Abstract
Background: Age has been identified as an independent risk factor for cardiovascular diseases. In addition, autonomic imbalance toward sympathetic preponderance has been shown to facilitate the occurrence of heart disease. Here, we aimed to assess autonomic modulation of cardiovascular parameters during normal ageing applying well‐established linear and novel nonlinear parameters.
Methods: Linear and nonlinear measures of heart rate variability and complexity as well as measures of QT interval variability and baroreflex sensitivity were obtained from a total of 131 healthy, medication‐free participants from a continuous age range between 20 and 90 years, who were allocated to three different age groups.
Results: Heart rate variability and complexity significantly decreased with age, while regularity of heart rate time series increased. In addition, QT interval variability linearly increased with age, while baroreflex sensitivity showed a pronounced decrease. Overall, concerning effects of ageing, linear and nonlinear parameters showed equal differentiation between groups.
Conclusion: These data indicate a shift of autonomic balance toward sympathetic predominance in higher age groups, limiting the reactiveness of the cardiovascular system to adjust to different demands and increasing the risk for developing tachyarrhythmias.
Ann Noninvasive Electrocardiol 2010;15(2):165–174
Keywords: age, heart rate variability, baroreflex, QT interval, gender, lifestyle, healthy, human
Age is one of the major independent risk factors for cardiovascular diseases. 1 A careful examination of the changes occurring in the cardiovascular system during ageing reveals an increased stiffness of the vasculature, degenerative processes in the heart, 2 and a significant shift in autonomic modulation of heart rate toward a sympathetic predominance. 3 In adolescence and in physically fit subjects, there is usually a vagal predominance. There are several reasons for the vagally mediated modulation of heart rate to be beneficial. Tonic vagal outflow limits energy consumption by the heart, together with a reduction in oxygen radicals. Lower levels of vagally modulated HRV have been demonstrated to be associated with increased cardiovascular morbidity and mortality in the elderly. 4 Because an intact autonomic modulation of the heart rate makes the system more reactive toward different strains, it eventually stabilizes body homeostasis. 5
Several studies investigated changes of certain parameters of cardiovascular modulation with age, mainly showing a decrease in parasympathetic modulation, with time courses dependent on the parameter assessed. 3 , 6 , 7 , 8 Overall, classical linear parameters from time and frequency domains of HRV have been used in these investigations. However, heart rate time series are not modulated in a linear fashion, and the manifold regulatory systems involved rather produce a very complex pattern, which can thus be depicted only in part by these parameters. In recent years, several nonlinear parameters for HRV assessment have been developed that have—at least in some fields—improved predictability of heart diseases, but also the differentiation between healthy controls and patients with different somatic and psychiatric diseases. 9 , 10 The latter parameters, however, have only rarely been investigated in regards to ageing. 11 Hence, we sought to recruit a well‐proportioned study population with healthy subjects from different ages in order to gather information on the natural course of such nonlinear parameters during life. In particular, we investigated—besides the classical linear parameters for comparability—measures of heart rate complexity (compression entropy, probability of high and low variability, and fractal dimension [FD] of RR intervals), alterations and complexity of QT interval time series (QT variability index and FD of QT intervals), and linear as well as nonlinear measures of baroreflex sensitivity obtained from short‐term recordings (30 minutes). Subjects were allocated to three different age groups, i.e., <30 years of age, 30–60 years of age, or >60 years of age, respectively.
MATERIALS AND METHODS
Participants
A total of 170 healthy participants were screened for this study. Subjects were recruited from hospital staff, medical students, and from the general community using flyer and newspaper advertisement. In order to exclude medical conditions that might putatively influence heart rate or blood pressure modulation, every participant was asked to fill in a standardized questionnaire in which characteristic symptoms were stated and which had to be confirmed or excluded. In addition, a thorough clinical and psychiatric investigation was performed. According to this assessment, anyone with cardiovascular and respiratory diseases, diabetes, neuropathy, and psychiatric pathology was excluded from the study. The subjects were also free from any medication. Applying these criteria, 39 out of the 170 originally screened participants had to be excluded from the study, resulting in a study population of 131. These were then allocated to the following three subgroups: <30 years of age (n = 48); 30–60 years of age (n = 53); >60 years of age (n = 30). For further characteristics of the study population, see Table 1. Participants were asked to refrain from smoking, eating or exercising 2 hours prior to the investigation. This study was carried out in accordance with the Declaration of Helsinki. After having been thoroughly informed about the nature of the procedures, all participants gave written informed consent to a protocol approved by the Ethics Committee of the Friedrich‐Schiller‐University, Jena.
Table 1.
Demographic Characteristics of the Study Population
| All Ages | <30 Years | 30–60 Years | >60 Years | |
|---|---|---|---|---|
| n | 131 | 48 | 53 | 30 |
| Male/Female | 75/56 | 26/22 | 32/21 | 17/13 |
| BMI | 24.1 ± 3.6 | 21.6 ± 2.4 | 25.4 ± 3.9 | 25.6 ± 2.4 |
| Smoking | ||||
| Smoker | 20 | 12 | 6 | 2 |
| Nonsmoker | 80 | 28 | 35 | 17 |
| Former smoker | 31 | 8 | 12 | 11 |
| Drinking | ||||
| Regular intake | 57 | 19 | 28 | 10 |
| Occasional intake | 74 | 29 | 25 | 20 |
| Sport/Physical activity | ||||
| Regular activity | 79 | 35 | 28 | 16 |
| No regular activity | 52 | 13 | 25 | 14 |
BMI = body mass index.
Data Acquisition and Preprocessing
Investigative procedures were performed in a quiet room, which was kept comfortably warm (22–24°C) between 1 and 6 p.m. Subjects were placed in supine position.
The electrocardiogram (high resolution, 1000 Hz) was recorded for 30 minutes from two separate adhesive monitoring electrodes (CNSystems, Medizintechnik GmbH Graz, Austria), which were placed on the chest wall to assure maximal R‐wave amplitude. From this, the device automatically extracted the RR intervals (beat‐to‐beat interval, BBI). Continuous blood pressure was also simultaneously recorded noninvasively from the third and fourth finger using the vascular unloading technique 12 and was corrected to absolute values using oscillometric blood pressure measurement. RR‐interval time series were afterwards filtered by an adaptive filter algorithm to replace and interpolate ventricular premature beats, artifacts and noise in order to generate normal‐to‐normal (NN) beat time series. All parameters explained below were calculated from BBI data obtained during the 30‐minute recording interval, except QT variability, for which another calculation algorithm had to be applied (see below).
Time and Frequency Domain Parameters of Heart Rate Variability (HRV)
In accordance with the suggestions of the HRV Task Force, 13 we computed measures of heart rate variability in the time and frequency domains. In particular, we obtained the coefficient of variation (cvNN), the standard deviation of NN intervals in consecutive 10‐minute intervals (SDANN10), and the square root of the mean of the squared differences of successive NN intervals (RMSSD) from the time domain. In addition, the quotient of low‐frequency (0.04–0.15 Hz) and high‐frequency components (HF 0.15–0.4 Hz) (LF/HF ratio) of the frequency domain was calculated as a probable measure of sympathovagal balance.
Nonlinear Compression Entropy
An approach to describe the entropy of a text was introduced in the framework of algorithmic information theory. Here, the entropy (complexity) of a given text is defined as the smallest algorithm that is capable of generating the text. Although it is theoretically impossible to develop such an algorithm, data compressors represent a sufficient approximation. In this study, we applied the LZ77 algorithm for loss‐less data compression introduced by Lempel and Ziv. 14 Its application in RR time series has been described in detail elsewhere. 9 , 15 The ratio of the compressed to the original time series length represents an index of entropy which is referred to as compression entropy Hc. Taken together, compression entropy indicates to which degree the data from BBI time series can be compressed using the detection of recurring sequences. The more frequent certain sequences occur and therefore the more regular these series are, the higher the compression rate.
Symbolic Dynamics for High and Low Variability
For analysis of symbolic dynamics, every single heart beat was compared to the preceding beat. Whenever the beat duration differed within a special time limit, this was encoded with the letter “0.” In contrast, when beats were different exceeding the given time limit the letter “1” was attributed (see Reference 16). As the time limit, 10 ms was used. From these data, letter sequences were analyzed. For low variability parameters (plvar), the occurrence of sequences containing six consecutive “0” were assessed, whereas for high variability parameters (phvar) six consecutive “1” were relevant. The time limit applied is always indicated by adding the respective number to the parameter assessed, e.g., plvar10 for low variability with a time limit of 10 ms. Taken together, in this model an increase of “000000” sequences resulting in increased values of plvar and a decrease in “111111” sequences leading to reduced values of phvar indicate reduced system complexity.
Fractal Dimension
Fractal dimension is based on the algorithm of Katz et al. 17 Waveforms (here: RR or QT intervals over time) are planar curves, i.e., ordered collections of (x, y) point pairs. One technique for numerically classifying waveforms assesses their fractal dimensionality, D. For waveforms:
with n = number of steps in the waveform (one less than the number of (x, y) point pairs), d = planar extent (diameter) of the waveform, and L = total length of the waveform. Overall, the higher the FD, the more irregular is the signal recorded and thus the more complexity is contained in its time series. Here, FD was calculated for both QT intervals and RR intervals.
QT Variability
In order to analyze a recording period with the fewest possible artifacts and errors and maximum stationarity, continuous 256‐second segments of ECG were identified from the segment between the 5th and the 15th minute of the original recordings and data from these were used to calculate the RR and QT intervals.
The QT variability algorithm applied here has been described by Berger and coworkers in detail and has been used by his and our groups in previous studies. 18 , 19 In brief, RR and QT interval data were detrended using the best‐fit line prior to the computation of spectral analyses. The mean RR (RR mean), detrended RR variance (DetrendRR), mean QT interval (QT mean), detrended QT variance (DetrendQT) of RR and QT intervals, and QT variance corrected for mean QT interval (QTVM) were calculated from the instantaneous RR and QT time series of 1024 points (256 seconds). Mean RR and mean QT intervals are in ms. The powers are corresponding squared values.
A normalized QT variability index was calculated as suggested by Berger et al.: 18
 |
Baroreflex Sensitivity
The baroreflex sensitivity (BRS) was assessed using the sequence method. 20 A detailed description has been published previously. 21 , 22 In brief, spontaneous sequences of at least three consecutive beats were analyzed, when an increased systolic blood pressure (SBP) of at least 1 mmHg caused an increased BBI of at least 5 ms (bradycardic sequence) or a decreased SBP caused a decreased BBI (tachycardic sequence). For each sequence, the regression between the three SBP values and three BBI values was calculated and the slope (tachycardic slope–tslope; bradycardic slope‐bslope) of the regression line was used as an index of BRS.
Nonlinear Joint Symbolic Dynamics
In order to assess heart rate and blood pressure dynamics in a more complex way, an analysis based on joint symbolic dynamics (JSD) was applied, which has been described in detail previously. 22 Here, the beat‐to‐beat changes of RR and SBP are each coarse‐grained to two different symbols: increasing values are coded as “1,” whereas decreasing and unchanged values are coded as “0,” respectively. Subsequently, short patterns of symbol sequences (words) are formed and its distribution properties are analyzed (probability of symmetric baroreflex‐like words–JSDsym, probability of diametric nonbaroreflex‐like words‐JSDdiam). Consequently, the dynamics between heart rate and blood pressure within three RR intervals (four heart beats) can be analyzed. Thus, a rough assessment of the overall RR and SBP short‐term interactions is obtained.
Statistical Analyses
For statistical analyses, SPSS for Windows (version 17.0, SPSS Inc, Chicago, IL, USA) was used. First, normal distribution of the parameters was assessed using the Kolmogorov–Smirnov test. In order to gather information on the effect of ageing on the above‐mentioned parameters, a multivariate analysis of variance (MANOVA) was performed with the between‐subject factor “group” (<30 years of age, 30–60 years of age, and >60 years of age), followed by univariate ANOVAs and post hoc Scheffé‐tests for comparison between groups. Since previous studies have shown an influence of blood pressure or body mass index on autonomic regulation, 23 additional MANCOVAs were performed using these parameters as covariates to control for confounding effects. In order to gain information whether linear or nonlinear parameters might better differentiate between age groups, we repeated the above‐mentioned MANOVA with linear parameters (cvNN, SDANN10, RMSSD, and LF/HF ratio) and with nonlinear parameters of heart rate variability only (Hc, plvar10, phvar10, FDRR).
In addition, cardiovascular parameters were correlated with age using Pearson rank correlation analyses. These analyses were performed for all participants as well as for male and female participants separately, and for all three groups separately.
Statistical significance was assumed for P < 0.05.
RESULTS
Multivariate Analyses
The MANOVA of all parameters revealed a significant main effect for the factor “group”[F(42,126) = 3.381; P < 0.001], indicating that all three age groups differed in the parameters assessed. This overall effect was still significant when considering body mass index [BMI; F(42,126) = 3.112; P < 0.001] or SBP [F(42,126) = 2.688; P < 0.001] as a covariate. The results obtained from follow‐up Scheffé analyses are displayed in Figure 1 and Table 2. When comparing the MANOVAs using linear or nonlinear parameters only, both indicated a highly significant difference between groups [F(8,248) = 7.059; P < 0.001 and F(8,248) = 7.615; P < 0.001, respectively].
Figure 1.
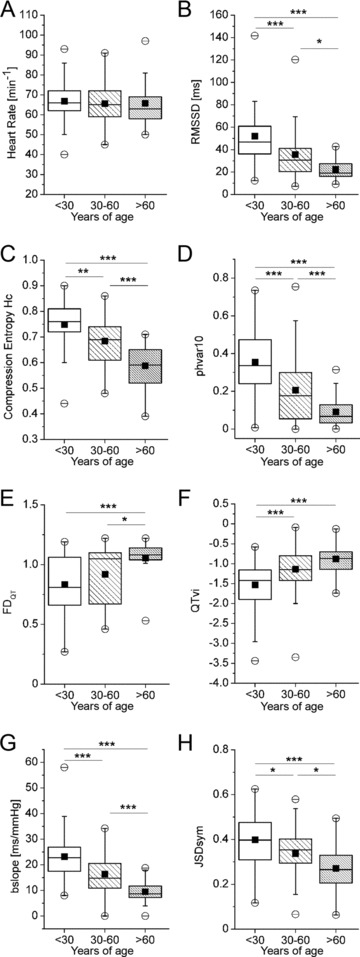
Comparison of cardiovascular autonomic parameters between different age groups. (A) Heart rate. (B) Root mean squared differences of successive normal‐to‐normal intervals (RMSSD). (C) Compression Entropy Hc. (D) Probability of high variability sequences with a time limit of 10 ms (phvar10). (E) Fractal dimension of QT interval time series (FDQT). (F) QT variability index (QTvi). (G) Bradycardic slope of baroreflex (bslope). (H) Joint symbolic dynamics of symmetric baroreflex‐like words (JSDsym). Data presented as box plots. Boxes indicate 25th and 75th percentile with the horizontal line indicating the median. ▪ mean. ○ 1st and 99th percentile. – minimum and maximum of data. *P < 0.05; **P < 0.01; ***P < 0.001.
Table 2.
Cardiovascular Parameters in Different Age Groups
| <30 Years | 30–60 Years | >60 Years | Significance | |
|---|---|---|---|---|
| Heart Rate Variability | ||||
| cvNN | 0.07 ± 0.02 | 0.05 ± 0.02 | 0.04 ± 0.02 | *, ††† |
| SDANN10 | 59.5 ± 22.7 | 47.3 ± 22.6 | 38.2 ± 18.8 | + |
| LF/HF ratio | 2.21 ± 2.19 | 3.22 ± 3.66 | 2.85 ± 3.42 | n.s. |
| FDRR | 1.09 ± 0.54 | 1.06 ± 0.38 | 1.22 ± 0.23 | n.s. |
| plvar10 | 0.019 ± 0.009 | 0.011 ± 0.034 | 0.033 ± 0.103 | + |
| QT interval parameters | ||||
| mean QT [ms] | 405.1 ± 57.8 | 411.5 ± 97.8 | 495.6 ± 115.8 | n.s. |
| Blood Pressure/Baroreflex | ||||
| Systolic BP [mmHg] | 120.4 ± 14.7 | 130.6 ± 23.2 | 130.7 ± 18.2 | * |
| Diastolic BP [mmHg] | 73.9 ± 9.9 | 86.3 ± 15.8 | 85.0 ± 12.4 | ***, ++ |
| tslope [ms/mmHg] | 21.1 ± 8.7 | 15.3 ± 7.2 | 10.8 ± 4.8 | ***, +++, † |
| JSDdiam | 0.022 ± 0.027 | 0.033 ± 0.030 | 0.047 ± 0.045 | ++ |
LF = low frequency band of heart rate variability; HF = high frequency band of heart rate variability; FD = fractal dimension; plvar10 = probability of low variability, time limit 10 ms; BP = blood pressure; tslope = tachycardic slope; JSD = joint symbolic dynamics. Data are presented as mean ± standard deviation. Significance levels: *P < 0.05; **P < 0.01; ***P < 0.001 for age groups <30 versus 30–60 years of age. The same levels apply to <30 versus >60 years of age (+) and 30–60 versus >60 years of age (†).
Linear Measures of Heart Rate Variability
Heart rate did not significantly differ between groups (Fig. 1A). Linear time domain measures of autonomic modulation of heart rate were significantly different between age groups, as indicated by RMSSD (Fig. 1B), whereas frequency band parameters showed no difference (LF/HF ratio, Table 2).
Complexity Measures of Heart Rate Modulation
Of the complexity measures examined, all except FD of RR intervals (FDRR, Table 2) and probability of low variability sequences (plvar10, Table 2) showed to be significantly different between groups. In particular, compression entropy Hc and probability of high variability sequences (phvar10) mirrored a dramatic decline with ageing (Fig. 1C and D), thus indicating reduced complexity in heart rate time series with increasing age.
Measures of QT Interval Time Series
While the mean duration of the QT interval showed no difference between groups (Table 2), its variability and the complexity of its time series did. Both FD of QT time series (FDQT, Fig. 1E) and the QT variability index (QTvi, Fig. 1F) increased with age. Since both measures indicate an increased repolarization lability, these findings might be interpreted in the light of an increased risk for arrhythmic events in older subjects.
Baroreflex Sensitivity
Both diastolic and systolic blood pressure significantly differed between the young and the middle‐aged as well as the old group, but not between the latter two (Table 2). BRS, however, showed a marked decline with ageing (Table 2). These findings indicate a diminished vagal reactivity toward changes in blood pressure in the elderly. In addition, joint symbolic dynamics (JSDsym and JSDdiam), which depict the interplay between heart rate and blood pressure in a more complex way, also significantly differ between groups (Fig. 1H and Table 2, respectively).
Correlations between Cardiac Autonomic Measures and Age
When including the complete study population, all of the above‐mentioned parameters correlate with age, except heart rate, blood pressure, LF/HF ratio, and FDRR. Taken together, parameters mainly indicating parasympathetic modulation are decreased, while those indicating sympathetic modulation are increased with age. As examples for the former, RMSSD (Fig. 2A), compression entropy Hc (Fig. 2B), and bradycardic slope of BRS (bslope, Fig. 2C) are depicted, while QTvi represents sympathetic modulation (Fig. 2D).
Figure 2.
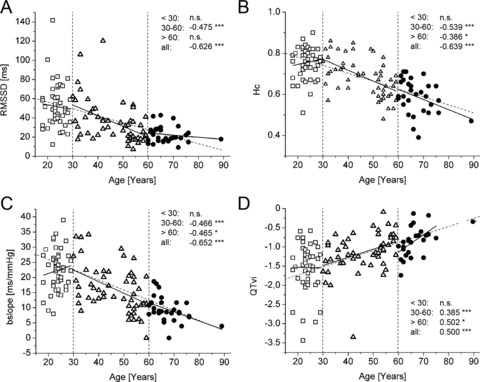
Correlations between cardiovascular autonomic parameters and age. (A) Root mean squared differences of successive normal‐to‐normal intervals (RMSSD). (B) Compression entropy, a complexity measure of heart rate time series, decreases similarly to RMSSD, but with higher correlation coefficients. (C) Decrease of baroreflex sensitivity with age as represented by bradycardic slopes (bslope). (A)–(C) are measures of vagal modulation of heart rate time series, which thus decrease in older age. (D) QT variability index, a measure of sympathetic influence at the level of the heart, increases with age, with an even enhanced steepness in the >60 years group. Correlation data are presented as r‐values and as linear fit regression lines for each age group separately (bold black lines) and for all age groups taken together (thin intermittent line). *P < 0.05; **P < 0.01; ***P < 0.001; n.s. – not significant.
Effects of Gender, Physical Activity, Drinking, and Smoking
When separating the complete sample population according to gender, two groups matched with respect to age are obtained (male: n = 75; mean age: 42.6 ± 17 years; female: n = 56; mean age: 42.8 ± 19 years). Of the parameters obtained, they only differ, however, in their mean heart rate, which is higher in women (68 ± 9; men: 64 ± 10; P < 0.02) and in the linear parameter LF/HF ratio (P < 0.01), but in none of the complexity and baroreflex measures obtained.
When separating the groups according to their self‐assessed physical activity, none of the parameters assessed showed to be significantly different. However, BMI was significantly lower in the subgroup that indicated regular physical activity (23.1 ± 2.2 as compared to 25.9 ± 2.3; P < 0.01) compared to those without.
When separating the sample according to regular/irregular drinking behavior, again the mean age was not significantly different between subgroups (41 ± 16 vs 39 ± 19). Of the parameters assessed, only the measures of BRS, i.e., bslope and tslope, were significantly reduced in those that indicated regular alcohol consumption (P < 0.02 and P < 0.004, respectively).
An effect of smoking was not calculated from the sample included in this study, since the mean age of smokers was significantly lower than that of nonsmokers (29 vs 43 years; P < 0.002).
DISCUSSION
In the current study, we examined changes in cardiovascular autonomic parameters as obtained from short‐term recordings with age. Applying linear and most notably nonlinear parameters of heart rate variability, QT interval variability, and BRS, the results of the present study show a decrease in classical time‐domain measures of HRV and, to a similar degree, in the novel nonlinear heart rate complexity parameters such as Hc and phvar10 with age. This decrease further corroborates a shift toward a sympathetically dominated modulation of heart rate time series, which has been detected from 24‐hour recordings previously. 3 , 11 This shift appears to start from an age of 30 onwards, when the correlation between loss in complexity/variability and age becomes linear. QT interval variability and complexity as measured by QTvi and FDQT, respectively, significantly increase during life, thus putatively making the heart more vulnerable for arrhythmic events. 24 BRS decreases with age. The latter might indicate a loss of afferent or efferent vagal modulation. In those subjects who reported regular alcohol intake, this decrease was more pronounced which is most likely due to vagal neuropathy. 25
Implications of Altered Autonomic Modulation
Overall, our data indicate a lifelong shift of autonomic balance toward sympathetic predominance beginning at 30 years of age. Prior to that age, no significant correlation was obvious for any of the parameters with age, most likely resembling the individual, yet gradual developmental increase in HRV in childhood and adolescence. 26 Negative sequelae of such an autonomic imbalance are as follows: while the vagal nerve usually limits energy expenditure and allows the organism to adjust to different demands very quickly, this ability is diminished when there is an increased sympathetic modulation. 5 In the current study, the major indicators for such a shift are decreased values of the linear measure RMSSD as well as the complexity measures Hc, plvar, and phvar (the latter indicating regularity, therefore being increased). While these measures contain information on higher centers of autonomic modulation of the cardiovascular system and the local regulation at the sinus node, parameters examining time series of the QT interval mainly depict the temporal fluctuation in ventricular repolarization, thus providing information on repolarization abnormalities. Hence, in contrast to general heart rate modulation, an increase in QT variability does not indicate a responsive system, but rather a very irregular duration of the QT interval in consecutive heart beats. 27 Late QT interval is the so‐called vulnerable phase for arrhythmias, because some parts of the myocardium are still refractory, while others are already capable of conducting action potentials. 24 Thus, an increased variability of QT interval duration indicates an increased risk for reentrant arrhythmias. The QT interval variability index, QTvi, 18 further indicates sympathetic influences at the heart, 28 therefore further explaining its increase with age. Interestingly, certain disease states such as schizophrenia or withdrawal from alcohol cause an even more pronounced increase in this parameter even at younger ages, possibly due to structural or functional abnormalities in the autonomic nervous system. 19 , 29 The latter is also true for BRS, which has also been shown to be decreased in these conditions. 21 , 22
Selection of the Study Population
Since our aim was to identify the effects of “normal” ageing on cardiovascular autonomic parameters, we took every effort to select a study population free from any somatic or psychiatric disease and free from any medication. This is of particular importance, since cardiovascular diseases such as coronary heart disease, 30 , 31 myocardial infarction, 31 and hypertension, 32 are all capable of accelerating the “autonomic ageing” as compared to the natural course described here, i.e., leading to an earlier and much steeper decrease in HRV and BRS. A similar accentuation has been described for psychiatric disorders, 16 , 21 , 29 which is why these were screened for and participants were excluded, when necessary. This rigorous exclusion of participants with any signs of diseases, which have been shown to alter autonomic balance, represents one of the major strengths of this study.
The selection process of completely healthy subjects proved particularly difficult for our age group > 60 years. Here, although the highest number of putative participants was screened (n = 59), the final number included was lowest (n = 30). While this selection might not be representative for this age group in general (in which certain morbidities occur with quite a high incidence), we can be reasonably certain that from the subjects included who were healthy, the effect of ageing alone might have resulted in the findings described above. One remaining uncertainty in this respect is putative occult cardiac disease, which might not have been detected by our clinical examination procedure, since this did not include electrocardiographic recordings during exercise or ultrasound. This might be capable of confounding the results obtained, at least in the older participants.
Value and Validity of Nonlinear Measures
While linear measures of heart rate variability have repeatedly been applied in order to examine the effect of age on autonomic function, 3 , 6 , 7 the use of novel nonlinear measures as shown here have only rarely been used (e.g., Reference 8). However, complex systems such as the cardiovascular system, which is controlled by many different regulatory mechanisms, may be better described when applying nonlinear algorithms, thus improving the sensitivity in diagnosing autonomic dysfunction, 10 particularly in diseases of the central nervous system such as psychiatric diseases. 16 , 33 , 34 In the current study in which we examined the influence of ageing, however, this superiority of nonlinear measures was not obvious.
Although the number of participants included in this study is too small to consider the obtained values as normative data for the respective age groups, one gets an idea about the range these parameters occur in healthy subjects. However, future studies including even more participants of different ethnic groups should be investigated and also meta‐analyses are warranted in order to get an even clearer picture on the validity and the putative clinical value of these parameters. This could be facilitated, if measures that contain algorithms for calculating nonlinear measures become commercially available.
Intriguingly, when looking at the correlations between RMSSD or bslope with age, both combined regression lines would cross “0” at an age of approximately 110–120 years of age, i.e., in the age range which is currently assumed to reflect the maximum biological age. 35
Influence of Gender and Lifestyle
Interestingly, there were no major gender differences in the population under study. This is well in line with previous data, which also showed few differences in linear measures such as LF/HF ratio 36 , 37 or QT variability. 38
Lifestyle has been shown to significantly affect physical fitness and likewise autonomic modulation. In particular, physical exercise training has been shown to improve autonomic modulation of heart rate and blood pressure parameters. 39 For drinking behavior, the described decrease in BRS could be corroborated here, 22 but none of the HRV measures showed to be different between the groups indicating different amounts of alcohol consumption.
Overall, the scarce additional information obtained for lifestyle factors limits the interpretation of our results to some degree as mentioned in this paragraph.
Conclusions
Linear and nonlinear parameters of heart rate variability, QT interval variability, and BRS are altered during normal ageing toward a sympathetically dominated autonomic state, which might be one of the main reasons for adverse cardiovascular events in higher ages, irrespective of concomitant disease states. The validity of these parameters and their putative predictive value for critical cardiovascular events should thus be validated in future longitudinal studies in different medical disorders. Eventually, such measures might be suitable for an additional risk stratification in patients with diseases of the cardiovascular system, considering the calculation of an “autonomic age,” which might contain information on autonomic nervous system reactivity.
Acknowledgments
Acknowledgments: The authors thank Dr. Vollandt (Institute for Medical Statistics, IMSID, University Hospital, Jena) for his advice on statistics.
Financial support: This study was in part funded by the IZKF of the University Hospital Jena.
Conflict of Interest: None declared.
REFERENCES
- 1. Pearson TA. New tools for coronary risk assessment: What are their advantages and limitations? Circulation 2002;105:886–892. [DOI] [PubMed] [Google Scholar]
- 2. Folkow B, Svanborg A. Physiology of cardiovascular aging. Physiol Rev 1993;73:725–764. [DOI] [PubMed] [Google Scholar]
- 3. De Meersman RE, Stein PK. Vagal modulation and aging. Biol Psychol 2007;74:165–173. [DOI] [PubMed] [Google Scholar]
- 4. Tsuji H, Venditti FJ, Jr ., Manders ES, et al Reduced heart rate variability and mortality risk in an elderly cohort. The Framingham Heart Study. Circulation 1994;90:878–883. [DOI] [PubMed] [Google Scholar]
- 5. Pincus SM. Assessing serial irregularity and its implications for health. Ann N Y Acad Sci 2001;954:245–267. [DOI] [PubMed] [Google Scholar]
- 6. Stein PK, Barzilay JI, Chaves PH, et al Heart rate variability and its changes over 5 years in older adults. Age Ageing 2009;38:212–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Agelink MW, Malessa R, Baumann B, et al Standardized tests of heart rate variability: Normal ranges obtained from 309 healthy humans, and effects of age, gender, and heart rate. Clin Auton Res 2001;11:99–108. [DOI] [PubMed] [Google Scholar]
- 8. Jokinen V, Syvanne M, Makikallio TH, et al Temporal age‐related changes in spectral, fractal and complexity characteristics of heart rate variability. Clin Physiol 2001;21:273–281. [DOI] [PubMed] [Google Scholar]
- 9. Baumert M, Baier V, Haueisen J, et al Forecasting of life threatening arrhythmias using the compression entropy of heart rate. Methods Inf Med 2004;43:202–206. [PubMed] [Google Scholar]
- 10. Voss A, Kurths J, Kleiner HJ, et al The application of methods of non‐linear dynamics for the improved and predictive recognition of patients threatened by sudden cardiac death. Cardiovasc Res 1996;31:419–433. [PubMed] [Google Scholar]
- 11. Pikkujämsä SM, Makikallio TH, Sourander LB, et al Cardiac interbeat interval dynamics from childhood to senescence: Comparison of conventional and new measures based on fractals and chaos theory. Circulation 1999;100:393–399. [DOI] [PubMed] [Google Scholar]
- 12. Penaz JVA, Teichmann W. Contribution to the continuous indirect blood pressure measurement. Z Gesamte Inn Med 1976;31:1030–1033. [PubMed] [Google Scholar]
- 13. Heart rate variability: Standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation 1996;93:1043–1065. [PubMed] [Google Scholar]
- 14. Lempel A, Ziv J. Universal algorithm for sequential data compression. IEEE Trans Inf Ther 1977;20:337–343. [Google Scholar]
- 15. Voss A, Baier V, Schulz S, et al Linear and nonlinear methods for analyses of cardiovascular variability in bipolar disorders. Bipolar Disord 2006;8:441–452. [DOI] [PubMed] [Google Scholar]
- 16. Bär KJ, Boettger MK, Koschke M, et al Non‐linear complexity measures of heart rate variability in acute schizophrenia. Clin Neurophysiol 2007;118:2009–2015. [DOI] [PubMed] [Google Scholar]
- 17. Katz MJ. Fractals and the analysis of waveforms. Comput Biol Med 1988;18:145–156. [DOI] [PubMed] [Google Scholar]
- 18. Berger RD, Kasper EK, Baughman KL, et al Beat‐to‐beat QT interval variability: Novel evidence for repolarization lability in ischemic and nonischemic dilated cardiomyopathy. Circulation 1997;96:1557–1565. [DOI] [PubMed] [Google Scholar]
- 19. Bär KJ, Boettger MK, Koschke M, et al Increased QT interval variability index in acute alcohol withdrawal. Drug Alcohol Depend 2007;89:259–266. [DOI] [PubMed] [Google Scholar]
- 20. Bertinieri G, Di Rienzo M, Cavallazzi A, et al A new approach to analysis of the arterial baroreflex. J Hypertens Suppl 1985;3:S79–S81. [PubMed] [Google Scholar]
- 21. Bär KJ, Boettger MK, Berger S, et al Decreased baroreflex sensitivity in acute schizophrenia. J Appl Physiol 2007;102:1051–1056. [DOI] [PubMed] [Google Scholar]
- 22. Bär KJ, Boettger MK, Boettger S, et al Reduced baroreflex sensitivity in acute alcohol withdrawal syndrome and in abstained alcoholics. Drug Alcohol Depend 2006;85:66–74. [DOI] [PubMed] [Google Scholar]
- 23. Pikkujämsä SM, Huikuri HV, Airaksinen KE, et al Heart rate variability and baroreflex sensitivity in hypertensive subjects with and without metabolic features of insulin resistance syndrome. Am J Hypertens 1998;11:523–531. [DOI] [PubMed] [Google Scholar]
- 24. Starmer CF. The cardiac vulnerable period and reentrant arrhythmias: Targets of anti‐ and proarrhythmic processes. Pacing Clin Electrophysiol 1997;20:445–454. [DOI] [PubMed] [Google Scholar]
- 25. Agelink MW, Malessa R, Weisser U, et al Alcoholism, peripheral neuropathy (PNP) and cardiovascular autonomic neuropathy (CAN). J Neurol Sci 1998;161:135–142. [DOI] [PubMed] [Google Scholar]
- 26. Korkushko OV, Shatilo VB, Plachinda Yu I, et al Autonomic control of cardiac chronotropic function in man as a function of age: Assessment by power spectral analysis of heart rate variability. J Auton Nerv Syst 1991;32:191–198. [DOI] [PubMed] [Google Scholar]
- 27. Haigney MC, Zareba W, Gentlesk PJ, et al QT interval variability and spontaneous ventricular tachycardia or fibrillation in the Multicenter Automatic Defibrillator Implantation Trial (MADIT) II patients. J Am Coll Cardiol 2004;44:1481–1487. [DOI] [PubMed] [Google Scholar]
- 28. Pohl R, Balon R, Jayaraman A, et al Effect of fluoxetine, pemoline and placebo on heart period and QT variability in normal humans. J Psychosom Res 2003;55:247–251. [DOI] [PubMed] [Google Scholar]
- 29. Bär KJ, Koschke M, Boettger MK, et al Acute psychosis leads to increased QT variability in patients suffering from schizophrenia. Schizophr Res 2007;95:115–123. [DOI] [PubMed] [Google Scholar]
- 30. Huikuri HV, Makikallio TH. Heart rate variability in ischemic heart disease. Auton Neurosci 2001;90:95–101. [DOI] [PubMed] [Google Scholar]
- 31. Nolan J, Batin PD, Andrews R, et al Prospective study of heart rate variability and mortality in chronic heart failure: Results of the United Kingdom heart failure evaluation and assessment of risk trial (UK‐heart). Circulation 1998;98:1510–1516. [DOI] [PubMed] [Google Scholar]
- 32. Singh JP, Larson MG, Tsuji H, et al Reduced heart rate variability and new‐onset hypertension: Insights into pathogenesis of hypertension: The Framingham Heart Study. Hypertension 1998;32:293–297. [DOI] [PubMed] [Google Scholar]
- 33. Boettger S, Hoyer D, Falkenhahn K, et al Nonlinear broad band dynamics are less complex in major depression. Bipolar Disord 2008;10:276–284. [DOI] [PubMed] [Google Scholar]
- 34. Boettger S, Hoyer D, Falkenhahn K, et al Altered diurnal autonomic variation and reduced vagal information flow in acute schizophrenia. Clin Neurophysiol 2006;117:2715–2722. [DOI] [PubMed] [Google Scholar]
- 35. Goggins WB, Woo J, Sham A, et al Frailty index as a measure of biological age in a Chinese population. J Gerontol A Biol Sci Med Sci 2005;60:1046–1051. [DOI] [PubMed] [Google Scholar]
- 36. Antelmi I, De Paula RS, Shinzato AR, et al Influence of age, gender, body mass index, and functional capacity on heart rate variability in a cohort of subjects without heart disease. Am J Cardiol 2004;93:381–385. [DOI] [PubMed] [Google Scholar]
- 37. Barantke M, Krauss T, Ortak J, et al Effects of gender and aging on differential autonomic responses to orthostatic maneuvers. J Cardiovasc Electrophysiol 2008;19:1296–1303. [DOI] [PubMed] [Google Scholar]
- 38. Krauss TT, Mauser W, Reppel M, et al Gender effects on novel time domain parameters of ventricular repolarization inhomogeneity. Pacing Clin Electrophysiol 2009;32(Suppl 1):S167–S172. [DOI] [PubMed] [Google Scholar]
- 39. Pichot V, Roche F, Denis C, et al Interval training in elderly men increases both heart rate variability and baroreflex activity. Clin Auton Res 2005;15:107–115. [DOI] [PubMed] [Google Scholar]


