Abstract
Background: The prognostic value of ST‐segment resolution (STR) after initiation of reperfusion therapy has been established by various studies conducted in both the thrombolytic and mechanic reperfusion era. However, data regarding the value of STR immediately prior to primary percutaneous coronary intervention (PCI) to predict infarct‐related artery (IRA) patency remain limited. We investigated whether STR prior to primary PCI is a reliable, noninvasive indicator of IRA patency in patients with ST‐segment elevation myocardial infarction (STEMI).
Methods: The study population consisted of STEMI patients who underwent primary PCI at our institution between 2000 and 2007. STR was analyzed in 12‐lead electrocardiograms recorded at first medical contact and immediately prior to primary PCI and defined as complete (≥70%), partial (70%− 30%), or absent (<30%).
Results: In 1253 patients with a complete data set, STR was inversely related to the probability of impaired preprocedural flow (Pfor trend < 0.001). Although the sensitivity of incomplete (<70%) STR to predict a Thrombolysis in Myocardial Infarction (TIMI) flow of <3 was 96%, the specificity was 23%, and the negative predictive value of incomplete STR to predict normal coronary flow was only 44%.
Conclusions: This study establishes the correlation between STR prior to primary PCI and preprocedural TIMI flow in STEMI patients treated with primary PCI. However, the negative predictive value of incomplete STR for detection of TIMI‐3 flow is only 44% and therefore should not be a criterion to refrain from immediate coronary angiography in STEMI patients.
Ann Noninvasive Electrocardiol 2010;15(2):107–115
Keywords: ST‐segment elevation myocardial infarction, primary percutaneous coronary intervention, ST‐segment resolution, preprocedural TIMI flow
Primary percutaneous coronary intervention (PCI) is superior compared to fibrinolysis regarding survival in patients with ST‐segment elevation myocardial infarction (STEMI). 1 , 2 This survival benefit is mainly due to a more effective restoration of coronary artery patency by means of coronary angioplasty. 3 , 4 , 5 , 6 Several survival scores have incorporated postprocedural Thrombolysis in Myocardial Infarction (TIMI) flow through the infarct‐related artery (IRA) as an important component for risk stratification of patients after primary PCI for STEMI. 7 , 8 Also, preprocedural TIMI flow has been recognized as an important predictor of clinical outcome. 9 An analysis of randomized trials emphasized the prognostic significance of adequate TIMI flow during diagnostic angiography prior to angioplasty. After multivariable analysis, preprocedural TIMI‐3 flow was a more powerful prognostic predictor than TIMI‐3 flow after angioplasty, 10 which underscores the importance of early flow restoration in STEMI patients.
While epicardial coronary flow has an important impact, reperfusion therapy aims at myocardial rather than just epicardial reperfusion. The relationship between epicardial as well as myocardial flow restoration and ST‐segment resolution (STR) has been established. Incomplete STR after reperfusion therapy, despite improved epicardial flow, is associated with impaired microvascular circulation and is an indicator of larger infarct size and increased mortality. 11 , 12 , 13 While there are extensive data on the prognostic value of postprocedural STR, data on the value of ST‐segment changes preceding primary PCI are limited. We sought to determine if early STR in STEMI patients at the time of emergency coronary angiography is a reliable, noninvasive indicator of coronary artery patency.
METHODS
Source Population
The data analyzed in our study were obtained from STEMI patients who underwent primary PCI at the Academic Medical Center – University of Amsterdam between November 1, 2000 and January 1, 2007. Generally, primary PCIs were performed according to current guidelines. Patients with an indication for primary PCI received aspirin (500 mg) and unfractionated heparin (5000 IU) during transportation to the catheterization laboratory. As per 2005, 300 mg of clopidogrel was added as pretreatment prior to primary PCI. Prehospital glycoprotein IIb/IIIa inhibition was not administered.
We obtained information on 1‐year vital status from the institutional follow‐up database of PCI patients. Patients are surveyed 1 year after PCI by means of a mailed, self‐administered questionnaire. Information on mortality was synchronized with computerized records from the National Death Index. We reviewed outpatients’ files and contacted general practitioners by telephone in case of conflicting or missing data.
Data Sources
We utilized the local electronic database at the catheterization laboratory to retrieve baseline demographic variables, procedural and angiographic information that had been prospectively collected and entered by specialized nurses and interventional cardiologists concurrently with routine patient care. This information included the operator's online assessment of antegrade flow using the TIMI scale 14 and preprocedural luminal obstruction.
ECG Collection and Analysis
For all STEMI patients who underwent primary PCI at our center, we retrospectively sought to collect 12‐lead electrocardiograms (ECGs) recorded at first medical contact, either by ambulance personnel, at a referring hospital without PCI facility, or at our emergency department. These ECGs at first medical contact were then matched with 12‐lead recordings obtained at our catheterization laboratory immediately prior to arterial puncture and emergency coronary angiography.
ECGs of included patients were analyzed by one experienced investigator (N.V.), unaware of the angiographic and outcome data. ST‐segment deviation was measured with a handheld caliper and magnifying glass at 80 milliseconds after the J‐point in all available leads. The TP‐segment was considered the preferred iso‐electric baseline and ST‐segment deviation was measured to the nearest 0.05 mV. We calculated the cumulative ST‐segment deviation as the total sum of the absolute value of ST‐segment deviations in all 12 standard leads of the ECG. We defined STR as the relative difference (in%) of the cumulative ST‐segment deviation between the 12‐lead ECG at first medical contact and the immediately preprocedural 12‐lead ECG. In case of missing leads or artifacts, patients were included if at least 50% of the corresponding, infarct‐related leads were suitable for ST‐segment analysis. According to validated algorithms, leads V1 to V6, I, and aVL were considered directly infarct‐related in anterior myocardial infarction (MI); II, III, aVF, V5, and V6 in nonanterior MI. We classified STR into complete (≥70%), partial (between 70% and 30%), or absent (<30%), according to Schröder et al. 15
Study Population
We included all STEMI patients who underwent primary PCI at our institution and from whom matched ECGs could be retrieved. We excluded patients who underwent primary PCI of the left main coronary artery or a bypass graft because of the distinctive ECG pattern observed in such cases. Patients who underwent primary PCI but whose matched ECGs did not meet the ESC/ACC criteria for STEMI 16 were excluded from ST‐segment analysis. Patients with recordings containing complete left bundle branch block, an accelerated idioventricular rhythm, a paced rhythm, or severe artifacts, which restrain from accurate ST‐segment evaluation, were also excluded.
Statistical Analyses
Normally distributed, continuous variables are expressed as means (±SD) and were compared using a Student's t‐test. Other continuous data are expressed as median with interquartile range (IQR). All categorical variables are depicted using absolute and relative frequency distributions and the chi‐square test was used to make a comparison. We performed a power calculation assuming a single one‐way contrast between the proportions in three groups (STR < 30%, 30%≤ STR < 70%, STR ≥ 70%) with unequal size using a logistic model. With current sample size (N = 1253), the study had 80% power to detect a trend of −0.035 using a 2‐sided test with a significance level of 0.05. With a receiver‐operating‐characteristic (ROC) analysis, we evaluated the diagnostic performance of STR at different cutoffs besides the established 30% and 70%. Because a high sensitivity is desirable for detection of impaired TIMI flow, we determined that an optimal cutoff should at least have a sensitivity of 90%.
Logistic regression analysis was used to determine whether preprocedural STR accurately predicts IRA patency after adjustment for potential confounding baseline characteristics. All variables were entered in block. All‐cause mortality‐rate estimates were calculated by the Kaplan–Meier method and compared using the log‐rank statistic. We defined a high‐risk subgroup of patients suffering an anterior STEMI and/or with advanced age (≥75 years). For all tests, differences were significant if the 2‐sided P‐value was less than 0.05. All analyses were performed using SPSS software package (Version 14.0; SPSS, Inc., Chicago, IL, USA).
RESULTS
Study Cohort
The study cohort consisted of 1253 (39%) patients with a complete data set, extracted from a population of 3185 patients who underwent primary PCI at our medical center. Missing ECGs at first medical contact or poor quality ECGs contributed largely to the selection of patients (Fig. 1). Baseline and procedural characteristics of the study cohort are depicted in Table 1.
Figure 1.
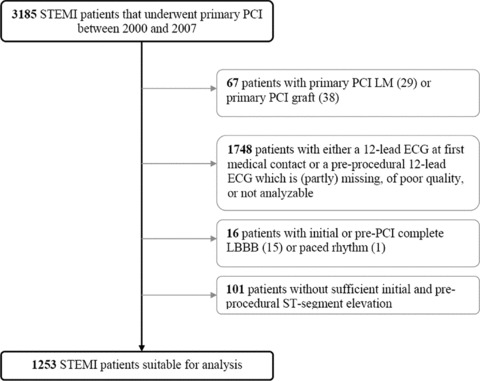
Selection study population. Overview of selection of patients suitable for inclusion in this study.
Table 1.
Baseline and Procedural Characteristics of Included STEMI Patients Who Underwent Primary PCI
| Characteristic | STEMI Patients Included (n = 1253) |
|---|---|
| Age (years [SD]) | 60 (13) |
| Male gender (%) | 72 |
| Current smoking (%) | 48 |
| Family history (%) | 44 |
| Diabetes mellitus (%) | 11 |
| BMI (kg/m2[SD]) | 27 (4) |
| Hypertension (%) | 33 |
| Hypercholesterolemia (%) | 24 |
| Previous MI (%) | 12 |
| Previous CABG (%) | 1 |
| Previous PCI (%) | 7 |
| Anterior MI (%) | 45 |
| Multivessel disease (%) | 32 |
| Pre‐PCI TIMI flow 0–2 (%) | 87 |
| Stent inserted (%) | 85 |
| IABP inserted (%) | 6 |
| Procedural success (%) | 97 |
| Total ischemic time (minutes [IQR]) | 176 (133–244) |
| Peak CK‐MB (μg/L [IQR])* | 260 (134–436) |
*Data available in 764 patients (61%). Standard deviations and interquartile ranges in parenthesis.
BMI = body mass index; CABG coronary artery bypass graft surgery; IABP = intraaortic balloon pump; IQR = interquartile range; MI = myocardial infarction; PCI = percutaneous coronary intervention; SD = standard deviation; STEMI = ST‐segment elevation myocardial infarction; TIMI = Thrombolysis in Myocardial Infarction.
Electrocardiography
The ECG at first medical contact was recorded at a median time of 99 minutes (IQR 63; 160) after symptom onset. The preprocedural ECG was performed at a median time of 64 minutes (IQR 50; 87) after the ECG recording at first medical contact and 10 minutes (IQR 4; 17) before restoration of IRA patency, which signifies a median contact‐to‐balloon time of 74 minutes.
On average 98% of the standard leads on each ECG could be analyzed. ECG analysis showed incomplete STR (<70%) or even additional ST‐segment elevation between the ECG at first medical contact and preprocedural ECG in the majority (74%) of patients.
Angiography
In 560 (45%) patients, emergency angiography showed anterior STEMI, while the majority (693 patients [55%]) suffered from nonanterior STEMI. Diagnostic angiography revealed impaired coronary flow (TIMI flow 0–2) in 1093 patients (87%).
Characteristics of included patients according to preprocedural TIMI flow are depicted in Table 2. Except for a higher percentage of current smokers and patients with a family history of coronary artery disease with preprocedural TIMI‐3 flow, baseline characteristics of the groups were comparable. On the other hand, preprocedural TIMI flow was significantly associated with procedural characteristics and prognostic indicators such as a preprocedural luminal obstruction, postprocedural TIMI‐3 flow, the need for intraaortic balloon pump insertion, total ischemic time and peak CK‐MB values.
Table 2.
Demographic and Procedural Characteristics According to Preprocedural TIMI Flow
| Characteristic | TIMI 0–1 (n = 957) | TIMI‐2 (n = 136) | TIMI‐3 (n = 160) | P |
|---|---|---|---|---|
| Age (years ± SD) | 60 (13) | 61 (13) | 60 (13) | 0.64 |
| Male gender (%) | 72 | 74 | 66 | 0.23 |
| Current smoking (%) | 47 | 45 | 59 | 0.02 |
| Family history (%) | 44 | 37 | 52 | 0.03 |
| Diabetes Mellitus (%) | 10 | 14 | 13 | 0.33 |
| BMI (kg/m2[SD]) | 27 (4) | 27 (4) | 26 (5) | 0.25 |
| Hypertension (%) | 33 | 28 | 34 | 0.45 |
| Hypercholesterolemia (%) | 23 | 27 | 22 | 0.63 |
| Previous MI (%) | 12 | 9 | 11 | 0.47 |
| Previous CABG (%) | 1 | 1 | 0 | 0.46 |
| Previous PCI (%) | 7 | 6 | 5 | 0.60 |
| Anterior MI (%) | 43 | 52 | 47 | 0.09 |
| Pre‐PCI luminal obstruction >90% (%) | 97 | 89 | 85 | <0.01 |
| Multivessel disease (%) | 32 | 35 | 31 | 0.67 |
| Stent inserted (%) | 83 | 93 | 91 | <0.01 |
| IABP inserted (%) | 7 | 6 | 1 | <0.01 |
| Post‐PCI TIMI‐3 flow (%) | 87 | 90 | 98 | <0.01 |
| Total ischemic time (minutes [IQR]) | 181 (134–250) | 170 (120–233) | 173 (139–234) | 0.04 |
| Peak CK‐MB (μg/L [IQR])* | 286 (155–471) | 156 (64–295) | 132 (54–291) | <0.01 |
*Data available in 764 patients. Standard deviations and interquartile ranges in parenthesis.
BMI = body mass index; CABG coronary artery bypass graft surgery; IABP = intraaortic balloon pump; IQR = interquartile range; MI = myocardial infarction; PCI = percutaneous coronary intervention; SD = standard deviation; STEMI = ST‐segment elevation myocardial infarction; TIMI = Thrombolysis in Myocardial Infarction.
Preprocedural STR
STR was associated with the probability of impaired TIMI flow during angiography prior to primary PCI. Categorical STR was inversely related to the probability of impaired preprocedural flow (Pfor trend < 0.001, Table 3). On the basis of ROC analysis (area under the curve 0.68, P < 0.001), we identified a range of STR cutoffs with acceptable diagnostic accuracy (Fig. 2). A sensitivity of at least 90% was achieved at cutoffs of 50% or higher, with negative likelihood ratios decreasing from 0.23 at 50% to 0.17 at a cutoff of 70%. The sensitivity of incomplete STR at a cutoff of 70% for identifying impaired coronary flow prior PCI was 96%.
Table 3.
Association between Preprocedural STR Prior to Primary PCI and Impaired Preprocedural Flow (TIMI 0–2)
| STR Pre‐PCI—All Patients | TIMI 0–2 | P* | ||
|---|---|---|---|---|
| <30% | (n = 930) | 92% | (853/930) |
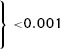
|
| 30–70% | (n = 242) | 81% | (195/242) | |
| >70% | (n = 81) | 56% | (45/81) | |
*Comparison by means of Cochran–Armitage test. Values are percentages (fractions) unless otherwise indicated.
PCI = percutaneous coronary intervention; STR = ST‐segment resolution; TIMI = Thrombolysis in Myocardial Infarction.
Figure 2.
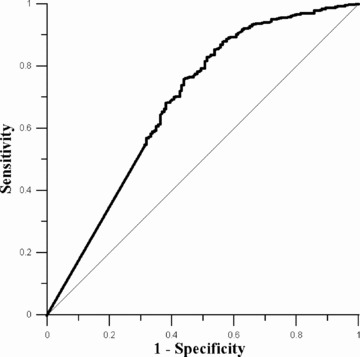
Accuracy of incomplete STR to predict impaired TIMI flow. ROC curve showing diagnostic accuracy of incomplete STR to predict a preprocedural TIMI flow of less than 3.
At a cutoff of 70%, only 36 of 81 patients with ≥70% STR showed TIMI‐3 flow through the IRA during emergency coronary angiography, which resulted in a negative predictive value of 44% and a specificity of 23% (Table 4). The test properties of preprocedural STR (with cutoffs at 30% and 70%) as a marker of impaired coronary flow prior to primary PCI are depicted in Table 4. Furthermore, 51 in a subgroup of 81 patients with complete STR prior to primary PCI did not have enough residual ST‐segment elevation to meet the current STEMI criteria. 16 Of these 51 patients with nonindicative preprocedural ECG, 28 (55%) had impaired flow during emergency angiography. Presence of ST‐segment elevation on the preprocedural ECG indicative for STEMI did not predict preprocedural impaired TIMI flow in this complete STR patient subgroup (57% vs 55%; P = 0.88, Table 5).
Table 4.
Test Properties of Pre‐PCI STR (at a 30% and 70% Cutoff) as a Marker of Impaired Flow (TIMI 0–2) Prior to PCI
| STR < 70% | STR < 30% | |||
|---|---|---|---|---|
| Sensitivity (%) | 96 | (1048/1093) | 78 | (853/1093) |
| Specificity (%) | 23 | (36/160) | 52 | (83/160) |
| PPV (%) | 89 | (1048/1172) | 92 | (853/930) |
| NPV (%) | 44 | (36/81) | 26 | (83/323) |
| PLR | 1.25 | 1.63 | ||
| NLR | 0.17 | 0.42 | ||
NLR = negative likelihood ratio; NPV = negative predictive value; PCI = percutaneous coronary intervention; PLR = positive likelihood ratio; PPV = positive predictive value; STR = ST‐segment deviation resolution; TIMI = Thrombolysis in Myocardial Infarction.
Table 5.
Association between Residual ST‐Segment Elevation Prior to Primary PCI and Impaired Preprocedural Flow (TIMI 0–2)
| TIMI 0–2 | P* | |||
|---|---|---|---|---|
| Residual ST‐Segment Elevation† on Pre‐PCI ECG—All Patients | ||||
| Present | (n = 1147) | 90% | (1033/1147) |
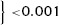
|
| Absent | (n = 106) | 57% | (60/106) | |
| Residual ST‐Segment Elevation† on Pre‐PCI ECG—Patients with STR ≥70% | ||||
| Present | (n = 30) | 57% | (17/30) |
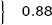
|
| Absent | (n = 51) | 55% | (28/51) | |
*Comparison by means of Cochran–Armitage test. Values are percentages (fractions) unless otherwise indicated.
†Residual ST‐segment elevation according to ECG criteria in Thygesen et al. 16
PCI = percutaneous coronary intervention; STR = ST‐segment resolution; TIMI = Thrombolysis in Myocardial Infarction.
Multivariable logistic regression identified complete STR prior to emergency angiography as an independent predictor of preprocedural TIMI‐3 flow with an odds ratio (OR) of 7.3 (95% CI 4.5–12.0; P < 0.001). It furthermore showed a relative increase of 2.9% in probability of preprocedural TIMI‐3 flow with each percent of improved STR (OR 1.029, 95% CI 1.02–1.04; P < 0.001).
Survival Analysis
Follow‐up was completed in 1247 patients (>99%). At 1 year after primary PCI, 68 patients (5%) had died. Complete (≥70%) STR prior to primary PCI did not predict 1‐year mortality (P = 0.83). Patients with preprocedural TIMI‐3 flow showed a trend toward improved long‐term survival compared to patients with impaired epicardial flow prior to primary PCI (P = 0.17), although statistical significance was not reached. In a subgroup analysis, TIMI‐3 flow prior to primary PCI showed to be particularly predictive for 1‐year survival in a high‐risk patient population (Fig. 3, Panel B). Presence of normal TIMI‐3 flow after primary PCI was more likely in patients who presented with normal flow prior to the procedure compared to patients with a preprocedural TIMI flow of 0–2 (98% vs 88%; P < 0.001).
Figure 3.
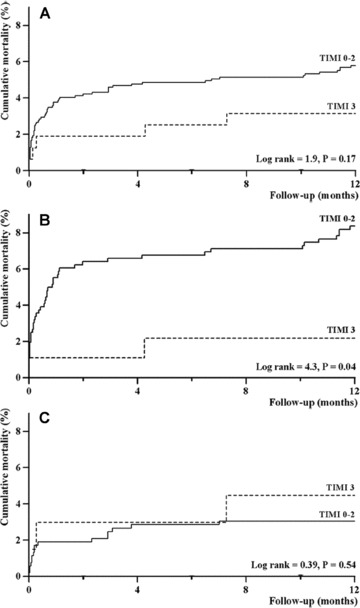
Association between preprocedural TIMI flow and 1‐year survival. Cumulative mortality curves as a function of preprocedural TIMI flow are depicted for the entire study cohort (Panel A), for a high‐risk patient group (anterior STEMI and/or age ≥ 75 years, Panel B), and for a low‐risk patient group (nonanterior STEMI and age < 75 years, Panel C). Impaired flow prior to primary PCI seems particularly predictive in high‐risk STEMI patients (Panel B, P = 0.04). PCI = percutaneous coronary intervention; STEMI = ST‐segment elevation myocardial infarction; TIMI = Thrombolysis in Myocardial Infarction.
DISCUSSION
To our knowledge, the present study is the first to investigate whether STR prior to primary PCI could be a real time pathophysiological indicator of epicardial reperfusion in patients suffering from STEMI. Despite the correlations shown in this study, clinical usefulness of STR to assess restoration of coronary flow is likely to be limited, because in more than half of primary PCI patients with complete preprocedural STR impaired coronary flow was found during angiography. Furthermore, even in patients with complete STR prior to primary PCI resulting in a preprocedural ECG nonindicative for STEMI, more than 50% had a preprocedural TIMI flow less than 3. Therefore, in STEMI patients with temporary ST‐segment elevation prior to primary PCI, this noninvasive measure should not withhold operators to assess flow through the IRA by emergency coronary angiography.
Preprocedural STR and TIMI Flow
Several studies showed the association between STR after pharmacological or mechanical therapy and long‐term mortality in STEMI patients. 13 , 17 , 18 , 19 , 20 , 21 , 22 Even in patients with documented, successful epicardial revascularization, absence of STR after initiation of therapy was associated with higher mortality, likely due to impaired microvascular reperfusion. As an indicator of IRA patency, correlation between STR and TIMI flow at 90 minutes after start of thrombolysis was investigated by De Lemos et al. A relatively low specificity of persistent ST‐segment elevation for IRA occlusion was found, triggering the need for more accurate noninvasive makers of reperfusion. 23 However, STR was evaluated at a median of 5.5 hours after symptom onset, which could have resulted in more extensive microvascular damage influencing ST‐segment recovery despite presence of IRA patency.
In the setting of facilitated PCI for acute STEMI, the FINESSE investigators confirmed the correlation between pre‐PCI STR and TIMI‐3 flow, although STR was not evaluated at the same time coronary angiography was performed and the median time from onset symptoms‐to‐balloon was longer than 4 hours. The reported nonlinear relationship between treatment delay and mortality benefit in patients with acute STEMI stresses the importance of IRA flow restoration within the first hours after symptom onset. 24
This study confirmed the correlation between STR prior to primary PCI and the occurrence of reperfusion of the IRA in STEMI patients. However, the diagnostic accuracy of STR to predict IRA patency was limited. More than 50% of patients with adequate STR (≥70%) showed a TIMI flow less than 3 during acute diagnostic angiography of the IRA. Even in a subgroup of patients with a pre‐PCI ECG without enough ST‐segment elevation to meet the electrocardiographic STEMI criteria, 55% had impaired flow during emergency angiography and herewith an indication for PCI. An explanation for these discrepancies could be the recruitment of a collateral circulation in response to prolonged ischemia or ischemia‐induced preconditioning, which could protect the threatened myocardium. 25 , 26 Furthermore, the ischemia‐related changes of transmembrane action potentials of individual cardiomyocytes during prolonged transmural ischemia could contribute to this observation. 27 , 28 In patients with an occluded coronary artery prior to primary PCI, the observation of adequate preprocedural STR could identify a subgroup with relatively benign prognosis, as suggested previously. 29 A potential survival benefit was not found in present study, mainly because of the limited number of events in this subgroup.
Serial ECG Recordings
We used serial 12‐lead ECG recordings to document ST‐segment changes over time. As a consequence, information on the dynamic behavior of the 12‐lead ECG over time, both spontaneously and in response to medication, was not available in this study. Continuous registration of the ECG provides more precise monitoring of the severity of transmural ischemia. This provides physicians with a more reliable assessment of maximum ST‐segment elevation and therefore a more accurate estimation of ST‐segment changes prior to the procedure. 30 However, in contrast to the nonocclusive pathogenesis of non‐ST‐elevation myocardial infarction, 31 the IRA is continuously occluded in the vast majority of STEMI patients during the first hours of ischemia. 32 This implies a more stable level of transmural ischemia resulting in less electrocardiographic variability over time. Furthermore, the use of serial ECG recordings to establish ST‐segment changes over time is easier to implement in daily practice and evaluation can be done instantly.
Limitations
Our data are essentially hypothesis generating. We did not randomize between immediate coronary angiography and a more conservative strategy in patients with complete STR prior to the procedure. A definite answer to the question whether immediate coronary angiography and subsequent PCI results in better outcome requires a prospective randomized controlled trial. Furthermore, ECG recordings were retrospectively collected. In our view, it is unlikely that the selection of patients in the retrospective study design has influenced the relation between STR and TIMI flow. Compared to the current study, higher rates of preprocedural TIMI‐3 flow in other studies may be explained by longer total ischemic times or the prehospital administration of triple antiplatelet therapy. 10 , 33
The extent of collateral circulation could have affected the association between preprocedural STR and TIMI flow in this study. Unfortunately, information regarding presence of collateral vessels during coronary angiography was not routinely collected. Also, the time interval of 10 minutes between the preprocedural ECG and emergency coronary angiography could have weakened the association between STR and pre‐PCI TIMI flow in this study. Furthermore, ST‐segment elevation and subsequent resolution may be difficult to assess in conditions that influence the ST‐segment, such as pericardial effusion or chronic pulmonary disease. However, these conditions are not very common in the early setting of primary PCI.
Regarding the association between preprocedural TIMI flow and 1‐year mortality, we would like to emphasize that the current dataset is underpowered and that mortality data are presented for descriptive purposes only. It goes without saying that a larger cohort is needed to investigate the possible association between preprocedural TIMI flow and long‐term survival in primary PCI patients presenting early after symptom onset.
Conclusion and Clinical Implication
This study establishes the correlation between STR prior to primary PCI and preprocedural TIMI flow in STEMI patients treated with primary PCI. However, STR is a poor indicator of spontaneous reperfusion and should likely not be used as a criterion to refrain from immediate coronary angiography in STEMI patients.
Conflicts of Interest: None of the authors have any conflict of interest regarding this subject.
Funding: None.
REFERENCES
- 1. Boersma E. Does time matter? A pooled analysis of randomized clinical trials comparing primary percutaneous coronary intervention and in‐hospital fibrinolysis in acute myocardial infarction patients. Eur Heart J 2006;27:779–788. [DOI] [PubMed] [Google Scholar]
- 2. Keeley EC, Boura JA, Grines CL. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: A quantitative review of 23 randomised trials. Lancet 2003;361:13–20. [DOI] [PubMed] [Google Scholar]
- 3. Grines CL, Cox DA, Stone GW, et al Coronary angioplasty with or without stent implantation for acute myocardial infarction. Stent Primary Angioplasty in Myocardial Infarction Study Group. N Engl J Med 1999;341:1949–1956. [DOI] [PubMed] [Google Scholar]
- 4. Stone GW, Brodie BR, Griffin JJ, et al Prospective, multicenter study of the safety and feasibility of primary stenting in acute myocardial infarction: In‐hospital and 30‐day results of the PAMI stent pilot trial. Primary Angioplasty in Myocardial Infarction Stent Pilot Trial Investigators. J Am Coll Cardiol 1998;31:23–30. [DOI] [PubMed] [Google Scholar]
- 5. Mehta RH, Harjai KJ, Cox D, et al Clinical and angiographic correlates and outcomes of suboptimal coronary flow inpatients with acute myocardial infarction undergoing primary percutaneous coronary intervention. J Am Coll Cardiol 2003;42:1739–1746. [DOI] [PubMed] [Google Scholar]
- 6. Stone GW, Grines CL, Cox DA, et al Comparison of angioplasty with stenting, with or without abciximab, in acute myocardial infarction. N Engl J Med 2002;346:957–966. [DOI] [PubMed] [Google Scholar]
- 7. De Luca G, Suryapranata H, Van‘t Hof AW, et al Prognostic assessment of patients with acute myocardial infarction treated with primary angioplasty: Implications for early discharge. Circulation 2004;109:2737–2743. [DOI] [PubMed] [Google Scholar]
- 8. Halkin A, Singh M, Nikolsky E, et al Prediction of mortality after primary percutaneous coronary intervention for acute myocardial infarction: The CADILLAC risk score. J Am Coll Cardiol 2005;45:1397–1405. [DOI] [PubMed] [Google Scholar]
- 9. Brodie BR, Stuckey TD, Hansen C, et al Benefit of coronary reperfusion before intervention on outcomes after primary angioplasty for acute myocardial infarction. Am J Cardiol 2000;85:13–18. [DOI] [PubMed] [Google Scholar]
- 10. Stone GW, Cox D, Garcia E, et al Normal flow (TIMI‐3) before mechanical reperfusion therapy is an independent determinant of survival in acute myocardial infarction: Analysis from the primary angioplasty in myocardial infarction trials. Circulation 2001;104:636–641. [DOI] [PubMed] [Google Scholar]
- 11. Buller CE, Fu Y, Mahaffey KW, et al ST‐segment recovery and outcome after primary percutaneous coronary intervention for ST‐elevation myocardial infarction: Insights from the Assessment of Pexelizumab in Acute Myocardial Infarction (APEX‐AMI) trial. Circulation 2008;118:1335–1346. [DOI] [PubMed] [Google Scholar]
- 12. McLaughlin MG, Stone GW, Aymong E, et al Prognostic utility of comparative methods for assessment of ST‐segment resolution after primary angioplasty for acute myocardial infarction: The Controlled Abciximab and Device Investigation to Lower Late Angioplasty Complications (CADILLAC) trial. J Am Coll Cardiol 2004;44:1215–1223. [DOI] [PubMed] [Google Scholar]
- 13. Van ‘t Hof AW, Liem A, De Boer MJ, et al Clinical value of 12‐lead electrocardiogram after successful reperfusion therapy for acute myocardial infarction. Zwolle Myocardial Infarction Study Group. Lancet 1997;350:615–619. [DOI] [PubMed] [Google Scholar]
- 14. The Thrombolysis in Myocardial Infarction (TIMI) Trial. Phase I findings. TIMI Study Group. N Engl J Med 1985;312:932–936. [DOI] [PubMed] [Google Scholar]
- 15. Schroder R, Dissmann R, Bruggemann T, et al Extent of early ST segment elevation resolution: A simple but strong predictor of outcome in patients with acute myocardial infarction. J Am Coll Cardiol 1994;24:384–391. [DOI] [PubMed] [Google Scholar]
- 16. Thygesen K, Alpert JS, White HD. Universal definition of myocardial infarction. J Am Coll Cardiol 2007;50:2173–2195. [DOI] [PubMed] [Google Scholar]
- 17. Anderson RD, White HD, Ohman EM, et al Predicting outcome after thrombolysis in acute myocardial infarction according to ST‐segment resolution at 90 minutes: A substudy of the GUSTO‐III trial. Global use of strategies to open occluded coronary arteries. Am Heart J 2002;144:81–88. [DOI] [PubMed] [Google Scholar]
- 18. De Lemos JA, Antman EM, Giugliano RP, et al Comparison of a 60‐ versus 90‐minute determination of ST‐segment resolution after thrombolytic therapy for acute myocardial infarction. In TIME‐II Investigators. Intravenous nPA for Treatment of Infarcting Myocardium Early‐II. Am J Cardiol 2000;86:1235–1237, A5. [DOI] [PubMed] [Google Scholar]
- 19. Lockwood E, Fu Y, Wong B, et al Does 24‐hour ST‐segment resolution postfibrinolysis add prognostic value to a Q wave? An ASSENT 2 electrocardiographic substudy. Am Heart J 2003;146:640–645. [DOI] [PubMed] [Google Scholar]
- 20. Lee AK, Sadick N, Ng A, et al Prognostic implication of ST‐segment resolution following primary percutaneous transluminal coronary angioplasty for ST‐elevation acute myocardial infarction. Intern Med J 2004;34:551–556. [DOI] [PubMed] [Google Scholar]
- 21. Matetzky S, Novikov M, Gruberg L, et al The significance of persistent ST elevation versus early resolution of ST segment elevation after primary PTCA. J Am Coll Cardiol 1999;34:1932–1938. [DOI] [PubMed] [Google Scholar]
- 22. Sorajja P, Gersh BJ, Costantini C, et al Combined prognostic utility of ST‐segment recovery and myocardial blush after primary percutaneous coronary intervention in acute myocardial infarction. Eur Heart J 2005;26:667–674. [DOI] [PubMed] [Google Scholar]
- 23. De Lemos JA, Antman EM, Giugliano RP, et al ST‐segment resolution and infarct‐related artery patency and flow after thrombolytic therapy. Thrombolysis in Myocardial Infarction (TIMI) 14 investigators. Am J Cardiol 2000;85:299–304. [DOI] [PubMed] [Google Scholar]
- 24. Boersma E, Maas AC, Deckers JW, et al Early thrombolytic treatment in acute myocardial infarction: Reappraisal of the golden hour. Lancet 1996;348:771–775. [DOI] [PubMed] [Google Scholar]
- 25. Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: A delay of lethal cell injury in ischemic myocardium. Circulation 1986;74:1124–1136. [DOI] [PubMed] [Google Scholar]
- 26. Yellon DM, Downey JM. Preconditioning the myocardium: From cellular physiology to clinical cardiology. Physiol Rev 2003;83:1113–1151. [DOI] [PubMed] [Google Scholar]
- 27. Downar E, Janse MJ, Durrer D. The effect of acute coronary artery occlusion on subepicardial transmembrane potentials in the intact porcine heart. Circulation 1977;56:217–224. [DOI] [PubMed] [Google Scholar]
- 28. Kleber AG, Janse MJ, Van Capelle FJ, et al Mechanism and time course of S‐T and T‐Q segment changes during acute regional myocardial ischemia in the pig heart determined by extracellular and intracellular recordings. Circ Res 1978;42:603–613. [DOI] [PubMed] [Google Scholar]
- 29. Shah A, Wagner GS, Granger CB, et al Prognostic implications of TIMI flow grade in the infarct related artery compared with continuous 12‐lead ST‐segment resolution analysis. Reexamining the “gold standard” for myocardial reperfusion assessment. J Am Coll Cardiol 2000;35:666–672. [DOI] [PubMed] [Google Scholar]
- 30. Krucoff MW, Johanson P, Baeza R, et al Clinical utility of serial and continuous ST‐segment recovery assessment in patients with acute ST‐elevation myocardial infarction: Assessing the dynamics of epicardial and myocardial reperfusion. Circulation 2004;110:e533–e539. [DOI] [PubMed] [Google Scholar]
- 31. Kerensky RA, Wade M, Deedwania P, et al Revisiting the culprit lesion in non‐Q‐wave myocardial infarction. Results from the VANQWISH trial angiographic core laboratory. J Am Coll Cardiol 2002;39:1456–1463. [DOI] [PubMed] [Google Scholar]
- 32. DeWood MA, Spores J, Notske R, et al Prevalence of total coronary occlusion during the early hours of transmural myocardial infarction. N Engl J Med 1980;303:897–902. [DOI] [PubMed] [Google Scholar]
- 33. Van ‘t Hof AW, Ten BJ, Heestermans T, et al Prehospital initiation of tirofiban in patients with ST‐elevation myocardial infarction undergoing primary angioplasty (On‐TIME 2): A multicentre, double‐blind, randomised controlled trial. Lancet 2008;372:537–546. [DOI] [PubMed] [Google Scholar]


