Abstract
Background: Paroxysmal atrial fibrillation (AF) recurs in up to one‐third of patients with atrioventricular nodal reentrant tachycardia (AVNRT) treated with slow pathway ablation. Therefore, identification of patients at risk for recurrence of AF after slow pathway ablation is important because of the necessity for additional therapies. The purpose of this study was to determine whether successful slow pathway ablation influences P wave parameters and whether these parameters predict the recurrence of paroxysmal AF in patients with both AVNRT and paroxysmal AF after ablation.
Methods: Thirty‐six patients with AVNRT and documented paroxysmal AF (Group 1) were compared to 36 age‐matched controls with AVNRT only (Group 2). P wave durations and P dispersion were measured before and after ablation.
Results: No significant differences were observed between P wave parameters observed before and after ablation. Maximum P wave durations (Pmax) and P dispersion (Pdisp) were significantly higher in Group 1 than in Group 2 (P < 0.001 for both) whereas minimum P wave durations did not differ between groups, both before and after ablation. Ten patients (28%) in Group‐1 had recurrence of AF during a mean follow‐up of 34 ± 11 months. Univariate predictors of AF recurrence were Pdisp ≥35.5 ms (P < 0.010), left atrial diameter >40 mm (P < 0.010), mitral or aortic calcification (P < 0.010), Pmax ≥112 ms (P < 0.050), valvular heart disease (P < 0.050), and atrial vulnerability (induction of AF lasting >30 second) after ablation (P < 0.050). However, only Pdisp ≥35.5 ms (P < 0.050) and left atrial diameter >40 mm (P < 0.010) were independent predictors of AF recurrences.
Conclusion: This study suggests that P wave dispersion could identify patients with AVNRT susceptible to recurrence of AF after slow pathway ablation.
Keywords: P wave dispersion, paroxysmal atrial fibrillation, atrioventricular nodal reentrant tachycardia, catheter ablation
Atrioventricular nodal reentrant tachycardia (AVNRT) is the most common form of paroxysmal regular supraventricular tachycardia in adults and accounts for 60% of these tachycardias. 1 On the other hand, atrial fibrillation (AF) is also the most common arrhythmia encountered in daily clinical practice with a significant potential risk for thromboembolism. 2 Although AF in patients with Wolff‐Parkinson‐White (WPW) syndrome has been studied in detail, little is known about patients with both AVNRT and paroxysmal AF, which is seen with a similar incidence in patients with AVNRT. 3 , 4 , 5 Therefore, identification of patients at risk for recurrence of AF after successful catheter ablation is important because of the necessity for additional therapies.
Two simple electrocardiographic markers, maximum P wave duration (Pmax) and P wave dispersion (Pdisp), have been used to evaluate the intraatrial and interatrial conduction times and the inhomogeneous propagation of sinus impulses which are well‐known electrophysiological (EP) characteristics of the atrium prone to AF. 6 , 7 However, to date, there is no study evaluating the predictive value of these two simple electrocardiographic markers in predicting the recurrence of paroxysmal AF in patients with both AVNRT and paroxysmal AF. The aims of this study were (1) to evaluate whether there are any changes in the values of Pmax, minimum P wave duration (Pmin), and Pdisp detected on surface ECG before and after successful catheter ablation for AVNRT, and (2) to determine whether P wave durations and Pdisp predict the recurrence of AF in patients with both AVNRT and paroxysmal AF after successful ablation.
MATERIALS AND METHODS
Patient Population
The study population consisted of two groups: 36 consecutive patients with a documented episode of paroxysmal AF before EP study and ablation were included in the study group (Group 1) and 36 age‐matched patients with AVNRT only undergoing EP study and ablation in the same period were enrolled in the study as control group (Group 2). Group 1 was further divided into two subgroups according to the presence [Group 1(+)] or absence [Group 1(−)] of recurrence of AF during follow‐up. Before catheter ablation, all patients provided medical history and underwent physical examination, 12‐lead surface ECG, chest X ray, and echocardiography. Patients using drugs known to affect P wave duration, history of noncardiac diseases capable of causing AF, like thyroid or chronic obstructive pulmonary disease, were excluded. The study was approved by institutional ethical committee and informed written consent was obtained from each patient.
EP Study and RF Catheter Ablation
EP study and catheter ablation were performed in a single session in all patients in the fasting, unsedated state and after discontinuation of all antiarrhythmic drugs for at least five half‐lives. Three multipolar, closely spaced (interelectrode space: 2 mm) electrode catheters were introduced from the right and left femoral veins and placed in the high right atrium, His bundle area and right ventricle. A steerable decapolar electrode catheter was placed in the coronary sinus to record the electrical activity around the posterior septum and coronary sinus.
The standard protocol consisted of high right atrial (A1A1) incremental pacing, usually starting from 700 ms and decreasing in steps of 10 ms until the atrioventricular (AV) node Wenckebach cycle length was reached, and single atrial extrastimulus (A1A2) testing with three different drive cycle lengths. A jump of the AH interval was defined as the difference between any consecutive AH intervals equal to or more than 50 ms during programmed or incremental atrial pacing. Atrial vulnerability was defined as inducibility during EP study of AF sustained for more than 30 seconds. 4 All EP data were collected with the patients unsedated and before infusion of any pharmacological stimulants. If tachycardia was not induced with this stimulation protocol under the baseline state, isoproterenol was infused to increase the heart rate by 20% to facilitate its induction and the stimulation protocol was repeated. AVNRT was diagnosed according to standard criteria. 1 , 8 , 9
Radiofrequency (RF) catheter ablation was done using 7‐Fr quadripolar deflectable catheters with 4‐mm tip electrodes (Marinr MC, Medtronic Co, Minneapolis, MN, USA). The technique of RF catheter ablation has been described previously. 10 Briefly, the ablation procedure was started at the lower margin of the coronary sinus ostium and the catheter was pulled toward the right atrium with continuous RF energy delivery to create a linear lesion with the integrated approach. The sites were considered optimal if the ratio of amplitudes of the atrial and ventricular electrograms was 0.1–0.5. Fifty watts of energy with a temperature limit of 70°C was applied at successful sites for 60–90 seconds.
Pmax and Pdisp Measurements in 12‐Lead Surface ECGs
Patients were discharged 48 hours after successful ablation. Digital 12‐lead surface ECG was recorded before and immediately after successful slow pathway ablation in all patients by using a Prucka Electrophysiology System (Prucka Engineering Inc., Sugar Land, TX, USA). All measurements of P wave were made on screen by two medically qualified observers who were unaware of the study hypothesis. The measurement of Pmax and Pmin was done as previously described. 6 , 7 Briefly, the onset of P wave was defined as the point of first visible upward departure from baseline for positive waveforms and as the point of first downward departure from baseline for negative waveforms. The return to the baseline was considered to be the end of the P wave. The Pmax measured in any of the 12 surface leads was used as the longest atrial conduction time. At least nine leads with clear P waves were utilized for measurements. In each lead, the mean values for three complexes were calculated. Pdisp was defined as the difference between Pmax and Pmin.
Intraobserver and interobserver coefficients of variation (SD of differences between two observations divided by the mean value and expressed as percent) were 2.1% and 2.2% for Pmax and 2.9% and 3.1% for Pdisp, respectively.
Follow‐up After Catheter Ablation
All patients were scheduled for a visit 4–6 weeks after hospital discharge and every 3 months thereafter in the first year and every 6 months in the following years. If palpitations recurred, patients were asked to obtain an ECG as soon as possible and contact our center. Long‐term efficacy was assessed clinically on the basis of the resting 12‐lead ECG recording, 24‐hour Holter monitoring and clinical symptoms. Recurrence of paroxysmal AF was defined as intermittent AF lasting for at least 1 minute separated by periods of sinus rhythm.
Statistical Analysis
Data were expressed as percentage for discrete variables and as mean ± standard deviation for continuous variables. Groups were compared by means of chi‐square analysis or Fisher's exact test when needed for discrete variables and with unpaired Student's t‐test for continuous variables. Continuous variables within groups before and after ablation were compared with paired Student's t‐test.
Multivariate analysis with a Cox proportional hazards model was used to test whether the recurrence of paroxysmal AF was related to age, gender, duration of symptoms, frequency of palpitation, presence of hypertension, diabetes mellitus, structural heart disease, AF inducibility after ablation, Pdisp, and Pmax. A receiver‐operating characteristics (ROC) curve was constructed to identify the P wave cutoff values that differentiate patients with and without recurrence of AF with the best sensitivity and specificity levels. Statistical comparisons were performed using the statistical software package SPSS 10.01 (SPSS Inc., Chicago, IL, USA). In all statistical tests, calculated P values of less than 0.05 were considered significant.
RESULTS
Clinical and EP Characteristics of Groups
There was no difference between groups regarding basic clinical and echocardiographic variables, frequency and duration of symptoms (palpitation), or EP characteristics (Table 1). The patients in the study group had 5 ± 2 (range 2–11) documented episodes of AF before ablation. The average elapsed time from the first AF episode to AVNRT ablation was 3.1 ± 1.5 years (range 1–6 years).
Table 1.
Basic Clinical and Electrophysiological Characteristics of Patients with (Group 1) and without (Group 2) Paroxysmal AF
| Group‐1 (n = 36) | Group‐2 (n = 36) | P | |
|---|---|---|---|
| Age (years) | 49 ± 17 | 48 ± 12 | NS |
| Male/Female (%) | 58/42 | 42/58 | NS |
| Heart rate (beat/min) | 75 ± 10 | 78 ± 9 | NS |
| Systemic hypertension n (%) | 11 (31) | 10 (28) | NS |
| Diabetes mellitus n (%) | 8 (22) | 5 (14) | NS |
| Structural heart abnormalities | |||
| Valve disease n (%) | 8 (22) | 5 (14) | NS |
| Coronary artery disease n (%) | 5 (14) | 4 (11) | NS |
| Left ventricular hypertrophy n (%) | 6 (17) | 3 (8) | NS |
| Left atrial diameter >40 mm n (%) | 7 (19) | 4 (11) | NS |
| Mitral or aortic calcification n (%) | 7 (19) | 3 (8) | NS |
| LVEF < 50% n (%) | 3 (8) | 1 (3) | NS |
| Duration of symptoms (years) | 10 ± 8 | 12 ± 7 | NS |
| Frequency of palpitations (attacks/month) | 2.1 ± 1.0 | 2.3 ± 0.9 | NS |
| TCL (ms) | 323 ± 30 | 332 ± 29 | NS |
| HRA‐VA interval (ms) | 70 ± 12 | 71 ± 10 | NS |
| His‐VA interval (ms) | 30 ± 8 | 32 ± 12 | NS |
| AV node WCL (ms) | 337 ± 29 | 343 ± 29 | NS |
| AV node antegrade ERP (ms) | 251 ± 31 | 261 ± 31 | NS |
| Atrial vulnerability before ablation n (%) | 32 (89) | 6 (17) | <0.001 |
| Recurrence of AVNRT n (%) | 1 (3) | 0 | NS |
| Follow‐up (months) | 34 ± 13 | 32 ± 11 | NS |
LVEF= left ventricular ejection fraction; TCL= tachycardia cycle length; AV= atrioventricular; WCL= Wenckebach cycle length; NS= not significant.
In 38 of 72 patients (53%), sustained AF lasting for more than 30 seconds was induced (atrial vulnerability) during the standard stimulation before ablation. Thirty‐two of these cases (89%) were in the study group, and 6 (17%) were in the control group (P < 0.001) (Table 1). AF converted spontaneously to sinus rhythm in the first 15 minutes in 35 of 38 patients (92%); sinus rhythm was restored with DC cardioversion in the remaining three patients.
P Wave Durations Before and After Slow Pathway Ablation in Groups
Pmax and Pdisp were significantly higher in Group 1 than in Group 2 both before and after ablation (before ablation: 108.8 ± 8.6 vs 100.2 ± 9.7 ms and 35.1 ± 8.0 vs 27.9 ± 8.1 ms, respectively, P < 0.001 for both; after ablation: 108.3 ± 10.2 vs 99.3 ± 10.0 ms and 35.4 ± 8.8 vs 27.6 ± 8.4 ms, respectively, P < 0.001 for both). Pmin values did not differ significantly between groups, both before and after ablation (before ablation: 73.8 ± 5.4 vs 72.3 ± 6.6 ms P = 0.300; after ablation: 72.9 ± 6.4 vs 71.8 ± 7.3 ms, P = 0.507). There was no significant difference between P wave durations calculated before and after ablation within each group (Fig. 1).
Figure 1.
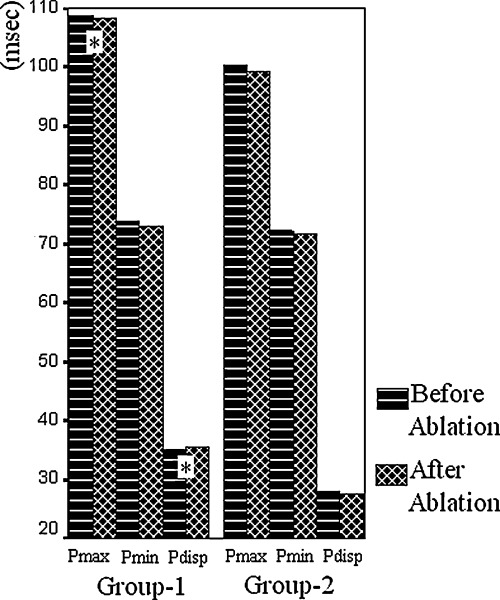
Intra‐ and intergroup comparisons of P wave durations and Pdisp before and after slow pathway ablation (*P < 0.001 as compared to Group 2).
Sixteen patients [10 patients in Group 1 (28%) and six patients in Group 2 (17%)] had residual slow pathway conduction with or without single echo beats after elimination of AVNRT. No significant difference was observed between the study and control groups with respect to the frequency of residual slow pathways (P > 0.050). Also, clinical features were not different in patients with and without residual slow pathway (P > 0.050). A subgroup analysis was done to assess the influence of slow pathway ablation on pre‐ and postablation P wave durations in patients with AVNRT and inducible AF (n = 30; 24 patients from Group 1 and 6 patients from Group 2). No significant difference was noted between pre‐ and postablation P wave durations in patients with AVNRT and inducible AF undergoing complete elimination of the slow pathway (107.6 ± 9.6 vs 107.4 ± 11.0 ms for Pmax; 73.5 ± 5.0 vs 73.3 ± 6.1 ms for Pmin and 34.1 ± 8.2 vs 34.2 ± 9.4 ms for Pdisp; P > 0.05 for all comparisons).
Clinical and EP Characteristics of Patients with and without Recurrence of Paroxysmal AF During Follow‐Up
During a mean follow‐up period of 28 ± 11 months (range 5–62), recurrence of AF was observed in 10 patients [Group 1(+); 28%] in Group 1. Most of the recurrences (n = 9) occurred within 1 year of catheter ablation. No statistical differences were observed between Group 1(+) and Group 1(−) regarding gender, presence of hypertension or diabetes mellitus, duration and frequency of symptoms before ablation, residual dual pathways, and the recurrence rate of AVNRT. In Group 1(+) patients, presence of valvular heart disease (P < 0.050), left atrial diameter >40 mm (P < 0.050), and atrial vulnerability after successful ablation (P < 0.010) were more frequent (Table 2).
Table 2.
Basic Clinical and Electrophysiological Characteristics of Patients with [Group 1(+)] and without [Group 1(−)] Recurrence of AF After Successful Ablation
| Group 1(+) (n = 10) | Group 1(−) (n = 26) | P | |
|---|---|---|---|
| Age (years) | 56 ± 16 | 46 ± 16 | NS |
| Male/Female (%) | 60/40 | 58/42 | NS |
| Systemic hypertension n (%) | 5 (50) | 6 (23) | NS |
| Diabetes mellitus n (%) | 2 (20) | 6 (23) | NS |
| Structural heart abnormalities | |||
| Valve disease n (%) | 5 (50) | 3 (12) | <0.050 |
| Coronary artery disease n (%) | 2 (20) | 3 (12) | NS |
| Left ventricular hypertrophy n (%) | 2 (20) | 4 (15) | NS |
| Left atrial diameter >40 mm n (%) | 5 (50) | 2 (8) | <0.050 |
| Mitral or aortic calcification n (%) | 4 (40) | 3 (12) | NS |
| LVEF < 50% n (%) | 2 (20) | 1 (4) | NS |
| Duration of symptoms (years) | 11 ± 9 | 9 ± 8 | NS |
| Frequency of palpitation before ablation (episodes/month) | 2.0 ± 0.7 | 2.2 ± 1.1 | NS |
| Atrial vulnerability after ablation n (%) | 7 (70) | 5 (19) | <0.010 |
| Residual dual pathway n (%) | 3 (30) | 7 (27) | NS |
| Recurrence of AVNRT n (%) | 0 | 1 (4) | NS |
LVEF = left ventricular ejection fraction; NS= not significant.
Prediction of AF Recurrence After Successful Catheter Ablation
Pdisp was significantly higher in Group 1(+) as compared to Group 1(−) (43.9 ± 8.5 vs 32.2 ± 6.4 ms, P < 0.001). This difference was related to the difference of Pmax between groups [119.2 ± 8.5 ms in Group 1(+) vs 104.1 ± 7.4 ms in Group 1(−), P < 0.001], as Pmin did not differ significantly (75.3 ± 3.6 vs 71.9 ± 7.1 ms, P = 0.162) (Fig. 2). A Pmax value of ≥112.0 ms separated Group 1(+) from Group 1(−) with a sensitivity of 80%, specificity of 87%, positive predictive value of 50%, and negative predictive value of 96%. A Pdisp value of ≥35.5 ms separated Group 1(+) from Group 1(−) with a sensitivity of 90%, specificity of 85%, positive predictive value of 50%, and negative predictive value of 98% (Fig. 3).
Figure 2.
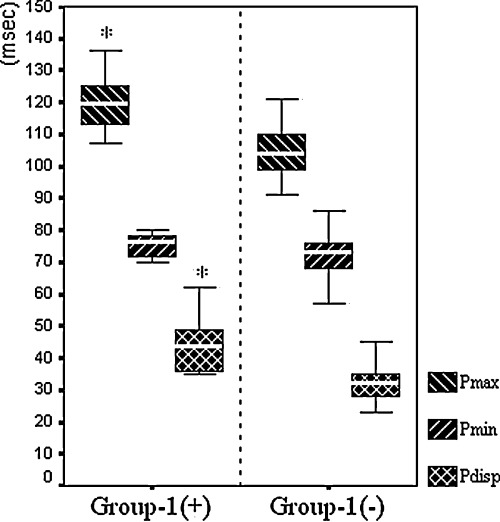
Pmax, Pmin, and Pdisp values, compared between patients with [Group 1(+)] and without [Group 1(−)] recurrence of AF after slow pathway ablation (*P < 0.001).
Figure 3.
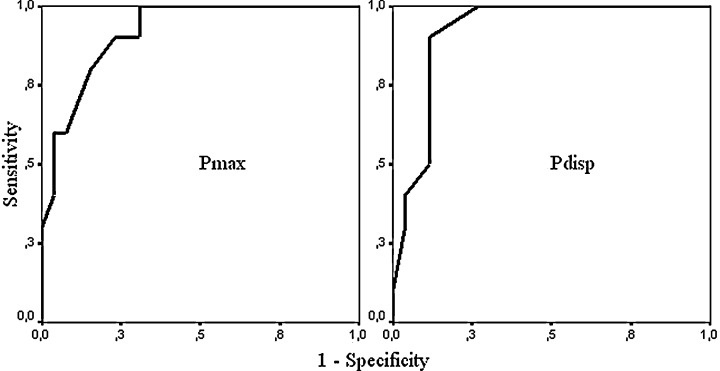
ROC curves for Pmax (area under curve: 0.917, 95% CI = 0.828–1.007, P < 0.001) and Pdisp (area under curve: 0.919, 95% CI = 0.830–1.009, P < 0.001).
In univariate analysis, significant predictors of AF were: Pdisp ≥ 35.5 ms (P < 0.010), Pmax ≥ 112 ms (P < 0.05), presence of valvular heart disease (P < 0.050), left atrial diameter >40 mm (P < 0.010), and atrial vulnerability after successful ablation (P < 0.050). However, using the multivariate analysis, only Pdisp ≥ 35.5 ms (P < 0.050) and left atrial diameter >40 mm (P < 0.010) remained significant as independent predictors of recurrence of AF after catheter ablation in patients with both AVNRT and paroxysmal AF (Table 3).
Table 3.
Univariate and Multivariate Predictors of AF Recurrence After Slow Pathway Ablation
| RR | 95% CI | P | |
|---|---|---|---|
| Univariate predictors | |||
| Left atrial diameter >40 mm | 17 | 3–87 | <0.010 |
| Pdisp ≥ 35.5 ms | 17 | 2–131 | <0.010 |
| Mitral or aortic calcification | 13 | 2–70 | <0.010 |
| Pmax ≥112 ms | 7 | 1–31 | <0.050 |
| Atrial vulnerability after ablation | 5 | 1–21 | <0.050 |
| Valvular heart disease | 4 | 1–13 | <0.050 |
| Multivariate predictors | |||
| Left atrial diameter >40 mm | 13 | 2–83 | <0.010 |
| Pdisp ≥35.5 ms | 12 | 1–101 | <0.050 |
RR = relative risk; CI= confidence interval.
DISCUSSION
Paroxysmal AF is the most common arrhythmia encountered in daily clinical practice with a significant potential risk for thromboembolism and its incidence is higher in patients with AVNRT, which is another common form of arrhythmia. 1 , 3 , 5 Although successful ablation of the slow pathway significantly decreases the incidence of paroxysmal AF in patients with both AVNRT and paroxysmal AF, AF can recur in up to 30% of patients after ablation. 11 However, to our knowledge, there has not been a simple noninvasive test developed to predict the recurrence of paroxysmal AF after successful RF catheter ablation in patients with AVNRT. This study is the first to show that Pdisp could be useful to predict recurrence of AF after successful catheter ablation.
Dilaveris et al. have first used Pdisp to evaluate the inhomogeneous and discontinuous atrial conduction in patients with lone paroxysmal AF. 6 They measured Pdisp in patients with history of paroxysmal AF and found higher Pdisp values in patients than in healthy controls (49 ± 15 ms vs 28 ± 7 ms). We have also found longer Pdisp values in patients with paroxysmal AF than in healthy subjects (44 ± 15 ms vs 27 ± 10 ms). 7 Therefore, studies show that Pdisp could be useful for separating patients with a history of paroxysmal AF from those without. However, there is no consensus about the cutoff value for Pdisp that separates patients who have a history of paroxysmal AF from healthy subjects. Dilaveris et al. identified 40 ms as cutoff value of Pdisp to separate patients with paroxysmal AF from controls, while we used 32.5 ms in another study, which excluded patients with structural heart disease. 12 The differences with respect to the cutoff values between these studies may be due to the patients' clinical characteristics and measurement methods used. In the present study, we have included patients with structural heart disease and found that a Pdisp value of ≥35.5 ms and a Pmax value of ≥112 ms can be used with reasonable positive and negative predictive values for detection of patients at risk for recurrence of AF after successful catheter ablation of AVNRT.
The incidence of paroxysmal AF is higher in patients with AVNRT than in normal population and is around 18%. 5 In a study where atrial vulnerability was evaluated with atrial transesophageal stimulation to induce AF sustainable for more than 1 minute, atrial vulnerability was found to be higher in patients with AVNRT than in normal population and in the same range as in patients with WPW syndrome. 3 The reasons for frequent occurrence of AF in patients with AVNRT have not yet been fully explained. The correlation between the presence of intraatrial conduction abnormalities and the induction of AF has been well documented. 13 , 14 D'Este et al. suggested that the greater atrial vulnerability to AF found in patients with AVNRT may be due to the presence of double nodal pathways. 3 Papageorgiou et al. showed that the conduction time to the region of the posterior triangle of Koch was significantly prolonged and the local slow pathway electrogram durations were broader only in patients with AF inducibility, signs which suggest a potential role of the slow pathway region in the inducibility of AF. 15 In addition, Chen et al. have shown in patients with AVNRT that mean atrial electrogram duration at the ablation site during high right atrial pacing was shorter after slow pathway ablation as compared to before. On the other hand, they have noticed no changes in patients with residual slow pathways, suggesting that the presence of slow pathway is an important factor in determining the local conduction properties at the posterior triangle of Koch. 16 It has been suggested that the presence of nonuniform anisotropic characteristics of the posterior triangle of Koch may be critical for AF induction. 15 However, after successful catheter ablation of the slow pathway, episodes of AF persist in up to 30% of patients, predominantly in those with structural heart disease. 11 , 17 We have not observed any significant changes among Pdisp, Pmax, and Pmin values calculated before and after slow pathway ablation, suggesting that the presence of slow antegrade AV nodal pathways do not render the atria prone to develop AF. It seems that, especially in those without structural heart disease, AVNRT acts as a main trigger for AF. The shortened atrial refractoriness during tachycardia resulting from atrial stretch caused by simultaneous contraction of the atria and ventricles, increased heart rate and vagal activation could be responsible for this triggering effect. 18 , 19 , 20 Brugada et al. reported four patients with documented AF, which was later proven with EP study to start with degeneration of AVNRT and did not recur after successful slow pathway ablation during a mean follow‐up of 14 ± 6 months. None of those patients had structural heart disease. 21
Study Limitations
Seasonal variation of P wave durations may have affected the predictive value of the cutoff point used in our study. However, the data collection process for this study had been started before the study about the seasonal variation of Pdisp was reported by Kose et al. 22
There is the possibility that episodes of AF may have occurred coincidentally as a result of structural heart disease or other reasons independent of AVNRT in some cases. However, the main purposes of this study were to investigate whether there are any changes in the value of Pmin, Pmax, and Pdisp observed before and after successful slow pathway ablation and whether these noninvasive parameters can predict the recurrence of paroxysmal AF in patients with both AVNRT and paroxysmal AF.
CONCLUSION
P dispersion could be utilized in the identification of patients at high risk for recurrence of paroxysmal AF after successful ablation of AVNRT, leading to closer follow‐up and planning of prophylactic management and in providing appropriate patient education.
REFERENCES
- 1. Elvas L, Gursoy S, Brugada J, et al Atrioventricular nodal reentrant tachycardia: A review. Can J Cardiol 1994;10:342–348. [PubMed] [Google Scholar]
- 2. Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: The Framingham Study. Stroke 1991;22:983–988. [DOI] [PubMed] [Google Scholar]
- 3. D'Este D, Pasqual A, Bertaglia M, et al Evaluation of atrial vulnerability with transoesophageal stimulation in patients with atrioventricular junctional reentrant tachycardia. Comparison with patients with ventricular pre‐excitation and with normal subjects. Eur Heart J 1995;16:1632–1636. [DOI] [PubMed] [Google Scholar]
- 4. Hamer ME, Wilkinson WE, Clair WK, et al Incidence of symptomatic atrial fibrillation in patients with paroxysmal supraventricular tachycardia. J Am Coll Cardiol 1995;25:984–988. [DOI] [PubMed] [Google Scholar]
- 5. Hurwitz JL, German LD, Packer DL, et al Occurrence of atrial fibrillation in patients with paroxysmal supraventricular tachycardia due to atrioventricular nodal reentry. Pacing Clin Electrophysiol 1990;13:705–710. [DOI] [PubMed] [Google Scholar]
- 6. Dilaveris PE, Gialafos EJ, Sideris SK, et al Simple electrocardiographic markers for the prediction of paroxysmal idiopathic atrial fibrillation. Am Heart J 1998;135:733–738. [DOI] [PubMed] [Google Scholar]
- 7. Aytemir K, Ozer N, Atalar E, et al P wave dispersion on 12‐lead electrocardiography in patients with paroxysmal atrial fibrillation. Pacing Clin Electrophysiol 2000;23:1109–1112. [DOI] [PubMed] [Google Scholar]
- 8. Haissaguerre M, Gaita F, Fischer B, et al Elimination of atrioventricular nodal reentrant tachycardia using discrete slow potentials to guide application of radiofrequency energy. Circulation 1992;85:2162–2175. [DOI] [PubMed] [Google Scholar]
- 9. Leitch J, Klein GJ, Yee R, et al Invasive electrophysiologic evaluation of patients with supraventricular tachycardia. Cardiol Clin 1990;8:465–477. [PubMed] [Google Scholar]
- 10. Kose S, Amasyali B, Aytemir K, et al Atrioventricular nodal reentrant tachycardia with multiple discontinuities in the atrioventricular node conduction curve: Immediate success rates of radiofrequency ablation and long‐term clinical follow‐up results as compared to patients with single or no AH‐jumps. J Interv Card Electrophysiol 2004;10:249–254. [DOI] [PubMed] [Google Scholar]
- 11. Delise P, Gianfranchi L, Paparella N, et al Clinical usefulness of slow pathway ablation in patients with both paroxysmal atrioventricular nodal reentrant tachycardia and atrial fibrillation. Am J Cardiol 1997;79:1421–1423. [DOI] [PubMed] [Google Scholar]
- 12. Aytemir K, Amasyali B, Kose S, et al Maximum p‐wave duration and p‐wave dispersion predict recurrence of paroxysmal atrial fibrillation in patients with wolff‐Parkinson‐white syndrome after successful radiofrequency catheter ablation. J Interv Card Electrophysiol 2004;11:21–27. [DOI] [PubMed] [Google Scholar]
- 13. Buxton AE, Waxman HL, Marchlinski FE, et al Atrial conduction: Effects of extrastimuli with and without atrial dysrhythmias. Am J Cardiol 1984;54:755–761. [DOI] [PubMed] [Google Scholar]
- 14. Cosio FG, Palacios J, Vidal JM, et al Electrophysiologic studies in atrial fibrillation: Slow conduction of premature impulses: A possible manifestation of the background for reentry. Am J Cardiol 1983;51:122–130. [DOI] [PubMed] [Google Scholar]
- 15. Papageorgiou P, Monahan K, Boyle NG, et al Site‐dependent intra‐atrial conduction delay. Relationship to initiation of atrial fibrillation. Circulation 1996;94:384–389. [DOI] [PubMed] [Google Scholar]
- 16. Chen YJ, Tai CT, Hsieh MH, et al Dependence of electrogram duration in right posteroseptal atrium and atrium‐pulmonary vein junction on pacing site: Mechanism and implications regarding atrioventricular nodal reentrant tachycardia and paroxysmal atrial fibrillation. J Cardiovasc Electrophysiol 2000;11:506–515. [DOI] [PubMed] [Google Scholar]
- 17. Weiss R, Knight BP, Bahu M, et al Long‐term follow‐up after radiofrequency ablation of paroxysmal supraventricular tachycardia in patients with tachycardia‐induced atrial fibrillation. Am J Cardiol 1997;80:1609–1610. [DOI] [PubMed] [Google Scholar]
- 18. Calkins H, El‐Atassi R, Kalbfleisch S, et al Effects of an acute increase in atrial pressure on atrial refractoriness in humans. Pacing Clin Electrophysiol 1992;15:1674–1680. [DOI] [PubMed] [Google Scholar]
- 19. Calkins H, Maughan WL, Kass DA, et al Electrophysiological effect of volume load in isolated canine hearts. Am J Physiol 1989;256:H1697–H1706. [DOI] [PubMed] [Google Scholar]
- 20. Zipes DP, Mihalick MJ, Robbins GT. Effects of selective vagal and stellate ganglion stimulation of atrial refractoriness. Cardiovasc Res 1974;8:647–655. [DOI] [PubMed] [Google Scholar]
- 21. Brugada J, Mont L, Matas M, et al Atrial fibrillation induced by atrioventricular nodal reentrant tachycardia. Am J Cardiol 1997;79:681–682. [DOI] [PubMed] [Google Scholar]
- 22. Kose S, Aytemir K, Can I, et al Seasonal variation of P‐wave dispersion in healthy subjects. J Electrocardiol 2002;35:307–311. [DOI] [PubMed] [Google Scholar]


