 26 | – | SCD, CA | Ischemic | 9% | 24 |
| Sarzi Braga et al. 13 | 46 | 59 | 89 | 29 | – | CD | Isc + Non−Isc | 15% | 19 |
| Bloomfield et al. 14 | 549 | 56 | 71 | 25 | 13 | Mortality, VT/VF, ICD shock | Isc + Non−Isc | 9% | 20 |
| Ikeda et al. 15 | 1003 | 64 | 79 | 55 | – | SCD, CA, VF | Ischemic | 2% | 32 |
| Baravelli et al. 16 | 70 | 65 | 72 | 29 | 21 | SCD, HF death, VT/VF, ICD shock | Nonischemic | 16% | 19 |
| Cantillon et al.*, 17 | 286 | 65 | 78 | 26 | 61 | Mortality | Isc + Non−Isc | 15% | 24 |
| Chow et al. 18 | 768 | 67 | 82 | 27 | 51 | Mortality, ICD shock | Ischemic | 21% | 27 |
| ALPHA 19 | 446 | 59 | 78 | 30 | 8 | CD, VT/VF, ICD shock | Nonischemic | 6% | 19 |
| MASTER20 | 575 | 65 | 84 | 24 | 100 | Mortality | Ischemic | 12% | 25 |
| SCD−HeFT substudy21 | 490 | 59 | 76 | 24 | 34 | SCD, CA, VT/VF, ICD shock | Isc + Non−Isc | 15% | 30 |
| ABCD22 | 566 | 65 | 84 | 28 | 87 | SCD, VT/VF, ICD shock | Ischemic | 7% | 23 |
| Overall | 5681 | 62 | 79 | 32 | 39 | 10% | 26 |
CA = cardiac arrest; CD = cardiac death; FU = follow‐up; EF = ejection fraction; HF = heart failure; ICD = implantable cardioverter defibrillator; Isc = ischemic; m = months; Non‐Isc = nonischemic; SCD = sudden cardiac death; VT = ventricular tachycardia; VF = ventricular fibrillation; y = years. *Study duration was 38 months, while the mortality rate was based on 24 months results.
Table 2 shows the PPV, NPV, RR and their corresponding 95% CI for each study, and the overall results yielded by the DerSimonian and Laird method (random effect suggested by the heterogeneity test: I2= 48.6%, P = 0.018). The summary PPV during the average 26‐month follow‐up was 14% (95% CI: 13–15); NPV was 95% (95% CI: 94–96), and the univariate RR was 2.35 (95% CI: 1.68–3.28) (Fig. 1). Figure 1 shows a forest plot depicting the contribution of each study to the meta‐analysis, as represented by the area of the box. On using the Egger method, publication bias is shown by Kendall's Tau, 1‐sided P = 0.023.
Table 2.
Results of 15 Prospective Studies of Microvolt T‐Wave Alternans
| Author | PPV (%) (95% CI) | NPV (%) (95% CI) | RR (95% CI) |
|---|---|---|---|
| Klingenheben et al. 8 | 18 (10−28) | 100 (89−100) | 12.24 (0.75−199.96) |
| Tapanainen et al. 9 | 1 (0−5) | 99 (96−100) | 1.41 (0.09−22.31) |
| Grimm et al. 10 | 16 (11−22) | 90 (81−96) | 1.67 (0.77−3.62) |
| Hohnloser et al. 11 | 16 (9−24) | 94 (80−99) | 2.64 (0.64−10.90) |
| Hohnloser et al. 12 | 13 (7−21) | 100 (90−100) | 9.47 (0.58−155.88) |
| Sarzi Braga et al. 13 | 21 (9−39) | 100 (75−100) | 6.18 (0.38−100.99) |
| Bloomfield et al. 14 | 13 (10−17) | 98 (95−99) | 6.17 (2.26−16.86) |
| Ikeda et al. 15 | 6 (3−9) | 100 (99−100) | 14.59 (4.26−49.99) |
| Baravelli et al. 16 | 23 (11−38) | 93 (78−99) | 3.38 (0.79−14.49) |
| Cantillon et al.*, 17 | 17 (12−23) | 90 (82−95) | 1.68 (0.84−3.37) |
| Chow et al. 18 | 25 (22−29) | 87 (82−91) | 1.89 (1.34−2.67) |
| ALPHA19 | 9 (6−12) | 98 (94−100) | 4.39 (1.35−14.32) |
| MASTER20 | 13 (10−17) | 90 (85−93) | 1.29 (0.80−2.08) |
| SCD−HeFT substudy21 | 17 (13−21) | 88 (81−93) | 1.40 (0.84−2.35) |
| ABCD22 | 8 (6−11) | 96 (91−98) | 1.86 (0.84−4.14) |
| Overall | 14 (13−15) | 95 (94−96) | 2.35 (1.68−3.28) |
Figure 1.
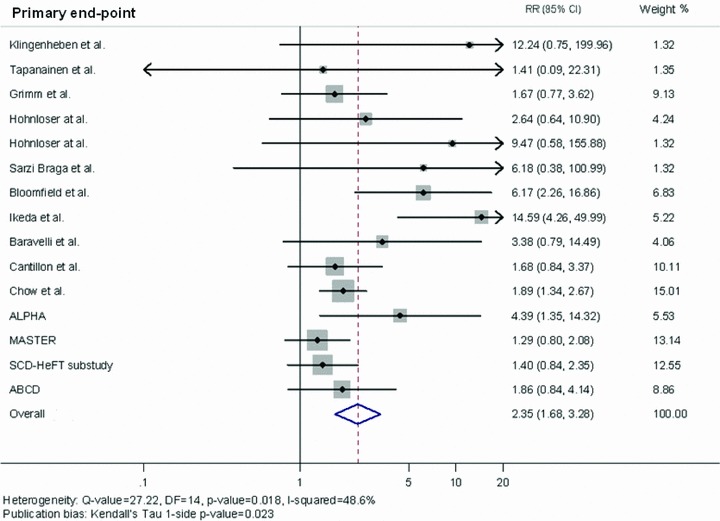
Summary estimate of the relative risk of abnormal microvolt T‐wave alternans for cardiac death or ventricular tachyarrhythmic events in the studies included in the meta‐analysis. The contribution of each study to the meta‐analysis is represented by the area of the box.
Table 3 shows the PPV, NPV and RR and their corresponding 95% CI for each study, and the overall results yielded by the DerSimonian and Laird method (random effect suggested by the heterogeneity test: I2= 60.3%, P = 0.019) in 7 prospective studies conducted in patients with ischemic cardiomyopathy. In the subgroup of patients affected by cardiomyopathy with ischemic etiology, the summary PPV during an average 23‐month follow‐up was 13% (95% CI: 11–14); NPV was 96% (95% CI: 95–97), and the univariate RR was 2.41 (95% CI: 1.37–4.25) (Fig. 2, Panel A). Figure 2 (Panel A) shows a forest plot indicating the contribution to the meta‐analysis of each study conducted in patients with ischemic cardiomyopathy, as represented by the area of the box. On using the Egger method, no evidence of publication bias emerged (Kendall's Tau 1‐sided P = 0.548).
Table 3.
Results of the Prospective Studies of Microvolt T‐Wave Alternans, Divided by Etiology
| Author | PPV (%) (95% CI) | NPV (%) (95% CI) | RR (95% CI) |
|---|---|---|---|
| Ischemic Etiology | |||
| Tapanainen et al. 9 | 1 (0−5) | 99 (96−100) | 1.41 (0.09−22.31) |
| Hohnloser et al. 12 | 13 (7−21) | 100 (90−100) | 9.47 (0.58−155.88) |
| Sarzi Braga et al. 13 | 29 (8−58) | 100 (63−100) | 5.40 (0.33−89.02) |
| Ikeda et al. 15 | 6 (3−9) | 100 (99−100) | 14.59 (4.26−49.99) |
| Chow et al. 18 | 25 (22−29) | 87 (82−91) | 1.89 (1.34−2.67) |
| MASTER20 | 13 (10−17) | 90 (85−39) | 1.29 (0.80−2.08) |
| ABCD22 | 8 (6−11) | 96 (91−98) | 1.86 (0.84−4.14) |
| Overall | 13 (11−14) | 96 (95−97) | 2.41 (1.37−4.25) |
| Nonischemic etiology | |||
| Grimm et al. 10 | 16 (11−22) | 90 (81−96) | 1.67 (0.77−3.62) |
| Hohnloser et al. 11 | 16 (9−24) | 94 (80−99) | 2.64 (0.64−10.90) |
| Sarzi Braga et al. 13 | 21 (9−39) | 100 (48−100) | 2.65 (0.17−40.51) |
| Baravelli et al. 16 | 23 (11−38) | 93 (78−99) | 3.38 (0.79−14.42) |
| ALPHA19 | 9 (6−13) | 98 (94−99) | 4.40 (1.35−14.33) |
| Overall | 14 (12−15) | 96 (92−95) | 2.58 (1.51−4.38) |
Figure 2.
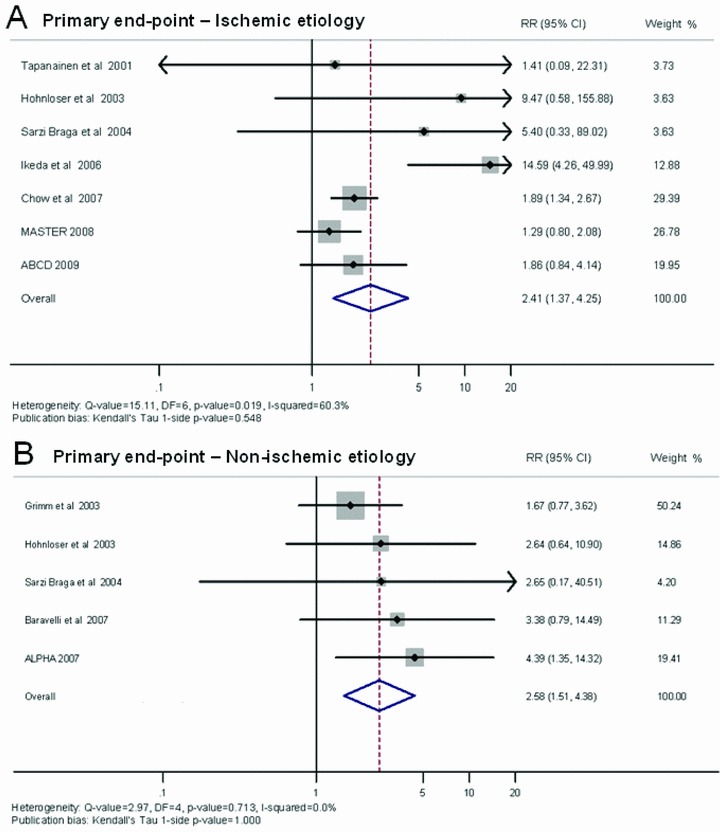
Summary estimate of the relative risk of abnormal microvolt T‐wave alternans in subjects with ischemic etiology (A) and in subjects with nonischemic etiology (B). The contribution of each study to the meta‐analysis is represented by the area of the box.
Table 3 shows the PPV, NPV and RR and their corresponding 95% CI for each study, and the overall results yielded by the Mantel–Haenszel method (fixed effect suggested by the heterogeneity test: I2= 0.0%, P = 0.713) in 5 prospective studies conducted in patients with nonischemic cardiomyopathy. In this subgroup of patients, the summary estimate of PPV was 14% (95% CI: 12–15); NPV was 96% (95% CI: 92–95), and the univariate RR was 2.58 (95% CI: 1.51–4.38). Figure 2 (Panel B) shows a forest plot depicting the contribution to the meta‐analysis of each study conducted in patients with nonischemic cardiomyopathy, as represented by the area of the box. On using the Egger method, no evidence of publication bias emerged (Kendall's Tau 1‐sided P value = 1.000).
When we grouped the studies together in terms of their end points, the high‐ICD group showed an average PPV of 15% (95% CI: 14–17), NPV of 90% (95% CI: 88–92), and RR of 1.47 (95% CI: 1.15–1.89) (Table 4). By contrast, the low‐ICD group, as shown in Table 4, had an average PPV of 13% (95% CI: 11–14), NPV of 98% (95% CI: 97–98), and RR of 3.50 (95% CI: 2.53–4.84). Figure 3 shows a forest plot indicating the contribution to the meta‐analysis of each study conducted in the low‐ICD (Panel A) and high‐ICD group (Panel B), as represented by the area of the box. On using the Egger method, publication bias is shown by Kendall's Tau, 1‐sided P value = 0.297 for low ICD group and P value = 0.027 for high ICD group.
Table 4.
Results of the Prospective Studies of Microvolt T‐Wave Alternans, Divided by Low ICD Group and High ICD group
| Author | PPV (%) (95% CI) | NPV (%) (95% CI) | RR (95% CI) |
|---|---|---|---|
| Low ICD Group | |||
| Klingenheben et al. 8 | 18 (10−28) | 100 (89−100) | 10.43 (0.64−170.66) |
| Tapanainen et al. 9 | 1 (0−5) | 99 (96−100) | 1.41 (0.09−22.25) |
| Grimm et al. 10 | 16 (11−22) | 90 (81−96) | 1.58 (0.72−3.43) |
| Hohnloser et al. 12 | 13 (7−21) | 100 (90−100) | 8.41 (0.51−138.51) |
| Sarzi Braga et al. 13 | 21 (9−39) | 100 (75−100) | 5.12 (0.31−84.04) |
| Bloomfield et al. 14 | 13 (10−17) | 98 (95−99) | 5.57 (2.04−15.24) |
| Ikeda et al. 15 | 6 (3−9) | 100 (99−100) | 13.84 (4.04−47.43) |
| Chow et al. − No ICD pts18 | 27 (21−34) | 88 (83−93) | 2.02 (1.26−13.46) |
| ALPHA 19 | 9 (6−12) | 98 (94−100) | 4.13 (1.27−13.46) |
| Overall | 13 (11−14) | 98 (97−98) | 3.16 (2.28−4.38) |
| High ICD group | |||
| Hohnloser et al. 11 | 16 (9−24) | 94 (80−99) | 2.64 (0.64−10.90) |
| Cantillon et al. 17 | 17 (12−23) | 90 (82−95) | 1.68 (0.84−3.37) |
| Chow et al. − ICD pts18 | 24 (20−29) | 83 (72−90) | 1.40 (0.82−2.38) |
| MASTER 20 | 13 (10−17) | 90 (85−93) | 1.29 (0.80−2.08) |
| SCD−HeFT substudy 21 | 17 (13−21) | 88 (81−93) | 1.40 (0.84−2.35) |
| ABCD 22 | 8 (6−11) | 96 (91−98) | 1.86 (0.84−4.14) |
| Overall | 15 (14−17) | 90 (88−92) | 1.47 (1.15−1.89) |
Figure 3.
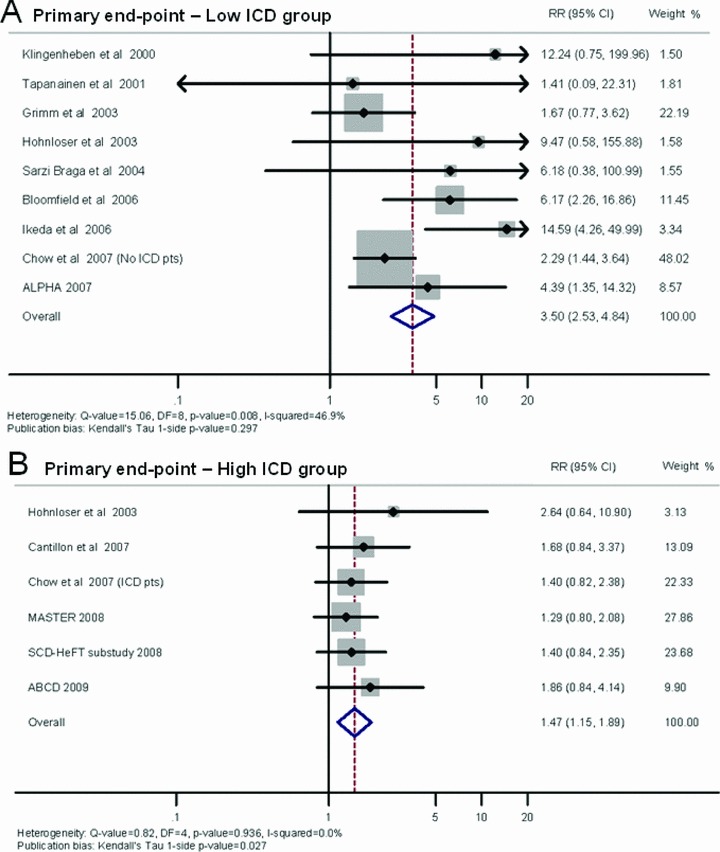
Summary estimate of the relative risk of abnormal microvolt T‐wave alternans in the studies included in the low‐ICD group (A) and in the high‐ICD group (B). The contribution of each study to the meta‐analysis is represented by the area of the box.
Figure 4 shows a forest plot depicting the RR for SCD or tachyarrhythmic event of an abnormal MTWA. The average RR was 2.40 (95% CI: 1.54–3.74). On using the Egger method, publication bias is shown by Kendall's Tau, 1‐sided P value = 0.003).
Figure 4.
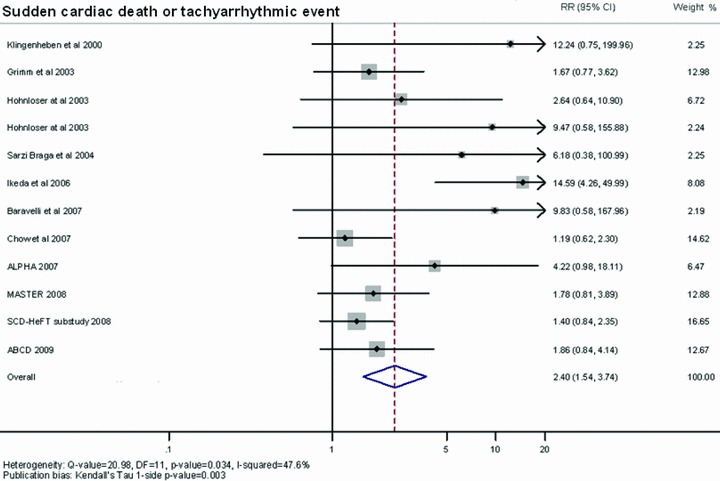
Summary estimate of the relative risk for sudden cardiac death or tachyarrhythmic event of abnormal microvolt T‐wave alternans. The contribution of each study to the meta‐analysis is represented by the area of the box.
When we divided the studies in terms of the proportion of events occurred in indeterminate patients, the low‐indeterminate group showed an average RR of 5.38 (95% CI: 1.88–15.40) (Fig. 5). By contrast, the high‐indeterminate group had an average RR of 1.56 (95% CI: 1.07–2.27). Figure 5 shows a forest plot indicating the contribution to the meta‐analysis of each study conducted in the low‐indeterminate (Panel A) and high‐indeterminate group (Panel B), as represented by the area of the box. On using the Egger method, no evidence of publication bias emerged (Kendall's Tau, 1‐sided P value = 0.734 for low indeterminate group and P value = 0.296 for high indeterminate group).
Figure 5.
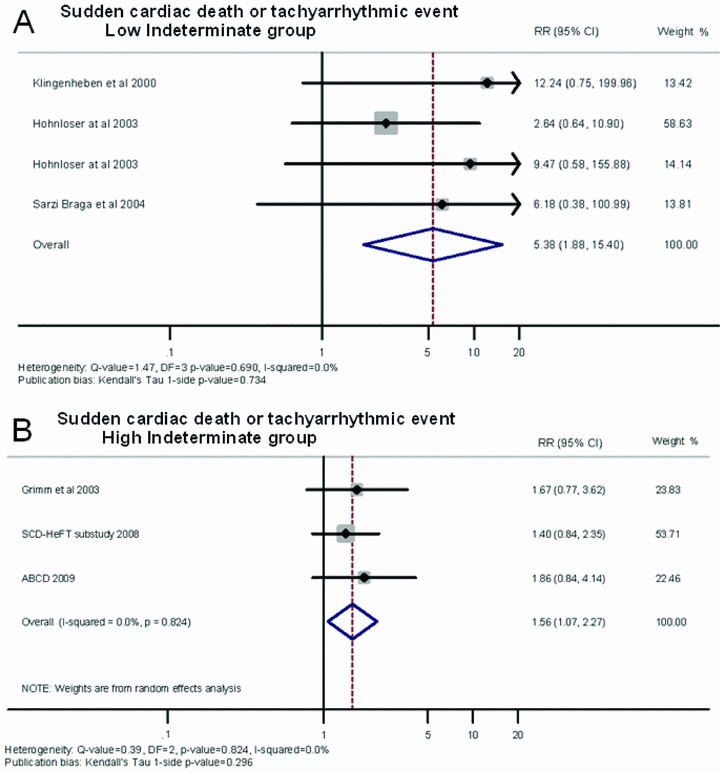
Summary estimate of the relative risk of abnormal microvolt T‐wave alternans in the studies included in the low‐indeterminate (A) and in the high‐indeterminate group (B). The contribution of each study to the meta‐analysis is represented by the area of the box.
When we grouped the studies together depending upon whether beta‐blockers were withheld prior to MTWA screening, the beta‐blockers group showed an average PPV of 10% (95% CI: 8–12), NPV of 99% (95% CI: 98–99), and RR of 5.88 (95% CI: 3.38–10.23). By contrast, the group in which beta‐blocker therapy was withheld had an average PPV of 16% (95% CI: 15–18), NPV of 90% (95% CI: 87–92), and RR of 1.63 (95% CI: 1.30–2.04). Figure 6 shows a forest plot indicating the contribution to the meta‐analysis of each study conducted in the beta‐blockers group (Panel A) and without beta‐blockers group (Panel B), as represented by the area of the box. On using the Egger method, publication bias is shown by Kendall's Tau, 1‐sided P value = 0.851 for beta‐blockers group and P value = 0.806 for without beta‐blockers group.
Figure 6.
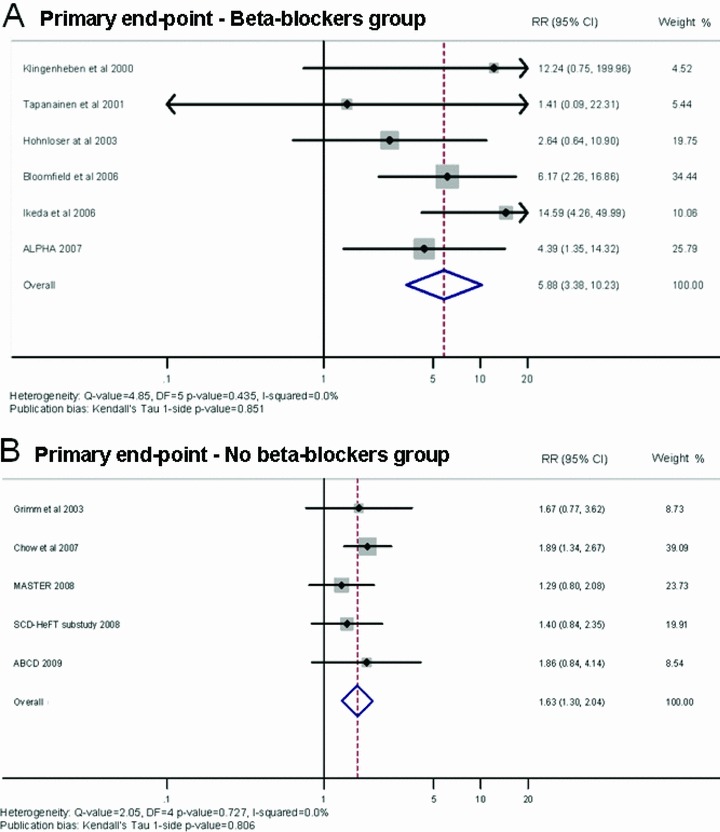
Summary estimate of the relative risk of abnormal microvolt T‐wave alternans in the studies including patients undergoing microvolt T‐wave alternans test with beta‐blockers (A) and without beta‐blockers (B). The contribution of each study to the meta‐analysis is represented by the area of the box.
DISCUSSION
Main Findings
In this meta‐analysis, MTWA testing by means of the spectral analytic method was evaluated as a predictor of ventricular arrhythmias and mortality in patients with ischemic and nonischemic cardiomyopathy. In our study, which comprised 5681 patients, the following were observed: (1) MTWA showed a very high NPV; a negative MTWA result predicted a 5% risk of severe arrhythmic events or mortality during an average follow‐up of 26 months. (2) A positive MTWA result determined an RR of 2.35 of clinically relevant events (death or significant ventricular arrhythmias). The summary PPV was low (14%). (3) The predictive value of MTWA was similar in patients with ischemic and nonischemic cardiomyopathy. (4) In the group of studies in which few patients had implanted ICD (low‐ICD group), the univariate RR was 3.50, whereas in the high‐ICD group, the univariate RR was 1.47. (5) The average RR for SCD or tachyarrhythmic events of an abnormal MTWA was 2.40, similar to that for cardiac death. (6) The low‐indeterminate group showed an average RR of 5.38, whereas the high‐indeterminate group had an average RR of 1.56. (7) In the group of studies in which beta‐blockers were withheld prior to MTWA screening, the univariate RR was 1.63, whereas in the beta‐blockers group, the univariate RR was 5.88.
Previous Meta‐Analyses
This meta‐analysis first examined the role of MTWA in the specific identification of tachyarrhythmic events or SCD, breaking down the analysis into cardiac death and SCD. Our results showed that the RR was similar, both for SCD and cardiac death. Furthermore, we divided the studies in terms of the proportion of events occurred in indeterminate patients, and noteworthy an abnormal MTWA test was associated with a 5‐fold increased risk for cardiac mortality in the low‐indeterminate group compared with 1.5‐fold increased risk in the high‐indeterminate group.
To our knowledge, five meta‐analyses have investigated the predictive role of MTWA in the risk stratification of ventricular tachyarrhythmic events and mortality. 25 , 26 , 27 , 28 , 29 In the first meta‐analysis, Gehi et al. 26 included heterogeneous populations in primary and secondary preventions, from healthy subjects to ischemic and nonischemic congestive heart failure patients. They found that MTWA predicted events with a univariate RR of 3.77. More recently, three meta‐analyses have been published. 25 , 27 , 28 De Ferrari et al. 28 showed that a nonnegative MTWA determines an RR of 2.99 in nonischemic dilated cardiomyopathy. Hohnloser et al. 25 compared primary prevention trials in which few patients had implanted ICDs with studies in which many patients had implanted ICDs. In the low‐ICD group, the hazard ratio associated with a nonnegative MTWA was 13.6, whereas in the high‐ICD group the hazard ratio was 1.6. van der Avoort et al. 27 performed a meta‐analysis in a population of 1946 patients with severe left ventricular dysfunction. They reported that the risk of mortality or severe arrhythmic events was higher in patients with a nonnegative MTWA, with an RR of 2.7. However, this meta‐analysis did not include some of the most recent clinical trials in ischemic and nonischemic cardiomyopathy. 19 , 20 , 21 , 22 A recent meta‐analysis 29 showed that an abnormal MTWA was associated with an RR of 5.39 when the beta‐blockers were continued during MTWA testing. In contrast, the association was much weaker in those studies where the beta‐blocker therapy was withheld prior to MTWA testing (RR = 1.40). In our study, an abnormal MTWA was associated with about a 6‐fold increased risk in studies in which beta‐blocker therapy was continued during MTWA testing, whereas an RR of 1.63 was found in those studies where the use of beta‐blocker therapy was withheld prior to MTWA assessment. Possible explanations of this finding are: (1) the reduction in MTWA amplitude after administration of beta‐blockers (reduction of false positive); (2) inability to achieve the target heart rate (increase of indeterminate tests) that can suggest poor functional capacity and higher risk of death.
Negative MTWA Studies
Grimm et al. 10 conducted a study on nonischemic cardiomyopathy patients, who were followed up for 52 months (the longest duration reported). They found that a negative MTWA test showed a trend toward decreased arrhythmic risk on univariate analysis (NPV 90%) but not on multivariate analysis. In their study, an indeterminate MTWA result was associated with the highest rate of arrhythmic events; however, no difference in event rates was seen between MTWA‐positive and ‐negative patients. Moreover, the study had a potential bias, in that a high percentage of patients were candidates for heart transplantation.
More recently, two larger trials have been performed on the SCD‐HeFT 21 and Madit II populations. 20 A prospective substudy of the SCD‐HeFT trial 21 found no significant differences in arrhythmic events or mortality between MTWA‐nonnegative (positive and indeterminate) and MTWA‐negative patients (HR 1.28). The MTWA‐negative patients had an event rate of 10.2% at 1 year. This study had two main limitations: (1) the rate of indeterminate MTWA results was higher (41%) than reported in previous studies; and (2) the primary end point was a ventricular tachyarrhythmic event, which was defined as SCD or an appropriate ICD shock. As observed by Ellenbogen et al., 30 approximately half of all ICD discharges are for arrhythmias that do not cause death. We can speculate that in some cases ICDs treat self‐terminating arrhythmias without administering shocks and in other cases the device therapy can be proarrhythmic. 31 , 32 Therefore, ICD shocks are an unreliable surrogate for SCD in clinical studies. 33 As observed in the meta‐analysis of Hohnloser et al., 25 in studies involving primary prevention patients with ICD, MTWA displayed a hazard ratio of only 1.6. Similarly, in our study, the RR was 1.54 in the high‐ICD group, whereas in the low‐ICD group it was 3.50. Therefore, MTWA may predict cardiac events that more closely correspond to SCD than to ICD‐related events.
In the MASTER trial, 20 conducted in Madit II‐type patients, the primary end point for MTWA was similar to that of the SCD‐HeFT substudy. In comparison with this latter, however, the rate of MTWA‐indeterminate results was very low. The MASTER trial found that MTWA did not predict ventricular tachyarrhythmic events over a mean follow‐up of 2.1 ± 0.9 years. However, a nonnegative MTWA test result was associated with a significantly increased total mortality (HR = 2.04).
Predictive Value of MTWA in Patients with Ischemic and Nonischemic Cardiomyopathy
In our meta‐analysis, the predictive value of MTWA was similar in patients with ischemic (RR = 2.41) and nonischemic cardiomyopathy (RR = 2.58).
Few studies have been performed in patients with only nonischemic cardiomyopathy, 10 , 11 , 16 , 19 the largest being the ALPHA study. 19 Salerno‐Uriarte et al. 19 performed MTWA testing in 446 patients with nonischemic cardiomyopathy (326 had idiopathic dilated cardiomyopathy), EF ≤ 40%, class NYHA II/III, and without an implanted ICD, who were followed up for 18 to 24 months. An abnormal MTWA test was observed 65.4% patients and was associated with a 4‐fold higher risk of cardiac death. More importantly, patients with normal MTWA had an extremely good outcome, with a 1.2 annual mortality rate and a 0.8 annual rate of arrhythmic death + life‐threatening arrhythmia. Accordingly, the NPV was 97.3 at 18 months.
In patients with ischemic cardiomyopathy, several studies 8 , 9 , 12 , 13 , 14 , 15 , 17 , 18 , 20 , 21 , 22 , 23 , 24 have tested a possible role of MTWA in the stratification of SCD. Some studies 20 , 21 found no significant differences in arrhythmic events or mortality between patients with negative and nonnegative MTWA. Other studies 8 , 12 , 13 , 14 , 15 showed that MTWA was a very powerful predictor of SCD, with an RR ranging from 6.17 to 14.59.
Recently, the ABCD 22 (Alternans Before Cardioverter Defibrillator) trial, a multicenter prospective study, enrolled 566 patients with EF ≤ 40% attributable to ischemic heart disease, and nonsustained ventricular tachycardia on Holter, and followed them up for a median of 1.9 years. All patients underwent both MTWA testing and electrophysiological study, with ICD implantation mandated in all patients in whom either MTWA or electrophysiological study was positive. The MTWA test was positive in 46% and indeterminate in 25%; electrophysiological study was positive in 40%. ICDs were implanted in 87% of all patients and in 97% of those with positive MTWA or electrophysiological study. Primary analysis showed that MTWA achieved 1‐year positive (9%) and negative (95%) predictive values that were comparable to those of electrophysiological study (11% and 95%, respectively). Interestingly, this trial showed that MTWA testing and electrophysiological study were complementary when applied in combination. Most patients (55%) had discordant MTWA and electrophysiological study results, suggesting that the two tests might analyze different aspects of the arrhythmogenic substrate (MTWA: repolarization; electrophysiological study: depolarization). Indeed, the lowest event rates (2.3%) were found when both MTWA and electrophysiological study were normal, whereas the highest event rates (12.6%) were seen in cases in which both tests were abnormal. The second interesting observation was that the predictive values of MTWA and electrophysiological study were time‐dependent. Specifically, MTWA was predictive of clinical outcomes at 6 months but not later in the follow‐up period (i.e., no longer predictive by 12 to 15 months); in contrast, electrophysiological study was a significant predictor of events at 9 months and for the remainder of the follow‐up period. This observation suggests that MTWA results seem to modify over time, probably owing to changes in the more unstable component of the arrhythmogenic substrate: repolarization.
MTWA testing seems to be a promising tool in the stratification of SCD in patients with ischemic cardiomyopathy and less severe ventricular dysfunction. Two such studies, performed in patients with a relatively preserved EF, were considered in our meta‐analysis. The study by Ikeda et al. 15 considered patients with EF ≥ 40% in the inclusion criteria. This study, which was performed in a very large population (1041 patients) with ischemic cardiomyopathy and preserved cardiac function (mean EF of 55%), showed an NPV of 99.6% after a relatively long follow‐up (32 ± 14 months). The RR was 14.59, the highest reported in any MTWA study. The other study was conducted in patients with ischemic cardiomyopathy 9 who had a mean EF of 45%; however, it also included patients with EF less than 30%. Sustained MTWA was found in 56 patients, none of whom died. However, the test was incomplete (inability to perform the exercise test or reach the required heart rate of 105 beats/min) in 35% of the patients enrolled, and, more importantly, an incomplete MTWA test was the most significant predictor of cardiac death (RR = 11.1). In patients with ischemic cardiomyopathy, Rashba et al. 23 found that MTWA was a highly significant predictor of events when EF was ≥ 30%. In contrast, MTWA had no prognostic value in subjects with EF < 30%. Moreover, electrophysiological study identified additional low‐risk patients within the subgroup with negative or indeterminate MTWA results (hazard ratio = 4.7), but did not offer incremental prognostic information in patients with positive MTWA. This study was not considered in our meta‐analysis, since only positive MTWA tests were classed as abnormal and indeterminate tests were considered separately. Moreover, in a recent study (abstract presentation) performed in subjects with previous myocardial infarction and EF > 40%, Radoi et al. 24 found that 37.5% of patients with positive MTWA were easily inducible on electrophysiological study. Furthermore, the high prognostic value of MTWA in subjects with preserved EF was confirmed by the REFINE 34 and FINCAVAS 35 trials, which analyzed MTWA by means of the time‐domain modified moving average analysis.
CONCLUSIONS
In our study, a positive MTWA test indicated an approximately 2.5‐fold higher risk of cardiac death and life‐threatening arrhythmia. Our meta‐analysis clearly shows that MTWA has a very high NPV. Thus, a normal MTWA test identifies patients who generally have a good prognosis. A screening test with high NPV but low PPV is clinically most useful in a population with a greatest a priori risk (e.g., low EF). This is very important, as one of the most relevant questions from a clinical and economic perspective is “who may benefit from ICDs?” in patients at high risk for SCD (low EF), not least because the liberalization of guidelines has raised ICD implantation rates. However, the data from our meta‐analysis must be interpreted cautiously, since the results concern a relatively short‐term follow‐up (average of 26 months).
The predictive value of MTWA seems to be particularly high in patients with ischemic cardiomyopathy and relatively preserved EF. 15 , 23 , 24 In these low risk populations, it is crucial to have higher PPV and this can be probably obtained combining MTWA with other diagnostic tests. This issue has been explored by two studies 22 , 23 that suggested MTWA testing and electrophysiological study as complementary when applied in combination. In fact, as recently observed by Amit et al., 36 these tests would predict distinct arrhythmia outcomes: MTWA was more predictive of unstable ventricular tachyarrhythmic events and electrophysiological study was more predictive of monomorphic ventricular tachyarrhythmic events. Future studies should explore the possibility of combining several predictors of SCD, particularly in patients without current ICD indications, in an effort to enhance PPV.
The predictive value of MTWA was similar in patients with ischemic and nonischemic cardiomyopathy. An abnormal MTWA showed a similar RR for cardiac death and for SCD.
In the low‐ICD group, the RR was significantly higher than in the high‐ICD group. The predictive value of MTWA is particularly high in studies with few events in indeterminate patients (5‐fold increased risk). Therefore, MTWA can be considered a predictor of the risk of cardiac events that more closely correspond to cardiac death than to ICD‐related events, and indeterminate results should be cautiously interpreted.
Finally, an abnormal MTWA was associated with about a 6‐fold increased risk in studies in which beta‐blocker therapy was continued during MTWA testing, whereas a weak relationship was found in those studies where the use of beta‐blocker therapy was withheld prior to MTWA screening. Therefore, to date it seems to be reasonable the strategy of continuation of anti‐adrenergic therapy during MTWA. Future trials should explore the predictive value of MTWA in patients both on and off beta‐blockers.
No financial support was received and no conflicts of interest exist.
REFERENCES
- 1. Moss AJ, Hall WJ, Cannom DS, et al Improved survival with an implanted defibrillator in patients with prior myocardial infarction, low ejection fraction, and asymptomatic nonsustained ventricular tachycardia. The Multicenter Automatic Defibrillator Trial (MADIT). N Engl J Med 1996;335:1933–1940. [DOI] [PubMed] [Google Scholar]
- 2. Buxton AE, Lee KL, Fisher JD, et al For the Multicenter Unsustained Tachycardia Trial Investigators. A randomized study of the prevention of sudden death in patients with coronary artery disease. N Engl J Med 1999;341:1882–1890. [DOI] [PubMed] [Google Scholar]
- 3. Moss AJ, Zareba W, Hall WJ, et al Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med 2002;346:877–883. [DOI] [PubMed] [Google Scholar]
- 4. Bardy GH, Lee KL, Mark DB, et al For the Sudden Cardiac Death in Heart Failure Trial (SCD‐HeFT) Investigators. Amiodarone or an implantable cardioverter‐defibrillator for congestive heart failure. N Engl J Med 2005;352:225–237. [DOI] [PubMed] [Google Scholar]
- 5. Rosenbaum DS, Jackson LE, Smith JM, et al Electrical alternans and vulnerability to ventricular arrhythmias. N Engl J Med 1994;330:235–241. [DOI] [PubMed] [Google Scholar]
- 6. Adachi K, Ohnishi Y, Yokoyama M. Risk stratification for sudden cardiac death in dilated cardiomyopathy using microvolt‐level T‐wave alternans. Jpn Circ J 2001;65:76–80. [DOI] [PubMed] [Google Scholar]
- 7. Schwab JO, Weber S, Schmitt H et al Incidence of T wave alternation after acute myocardial infarction and correlation with other prognostic parameters: results of a prospective study. Pacing Clin Electrophysiol 2001;24:957–961. [DOI] [PubMed] [Google Scholar]
- 8. Klingenheben T, Zabel M, D’Agostino RB, et al Predictive value of T‐wave alternans for arrhythmic events in patients with congestive heart failure. Lancet 2000;356:651–652 [DOI] [PubMed] [Google Scholar]
- 9. Tapanainen JM, Still AM, Airaksinen KEJ, et al Prognostic significance of risk stratifiers of mortality, including T wave alternans, after acute myocardial infarction: Results of a prospective follow‐up study. J Cardiovasc Electrophysiol 2001;12:645–652. [DOI] [PubMed] [Google Scholar]
- 10. Grimm W, Christ M, Bach J, et al Noninvasive arrhythmia risk stratification in idiopathic dilated cardiomyopathy. Results of the Marburg cardiomyopathy study. Circulation 2003;108:2883–2891. [DOI] [PubMed] [Google Scholar]
- 11. Hohnloser SH, Klingenheben T, Bloomfield D, et al Usefulness of microvolt T‐wave alternans for prediction of ventricular tachyarrhythmic events in patients with dilated cardiomyopathy: Results from a prospective observational study. J Am Coll Cardiol 2003;41:2220–2224. [DOI] [PubMed] [Google Scholar]
- 12. Hohnloser SH, Ikeda T, Bloomfield DM, et al T‐wave alternans negative coronary patients with low ejection and benefit from defibrillator implantation. Lancet 2003;362:125–126. [DOI] [PubMed] [Google Scholar]
- 13. Sarzi Braga S, Vaninetti R, Laporta A, et al T wave alternans is a predictor of death in patients with congestive heart failure. Int J Cardiol 2004;93:31–38. [DOI] [PubMed] [Google Scholar]
- 14. Bloomfield DM, Bigger JT, Steinman RC et al Microvolt T‐wave Alternans and risk of death or sustained ventricular arrhythmias in patients with left ventricular dysfunction. J Am Coll Cardiol 2006;47:456–463. [DOI] [PubMed] [Google Scholar]
- 15. Ikeda T, Yoshino H, Sugi K, et al Predictive value of microvolt T‐wave alternans for sudden cardiac death in patients with preserved cardiac function after acute myocardial infarction. J Am Coll Cardiol 2006;48:2268–2274. [DOI] [PubMed] [Google Scholar]
- 16. Baravelli M, Fantoni C, Rogiani S, et al Combined prognostic value of peak O2 uptake and microvolt level T‐wave alternans in patients with idiopathic dilated cardiomyopathy. Int J Cardiol 2007;121:23–29. [DOI] [PubMed] [Google Scholar]
- 17. Cantillon DJ, Stein KM, Markowitz SM et al Predictive value of microvolt T‐wave alternans in patients with left ventricular dysfunction. J Am Coll Cardiol 2007;50:166–173. [DOI] [PubMed] [Google Scholar]
- 18. Chow T, Kereiakes DJ, Bartone C, et al Prognostic utility of microvolt T‐wave alternans in risk stratification of patients with ischemic cardiomyopathy. J Am Coll 2006;47:1820–1827. [DOI] [PubMed] [Google Scholar]
- 19. Salerno‐Uriarte JA, De Ferrari GM, Klersy C et al Prognostic value of T‐wave alternans in patients with heart failure due to nonischemic cardiomyopathy. Results of the ALPHA Study. J Am Coll Cardiol 2007;50:1896–1904. [DOI] [PubMed] [Google Scholar]
- 20. Chow T, Kereiakes DJ, Onufer J, et al Does microvolt t‐wave alternans testing predict ventricular tachyarrhythmias in patients with ischemic cardiomyopathy and prophylactic defibrillators ? J Am Coll Cardiol 2008;52:1607–1615. [DOI] [PubMed] [Google Scholar]
- 21. Gold MR, Ip JH, Costantini O, et al Role of microvolt T‐wave alternans in assessment of arrhythmia vulnerability among patients with heart failure and systolic dysfunction: Primary results from the T‐wave Alternans Sudden Cardiac Death in Heart Failure Trial substudy. Circulation 2008;118:2022–2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Costantini O, Honloser SH, Kirk MM, et al The ABCD (Alternans Before Cardioverter Defibrillator) Trial. Strategies using T‐wave alternans to improve efficiency of sudden cardiac death prevention. J Am Coll Cardiol 2009;53:471–479. [DOI] [PubMed] [Google Scholar]
- 23. Rashba EJ, Osman AF, Macmurdy K, et al Enhanced detection of arrhythmia vulnerability using T wave alternans, left ventricular ejection fraction, and programmed ventricular stimulation: a prospective study in subjects with chronic ischemic heart disease. J Cardiovasc Electrophysiol 2004;15:170–176. [DOI] [PubMed] [Google Scholar]
- 24. Radoi N, Ionescu DD. Microvolt T wave alternans may be used as a risk stratifier in post myocardial infarction patients without left ventricular systolic dysfunction (abstract). Europace 2008;10(Suppl. 1):i16. [Google Scholar]
- 25. Hohnloser SH, Ikeda T, Cohen RJ. Evidence regarding clinical use of microvolt T‐wave alternans. Heart Rhythm 2009;6:S36–S44 [DOI] [PubMed] [Google Scholar]
- 26. Gehi AK, Stein RH, Metz LD, et al Microvolt T‐wave alternans for the risk stratification of ventricular tachyarrhythmic events: A meta‐analysis. J Am Coll Cardiol 2005;46:75–82 [DOI] [PubMed] [Google Scholar]
- 27. van der Avoort CJ, Filion KB, Dendukuri N, et al Microvolt T‐wave alternans as a predictor of mortality and severe arrhythmias with left‐ventricular dysfunction: A systematic review and meta‐analysis. BMC Cardiovasc Disord 2009;9:5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. De Ferrari GM, Sanzo A. T‐wave alternans in risk stratification of patients with nonischemic dilated cardiomyopathy: Can it help to better select candidates for ICD implantation? Heart Rhythm 2009;6:S29–S35 [DOI] [PubMed] [Google Scholar]
- 29. Chan PS, Gold MR, Nallamothu BK. Do beta‐blockers impact microvolt T‐wave alternans testing in patients at risk for ventricular arrhythmias? A meta‐analysis. J Cardiovasc Electrophysiol 2010;21:1009–1014. [DOI] [PubMed] [Google Scholar]
- 30. Ellenbogen KA, Levine JH, Berger RD, et al Are implantable cardioverter defibrillator shocks a surrogate for sudden cardiac death in patients with noischemic cardiomyopathy? Circulation 2006;113:776–782. [DOI] [PubMed] [Google Scholar]
- 31. Germano JJ, Reynolds M, Essebag V, et al Frequency and causes of implantable cardioverter‐defibrillator therapies: Is device therapy proarrhythmic? Am J Cardiol 2006;97:1255–1261. [DOI] [PubMed] [Google Scholar]
- 32. Sweeney MO, Ruetz LL, Belk P, et al Bradycardia pacing‐induced short‐long‐short sequences at the onset of ventricular tachyarrhythmias: A possible mechanism of proarrhythmia? J Am Coll Cardiol 2007;50:614–622. [DOI] [PubMed] [Google Scholar]
- 33. Connolly SJ. Use and misuse of surrogate outcomes in arrhythmia trials. Circulation 2006;113:764–766. [DOI] [PubMed] [Google Scholar]
- 34. Exner DV, Kavanagh KM, Slawnych MP, et al Non‐invasive risk assessment early after a myocardial infarction – The REFINE study. J Am Coll Cardiol 2007;50:2275–2284. [DOI] [PubMed] [Google Scholar]
- 35. Nieminen T, Lehtimaki T, Viik J et al T‐wave alternans predicts mortality in a population undergoing a clinically indicated exercise test. Eur Heart J 2007;28:2322–2327. [DOI] [PubMed] [Google Scholar]
- 36. Amit G, Rosembaum DS, Super DM, et al Microvolt T‐wave alternans and electrophysiologic testing predict distinct arrhythmia substrates: Implications for identifying patients at risk for sudden cardiac death. Heart Rhythm 2010;7:763–768. [DOI] [PubMed] [Google Scholar]


