Abstract
Background: The Selvester QRS score was developed as a method to estimate infarct size (IS) using the ECG and has been validated during the prereperfusion era. Few comparisons exist with contrast‐enhanced magnetic resonance imaging (ceMRI) in reperfused patients. This study evaluates the ability of the Selvester QRS score to estimate serial changes in IS during the acute and chronic phases of the infarct evolution in patients who have received reperfusion therapy.
Methods: Thirteen patients with acute myocardial infarction underwent serial ceMRI studies in the acute (<1 week) and chronic phase (>2 months) after their initial myocardial infarction. QRS scoring was performed on the corresponding ECGs. The correlation between ceMRI measurement and QRS score estimation of IS was determined at both time points and for the difference between the two phases.
Results: The mean IS was 20.1 ± 11.0% of total left ventricular mass (% LV) in the acute phase and 13.3 ± 6.4% LV in the chronic phase ceMRI. The mean IS estimated by Selvester QRS score in the acute and chronic phases were 18.7 ± 8.2% and 16.4 ± 8.5% LV, respectively. A modest correlation was found for the acute (r = 0.57) and chronic phase IS (r = 0.54). However, there was no correlation for the difference in IS between the acute and chronic phases.
Conclusions: In this pilot study, the Selvester QRS score correlates modestly to IS by ceMRI during both the acute and chronic phases of the infarction process. The serial changes over time in the Selvester QRS score and IS by ceMRI show no correlation.
Keywords: acute myocardial infarction (AMI), infarct size (IS), electrocardiography, magnetic resonance imaging
In the past three decades, mortality rates from coronary heart disease (CHD) have declined by more than 50%. A significant part of this decline is due to the introduction of reperfusion therapy for acute myocardial infarction (AMI). 1 , 2 Reperfusion limits final infarct size (IS); not only by salvaging viable myocardium, but also by favorably influencing infarct healing. 3 , 4 After AMI, the size of the infarct yields valuable prognostic information: IS directly relates to the occurrence of adverse cardiac outcomes such as mortality, congestive heart failure, and ventricular arrhythmia. 4 , 5 , 6 , 7 Quantifying IS may also provide useful information about the effectiveness of the initial treatment as well as influence the selection of appropriate subsequent treatment. It may also be an early outcome measure in the investigation of experimental therapies. 8
IS changes temporally as healing occurs. In the first few days after the acute infarction, there is preserved wall thickness and the size of the infarcted area may be somewhat increased due to the edema of inflammation and the hemorrhage of reperfusion. 9 Gradually, the necrotic and cellular material is replaced by fibrotic, collagenous scar tissue, and the completion of this process generally requires approximately 2 months. 10 Many factors can influence the progression of this process. Because of the importance of the scar formation and the LV remodeling process in evolving myocardial infarction, assessment of serial changes in IS is of clinical relevance.
Five diagnostic modalities are available clinically to estimate the size of myocardial infarcts: 8 electrocardiography, cardiac biomarkers, and echocardiography provide indirect quantification of myocardial damage, whereas radionuclide imaging and contrast‐enhanced magnetic resonance imaging (ceMRI) are able to directly quantify IS. 7 , 11 Electrocardiography has some clear advantages over the other methods: it is noninvasive, inexpensive, and readily available. Previously, various ECG scoring codes for estimating IS have been developed. Selvester et al. 12 developed a QRS scoring system based on computer simulation of human heart activation. 13 The original criteria were later validated by postmortem anatomic studies and modified so that every criterion achieved at least 95% specificity for presence of myocardial infarction. 14 , 15 , 16 , 17 , 18 The modified Selvester QRS scoring system has been found to be superior to other electrocardiographic scoring systems in estimating the size of single infarcts in the left ventricle. 19
Advances in cardiac magnetic resonance imaging have provided new possibilities in visualizing myocardial infarctions. ceMRI uses gadolinium‐based contrast media to visually measure IS, 20 , 21 , 22 , 23 , 24 and correlates closely with pathology in ex vivo animal studies. 20 , 21 , 22 , 23 , 24 Comparing ceMRI with ECG data allows validation of the accuracy of the Selvester score in estimating final IS. A good correlation (r = 0.79) between ceMRI and Selvester QRS score estimations of IS previously has been found by Engblom et al. 25 in patients 1 week following reperfused AMI.
The aim of this study is to determine the comparative capabilities of the Selvester QRS scoring system and ceMRI to estimate evolutionary changes in IS between the acute and chronic phases.
METHODS
Patient Selection
Patients with their initial acute ST elevation myocardial infarction were recruited for enrollment in the Johns Hopkins Magnetic Resonance Imaging study, and their data entered into a computerized database. Myocardial infarction was confirmed by elevated cardiac biochemical marker levels. All patients received reperfusion therapy and underwent serial ceMRI in the acute (<1 week) and chronic (>2 months) phases after hospital admission. The files of standard 12‐lead ECGs were searched for available recordings within 1 day in the acute phase and 4 months in the chronic phase. The duration of this time window was selected based on the availability of ECGs. Patients with ECG signs of left ventricular hypertrophy, fascicular or bundle branch block, or paced rhythms; and those with either ECG or ceMRI signs of myocardial infarction, either before or following the acute event were excluded. All patients signed an Institutional Review Board approved informed consent form.
ECG Analysis
For each patient, all available clinically indicated standard 12‐lead ECGs were evaluated for serial changes characteristic of AMI. In the ECGs recorded closest to the corresponding acute and chronic phase ceMRI scans, IS was estimated using the 50‐criteria/31‐point Selvester QRS scoring system (Fig. 1). Scoring was performed manually by two investigators, blinded to the ceMRI results; final score was based on consensus. The criteria for every point have been developed to represent 3% of the total left ventricular mass (% LV). 12
Figure 1.
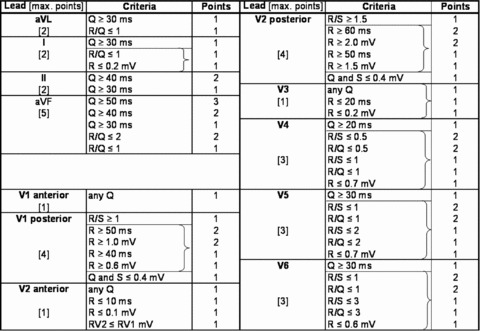
The 50‐criteria/31‐point Selvester QRS scoring system. Each of the 50 QRS criteria correspond to approximately 3% of the left ventricular mass. Brackets: only the criterion yielding the highest amount of points is counted.
ceMRI Acquisition and Analysis
All patients were imaged using a 1.5 T clinical MRI scanner (Signa CV/I, GE Healthcare Technologies, Waukesha, WI, USA). After localization of the heart, 8 to 10 short‐axis slices were obtained to cover the entire LV from base to apex. For IS quantification, delayed‐enhancement images were acquired 10 to 15 minutes after a bolus injection of 0.2 mmol/kg gadodiamide, using an inversion recovery fast gradient‐echo pulse sequence. 21 , 23 Imaging parameters 21 , 23 were: TR 5.4 ms, TE 1.3 ms, 36–40 cm FOV, 8‐mm slice thickness, matrix 256 × 192, TI 175–250 ms (adjusted to null the signal of normal myocardium), 2 NEX, flip angle 20°. All MRI postprocessing analyses were performed using software package, CINEtool (GE Healthcare Technologies). IS was calculated as the mass of the hyperenhanced area as % LV using the full‐width half‐maximum criterion. 24 In the acute phase ceMRI studies, this included the hypoenhanced region of microvascular obstruction, if present. 21 , 23
Statistical Analysis
All IS measurements (in % LV) were expressed as mean ± standard deviation. The time intervals between hospital admission, ceMRI studies, and ECG recordings were expressed as a median (minimal − maximal). To assess significance of the difference between acute and chronic phase IS, the paired t‐test was used. The Pearson product‐moment correlation coefficient (r) was used to define the linear correlation between ceMRI measurements and ECG estimations of IS. To illustrate the correlation between these two methods, Bland‐Altman plots were used.
RESULTS
The mean patient age was 52 ± 7 years, and 77% were male. The acute phase ceMRI study was performed in the first week after the AMI, and the chronic phase ceMRI study was performed after a median of 7 months (range 2–17 months). The maximum time interval between the ceMRI study and the closest available ECG was 1 day for the acute phase, and 115 days for the chronic phase.
Mean IS, measured as % LV, averaged 20.1 ± 11.0% on the acute phase ceMRI study and 13.3 ± 6.4% on the chronic phase ceMRI (Fig. 2). This represented a significant (P < 0.01) decrease in mean IS over time of 6.8 ± 7.9% LV. The mean acute and chronic phase IS estimated by Selvester QRS score were 18.7 ± 8.2% and 16.4 ± 8.5% LV, respectively (Fig. 2). This corresponded to a mean decrease in IS of 2.3 ± 5.4% LV, which was not statistically significant.
Figure 2.
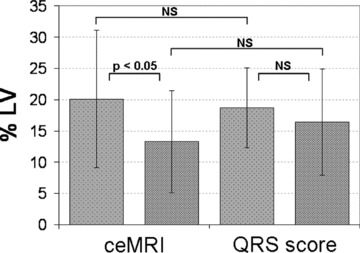
Mean infarct sizes measured by ceMRI and estimated by Selvester QRS score, in the acute (<1 week) and chronic (>2 months) phase after acute myocardial infarction. % LV = percentage of left ventricular mass; NS = not significant.
There was a correlation between IS by the Selvester QRS score and by ceMRI in both the acute (r = 0.59, P < 0.05) and chronic (r = 0.54, P < 0.05) phases after AMI (Fig. 3). However, there was no correlation (r =−0.07) between serial ceMRI changes and Selvester QRS score changes (Fig. 3).
Figure 3.
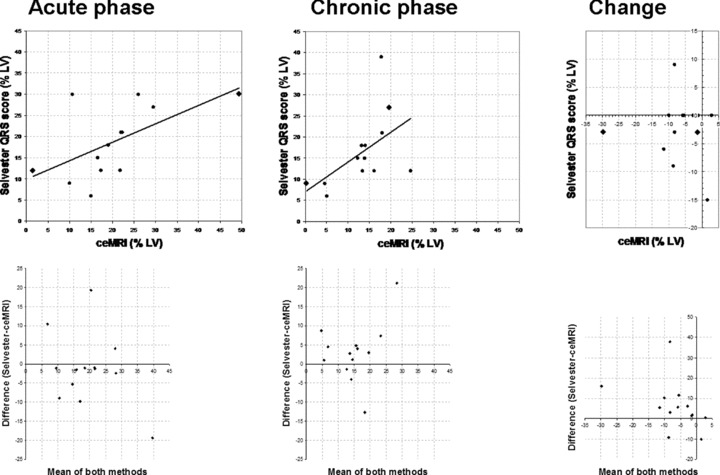
Top: correlation between infarct size measurements by the Selvester QRS score and contrast‐enhanced MRI (ceMRI) in the acute (left) and chronic phases (mid) after acute myocardial infarction, and the change (right) between these two measurements. The two patients with the largest time intervals between ceMRI and ECG are depicted as a diamond. Bottom: Bland‐Altman plots of these correlations.
A decrease of >1 point in Selvester QRS score was present in 3 patients, and in 2 of these a decrease in IS was also found on ceMRI. The QRS score showed ≤1 point change in the other 10 patients. Only 3 of these changed <3% LV on ceMRI, whereas the other 7 patients showed a decrease in IS ranging from 5.5% to 29.8% LV. Thus, of the 10 patients with decreased IS on ceMRI of ≥3% LV, 4 showed a concordant decrease by ECG, but 6 showed no significant decrease in IS (Fig. 4).
Figure 4.
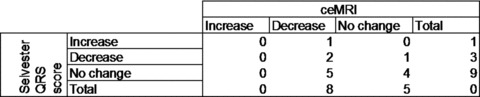
Patient numbers in groups divided by increase or decrease by Selvester QRS score and ceMRI. Increase or decrease was defined as a change of >1 point in Selvester QRS score or >3% LV on ceMRI.
DISCUSSION
To our knowledge, this is the first study to compare the Selvester QRS score to ceMRI documented sizes of myocardial infarction at both the acute and chronic phases. The acute phase was defined as the first week after the AMI. We assumed that after 2 months, minimal further change would occur in the extent of the scar tissue; this was therefore regarded as the chronic phase. Since reperfusion therapy affects both the initial IS and the subsequent healing process, 3 , 4 it can be useful to determine the amount of change in IS over time.
This study was performed to evaluate whether the Selvester QRS score might prove a valuable tool to accomplish this. Though not as direct as ceMRI, the ECG is inexpensive and easily accessible to the clinician. The Selvester QRS score does have known limitations: it cannot be accurately performed in the presence of common confounding factors such as bundle branch blocks and left ventricular hypertrophy.
The correlations between ceMRI and Selvester QRS scores in both the acute and chronic phases were weaker than in an earlier study by Engblom et al. 24 In addition, there was no correlation found for quantitative change in IS over time. The majority of patients showed a decrease in IS measured by ceMRI, consistent with prior reports of infarct shrinkage due to healing and remodeling. In addition, the average IS decrease found on ceMRI was significantly larger than the decrease found using the Selvester QRS score, so the Selvester QRS score generally underestimates changes in IS compared to ceMRI.
Earlier studies have demonstrated that ceMRI correlates well with IS. Hence, the discordance between ceMRI and Selvester QRS score may indicate that the latter does not perform as well in the presence of acute reperfusion therapy. The Selvester QRS score was developed in an era prior to reperfusion therapy, and could not be analyzed in vivo in the acute phase. In general, there is agreement between electrophysiologic and anatomic methods about either resolution or lack of resolution of the infarction scar in half of this study population, but in the other half there is disagreement. These results indicate that in many patients the two methods provide information on different aspects of the postinfarction dynamic process.
The findings of this study might be influenced by several limitations. The sample size is small because of the challenge in obtaining follow‐up data from both of the study methods during the chronic phase of myocardial infarction. However, these unique data should serve to generate hypotheses for testing in larger populations. Indeed, recruitment of even this limited sample size required inclusion of some patients in whom there was a several month interval between the follow‐up ceMRI and ECG studies. Although the absence of interim clinical reinfarction was documented, this time interval could reduce the correlation between the two infarct sizing methods. All patients received reperfusion therapy that causes heterogeneous myocardial scar patterns due to a complex pathophysiologic process. This heterogeneity may also have affected the correlations found in this study.
Further studies with larger patient numbers and more consistent timing of data acquisition are required to understand the contributions of these ECG and ceMRI methods for monitoring the serial changes of myocardial infarcts during the acute, healing and chronic phases. Larger patient numbers also allow making subgroups for different infarct localizations. Since it is a relatively new method, ceMRI requires further standardization and optimization to serve as a gold standard for the less direct but more generally available methods, such as the standard 12‐lead electrocardiogram. 26 , 27
Acknowledgments
Acknowledgments: Julie M. Miller is a recipient of a Doris Duke Clinical Scientist Development Award. This work was supported by the Doris Duke Charitable Foundation.
Katherine C. Wu is supported by The Donald W. Reynolds Foundation and the National Heart, Lung, and Blood Institute, National Institutes of Health (K23 HL04444).
REFERENCES
- 1. Levy D, Thom TJ. Death rates from coronary disease—progress and a puzzling paradox. N Engl J Med 1998;339:915–917. [DOI] [PubMed] [Google Scholar]
- 2. Ribichini F, Ferrero V, Wijns W. Reperfusion treatment of ST‐elevation acute myocardial infarction. Prog Cardiovasc Dis 2004;47:131–157. [DOI] [PubMed] [Google Scholar]
- 3. Hochman JS, Choo H. Limitation of myocardial infarct expansion by reperfusion independent of myocardial salvage. Circulation 1987;75:299–306. [DOI] [PubMed] [Google Scholar]
- 4. Ertl G, Frantz S. Healing after myocardial infarction. Cardiovasc Res 2005;66:22–32. [DOI] [PubMed] [Google Scholar]
- 5. Sobel BE, Bresnahan GF, Shell WE, et al Estimation of infarct size in man and its relation to prognosis. Circulation 1972;46:640–648. [DOI] [PubMed] [Google Scholar]
- 6. Geltman EM. Infarct size as a determinant of acute and long‐term prognosis. Cardiol Clin 1984;2:95–103. [PubMed] [Google Scholar]
- 7. Miller TD, Christian TF, Hopfenspirger MR, et al Infarct size after acute myocardial infarction measured by quantitative tomographic 99mTc sestamibi imaging predicts subsequent mortality. Circulation 1995;92:334–341. [DOI] [PubMed] [Google Scholar]
- 8. Antman EM, Anbe DT, Armstrong PW, et al ACC/AHA guidelines for the management of patients with ST‐elevation myocardial infarction: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1999 Guidelines for the Management of Patients with Acute Myocardial Infarction). Circulation 2004;110:e82–e292. [PubMed] [Google Scholar]
- 9. Reimer KA, Jennings RB. The changing anatomic reference base of evolving myocardial infarction. Underestimation of myocardial collateral blood flow and overestimation of experimental anatomic infarct size due to tissue edema, hemorrhage and acute inflammation. Circulation 1979;60:866–876. [DOI] [PubMed] [Google Scholar]
- 10. Cotran RS, Kumar V, Collins T, et al Robbins Pathologic Basis of Disease. Philadelphia , PA , Saunders, 1999. [Google Scholar]
- 11. Yuasa K, Sugimura K, Kawamitsu H, et al Quantification of occlusive and reperfused myocardial infarct size with Gd‐DTPA‐enhanced MR imaging. Eur J Radiol 1993;17:150–154. [DOI] [PubMed] [Google Scholar]
- 12. Selvester R, Wagner J, Rubin H. Quantitation of myocardial infarct size and location by electrocardiogram and vectorcardiogram In: Snellin H. (ed.): Boerhave Course in Quantitation in Cardiology. The Netherlands , Leyden University Press, 1972, pp. 31–44. [Google Scholar]
- 13. Durrer D, Van Dam RT, Freud GE, et al Total excitation of the isolated human heart. Circulation 1970;41:899–912. [DOI] [PubMed] [Google Scholar]
- 14. Wagner GS, Freye CJ, Palmeri ST, et al Evaluation of a QRS scoring system for estimating myocardial infarct size. I. Specificity and observer agreement. Circulation 1982;65:342–347. [DOI] [PubMed] [Google Scholar]
- 15. Ideker RE, Wagner GS, Ruth WK, et al Evaluation of a QRS scoring system for estimating myocardial infarct size. II. Correlation with quantitative anatomic findings for anterior infarcts. Am J Cardiol 1982;49:1604–1614. [DOI] [PubMed] [Google Scholar]
- 16. Roark SF, Ideker RE, Wagner GS, et al Evaluation of a QRS scoring system for estimating myocardial infarct size. III. Correlation with quantitative anatomic findings for inferior infarcts. Am J Cardiol 1983;51:382–389. [DOI] [PubMed] [Google Scholar]
- 17. Ward RM, White RD, Ideker RE, et al Evaluation of a QRS scoring system for estimating myocardial infarct size. IV. Correlation with quantitative anatomic findings for posterolateral infarcts. Am J Cardiol 1984;53:706–714. [DOI] [PubMed] [Google Scholar]
- 18. Sevilla DC, Wagner NB, White RD, et al Anatomic validation of electrocardiographic estimation of the size of acute or healed myocardial infarcts. Am J Cardiol 1990;65:1301–1307. [DOI] [PubMed] [Google Scholar]
- 19. Pahlm US, Chaitman BR, Rautaharju PM, et al Comparison of the various electrocardiographic scoring codes for estimating anatomically documented sizes of single and multiple infarcts of the left ventricle. Am J Cardiol 1998;81:809–815. [DOI] [PubMed] [Google Scholar]
- 20. Kim RJ, Fieno DS, Parrish TB, et al Relationship of MRI delayed contrast enhancement to irreversible injury, infarct age, and contractile function. Circulation 1999;100:1992–2002. [DOI] [PubMed] [Google Scholar]
- 21. Rochitte CE, Lima JA, Bluemke DA, et al Magnitude and time course of microvascular obstruction and tissue injury after acute myocardial infarction. Circulation 1998;98:1006–1014. [DOI] [PubMed] [Google Scholar]
- 22. Fieno DS, Kim RJ, Chen EL, et al Contrast‐enhanced magnetic resonance imaging of myocardium at risk: Distinction between reversible and irreversible injury throughout infarct healing. J Am Coll Cardiol 2000;36:1985–1991. [DOI] [PubMed] [Google Scholar]
- 23. Wu KC, Kim RJ, Bluemke DA, et al Quantification and time course of microvascular obstruction by contrast‐enhanced echocardiography and magnetic resonance imaging following acute myocardial infarction and reperfusion. J Am Coll Cardiol 1998;32:1756–1764. [DOI] [PubMed] [Google Scholar]
- 24. Amado LC, Gerber BL, Gupta SN, et al Accurate and objective infarct sizing by contrast‐enhanced magnetic resonance imaging in a canine myocardial infarction model. J Am Coll Cardiol 2004;44:2383–2389. [DOI] [PubMed] [Google Scholar]
- 25. Engblom H, Hedstrom E, Heiberg E, et al Size and transmural extent of first‐time reperfused myocardial infarction assessed by cardiac magnetic resonance can be estimated by 12‐lead electrocardiogram. Am Heart J 2005;150:920e1–920e9. [DOI] [PubMed] [Google Scholar]
- 26. Engblom H, Arheden H, Foster JE, et al Myocardial infarct quantification: Is magnetic resonance imaging ready to serve as a gold standard for electrocardiography? J Electrocardiol 2007;40:243–245. [DOI] [PubMed] [Google Scholar]
- 27. Wagner GS, Hakacova N. Electrocardiograpic measures of myocardial function and necrosis. J. Am Coll Cardiol Img in press. [DOI] [PubMed] [Google Scholar]


