Abstract
Background: Beat‐to‐beat QT interval variability (QTV) is associated with sudden cardiac death and New York Heat Association functional class severity. We sought to evaluate the relationship between QTV and left ventricular (LV) function in patients with previous myocardial infarction (MI).
Methods: Fifty‐nine patients with previous anterior MI were enrolled. LV ejection fraction (EF), LV end‐systolic volume index (LVESVI), and LV end‐diastolic volume index (LVEDVI) were measured by LV contrast angiography. QT interval was measured by automated analysis of 512‐beat records of 12‐lead electrocardiogram. The mean interval, standard deviation and variance in RR and QT intervals, and the QT variability index (QTVI) were calculated for each patient using two leads that corresponded with and without the infarction site. High‐frequency power, low‐frequency power, total‐frequency power, and the ratio of low‐frequency to high‐frequency power in RR and QT intervals were calculated.
Results: While measured indices of RR intervals and indices of QT intervals, which did not correspond with the infarction site, did not correlate with differences in LV function, measured indices of QT intervals, which corresponded with the infarction site, did correlate with differences in LV function. However, there were no correlations between the ratio of low‐frequency to high‐frequency power in QT intervals and EF or LVEDVI. Correlations between QTVI and LV function were observed, particularly between QTVI and LVESVI (r = 0.712, P < 0.0001).
Conclusion: In patients with previous anterior MI, there was variability in temporal dispersion of QT interval and a strong correlation between QTV corresponded with the infarcted site and LV function.
Keywords: beat‐to‐beat QT interval variability, QT variability index, myocardial infarction, left ventricular function, left ventricular end‐systolic volume index
QT interval variability (QTV) is a parameter of temporal dispersion of repolarization and may be a marker for increased risk of developing life‐threatening arrhythmias and sudden cardiac death. 1 Sarma et al. demonstrated that patients with coronary artery disease had abnormal coupling of heart rate and QT interval. 2 Other studies have shown that QT variability index (QTVI) in patients with heart disease was associated with increased risk of arrhythmic events and the presence of inappropriately large QT interval fluctuations among patients with dilated cardiomyopathy. 1 , 3 However, the relationship between temporal dispersion of QT intervals and left ventricular (LV) function in patients with previous myocardial infarction (MI) remains unknown. The purpose of the present study was to determine the level of QTV and its relationship with the LV contrast angiography (LVG) indices in patients with previous MI.
METHODS
Study Population and ECG Recording
The study population included 59 patients with previous anterior MI at least 6 months prior to enrollment. All patients had single‐vessel disease of descending coronary artery and underwent percutaneous transluminal coronary angioplasty. The sex and age distributions of the group are summarized in Table 1. The ethics committee of Hyogo College of Medicine approved the study protocol, and written informed consent was obtained from all patients.
Table 1.
Characteristics of Patients
| Variables | Number |
|---|---|
| Population | 59 |
| Age (years) | 64.4 ± 7.4 |
| Male/Female | 49/10 |
| Hypertension | 7 |
| Hyperlipidemia | 19 |
| Medication | |
| Nitrate | 34 |
| Angiotensin‐converting enzyme inhibitors | 16 |
| Angiotensin II receptor antagonists | 14 |
| Diuretics | 24 |
| Calcium antagonists | 17 |
| Prior VT/VF | 2 |
| Mean of QT interval | 429.6 ± 39.7 ms |
| Mean of RR interval | 880.6 ± 130.2 ms |
Data are presented as the mean ± SD.
VT = ventricular tachycardia; VF = ventricular fibrillation.
For QT and RR interval variability analysis, all patients underwent ECG for 512 beats while supine and awake during sinus rhythm in the morning (9 A.M.–12 M.). After patients rested for a minimum of 10 minutes in a quiet room, standard 12‐lead surface electrocardiograms were digitally sampled at a frequency of 500 Hz in each patient (FUKUDA DENSHI, FDX‐6531). The recorded ECG signal was stored in a memory card for off‐line proceeding by means of commercially available software (QTd1, Fukuda Denshi, Tokyo, Japan). The algorithm created the beat‐to‐beat RR and QT interval measurements automatically. Only patients with normal sinus rhythm were included in the study. The presence of atrial or ventricular ectopy was allowed unless such beats represented >5% of all beats. Patients with bundle branch block, accessory conducting pathways or those receiving class I or III antiarrhythmic agents or β‐blocker agents were excluded from the study. Diabetes mellitus was not present in this population. LVG was performed within 7 days of ECG assessment. Serum electrolytes and thyroid function were within normal limits.
Automatic ECG Analysis
The QT intervals for each lead and RR intervals were automatically calculated. The end of the T wave was detected at the point where the differentiated waveform returned to the ground line or isoelectric line. Any positive or negative deflection that measured >0.78 mV was measured. Thus, if the U wave was present, it was also included.
One lead (either V2 or V3) was selected, which best reflected the anterior infarction site and in which the end of the T wave was clearly recognized. Another lead (aVF) was selected which did not reflect the anterior infarction site. Large abrupt deflections in the resampled instantaneous RR and QT interval series resulting from ectopic beats were eliminated with a linear spline approach. Linear trends found in the heart rate and QT interval series over each 512‐beat epoch were corrected for subtraction of the best‐fit line. The 512 beat of data were analyzed for heart rate variability using methods described previously. 4 The mean interval, standard deviation, and variance in RR and QT intervals were computed from the respective beat series for each 512‐beat epoch for time‐domain measurements.
A normalized QTVI was then derived for each epoch according to following equation.
where QTv is the variance of QT interval, QTm is the mean QT interval, HRv is the variance of heart rate, and HRm is the mean heart rate.
The QTVI represents the log ratio between the QT interval and heart rate variability, each normalized by the squared mean of process. 3
In addition, the fast Fourier transform was calculated on 512 beat of RR and QT interval data. The power density spectrum was estimated by the sum of the squares of the magnitude of the fast Fourier transform on 512 beat. We computed power spectral densities on usable 512 beat and calculated four frequency‐domain measurements in RR and QT intervals: (1) low‐frequency power in RR and QT intervals (0.04–0.15 Hz), (2) high‐frequency power in RR and QT intervals (0.15–0.40 Hz), (3) total‐frequency power in RR and QT intervals (0.01–0.40 Hz), and (4) ratio of low‐frequency to high‐frequency power in RR and QT intervals.
Reproducibility of RR and QT Intervals for Automatic ECG Analysis
Variability of data in the measurements of RR and QT intervals was calculated from two ECG recordings (the first and the second ECG recordings taken at an identical time on the day following the first ECG recordings) by using 20 randomly selected patients. Reproducibility of RR and QT intervals for automatic ECG analysis was analyzed by the Bland‐Altman method. 5 The 95% limits of agreement were expressed in absolute values.
LV Contrast Angiography Analysis
Cardiac catheterization was performed in all patients. Single‐plane LVG was performed using a 30° right anterior oblique view. LV end‐diastolic volume and end‐systolic volume was calculated by the area‐length method from the single‐plane view. LV ejection fraction (EF) was calculated as the ratio (end‐diastolic volume—end‐systolic volume)/end‐systolic volume, and LV end‐diastolic volume index (LVEDVI) and left ventricular end‐systolic volume index (LVESVI) was determined from these data.
Statistical Analysis
Data are expressed as mean ± SD. The relationship between individual RR, QT interval indices and analyzed LVG parameters was evaluated by single regression analysis (Pearson's correlation).
All statistical analyses were performed using the Stat View 5.0 software (Abacus Concepts; SAS Institute, Cary, NC). For all calculations, P < 0.05 was considered statistically significant.
RESULTS
Characteristics of Patients
Fifty‐nine patients were recruited for this study. Mean age of the patients was 64.4 ± 7.4 years (range 40–77). Forty‐nine of the patients (83%) were men. The clinical characteristics of the study population are summarized in Table 1.
Correlations between Time‐Domain Indices of RR and QT Intervals and LVG Parameters
RR variability measurements and QTV measurements reflected the infarction site, and LVG parameters are summarized in Table 2. Univariate analysis revealed no correlation between variability of RR interval and LVG parameters or between mean QT interval and LVG parameters. Significant relationships were observed between standard deviation of QT interval and the LVG parameters, EF (r =−0.592, P < 0.0001), LVESVI (r = 0.580, P < 0.0001), and LVEDVI (r = 0.493, P < 0.0001). Correlation was also observed between variance of QT interval and the LVG parameters, EF (r =−0.510, P < 0.0001), LVESVI (r = 0.506, P < 0.0001), and LVEDVI (r = 0.459, P = 0.0003). QTVI was significantly correlated with LVEDVI (r = 0.553, P < 0.0001). A strong negative correlation was observed between QTVI and EF (r =−0.695, P < 0.0001). The strongest correlation was between QTVI and LVESVI (r = 0.712, P < 0.0001, Fig. 1).
Table 2.
Correlations between the Indices of RR and QT Intervals Corresponded with the Infarcted Site and LVG Parameters in the Time‐Domain Analysis
| EF (%) | LVESVI (mL/m2) | LVEDVI (mL/m2) | ||||
|---|---|---|---|---|---|---|
| Correlation Coefficient (r) | P Value | Correlation Coefficient (r) | P Value | Correlation Coefficient (r) | P value | |
| RRm | 0.108 | 0.415 | 0.016 | 0.907 | 0.113 | 0.396 |
| RR‐SD | 0.181 | 0.170 | −0.202 | 0.124 | −0.105 | 0.427 |
| RRv | 0.128 | 0.335 | −0.146 | 0.268 | −0.062 | 0.641 |
| QTm | −0.199 | 0.130 | 0.235 | 0.073 | 0.252 | 0.055 |
| QT‐SD | −0.592 | <0.0001 | 0.580 | <0.0001 | 0.493 | <0.0001 |
| QTv | −0.510 | <0.0001 | 0.506 | <0.0001 | 0.459 | 0.0003 |
| QTVI | −0.695 | <0.0001 | 0.712 | <0.0001 | 0.553 | <0.0001 |
LVG = left ventricular contrast angiography; EF = ejection fraction; LVESVI = left ventricular end‐systolic volume index; LVEDVI = left ventricular end‐diastolic volume index; QTVI = QT variability index; RR‐m = mean of RR interval; RR‐SD = standard deviation of RR interval; RRv = variance of RR interval; QTm = mean of QT interval; QT‐SD = standard deviation of QT interval; QTv = variance of QT interval.
Figure 1.
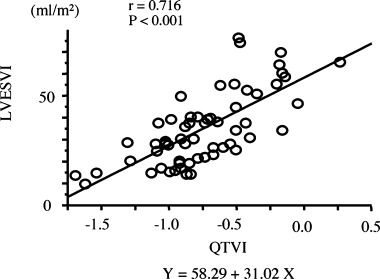
Correlation between LVESVI and QTVI‐reflected infarction site. Pearson's correlation coefficient is presented. LVESVI = left ventricular end‐systolic volume index; QTVI = QT variability index.
At aVF lead, which did not correspond with the infarction site, all time‐domain measures of the QT interval did not significantly correlate with LVG parameters, though we did not show detailed data in table. For typical example, there was poor correlation between EF and QTVI at aVF lead (r = 0.254, P = 0.096).
Correlations between Frequency‐Domain Indices of RR and QT Intervals and LVG Parameters
Frequency‐domain measures of RR or QT intervals reflected the infarction site, and LVG parameters are summarized in Table 3. Frequency‐domain measures of the RR interval did not correlate with LVG parameters. However, a significant relationship was found between high‐frequency power of QT interval and the LVG parameters, EF (r =−0.592, P < 0.0001), LVESVI (r = 0.621, P < 0.0001), and LVEDVI (r = 0.438, P = 0.0005). In addition, correlation was observed between low‐frequency power of QT interval and the LVG parameters, EF (r =−0.476, P = 0.0001), LVESVI (r = 0.515, P < 0.0001), and LVEDVI (r = 0.439, P = 0.0005), and between total power of QT interval and the LVG parameters, EF (r =−0.564, P < 0.0001), LVESVI (r = 0.632, P < 0.0001), and LVEDVI (r = 0.549, P < 0.0001). No significant correlation was found between ratio of low‐frequency to high‐frequency power of QT interval and LVG parameters.
Table 3.
Correlations between Indices of RR and QT Intervals Corresponded with the Infarcted Site and LVG Parameters in the Frequency‐Domain Analysis
| EF (%) | LVESVI (mL/m2) | LVEDVI (mL/m2) | ||||
|---|---|---|---|---|---|---|
| Correlation Coefficient (r) | P Value | Correlation Coefficient (r) | P Value | Correlation Coefficient (r) | P value | |
| RR‐HF | −0.049 | 0.711 | 0.007 | 0.960 | 0.042 | 0.752 |
| RR‐LF | 0.151 | 0.255 | −0.168 | 0.204 | −0.053 | 0.691 |
| RR‐TP | 0.087 | 0.513 | −0.118 | 0.374 | −0.076 | 0.567 |
| RR‐LF/HF | 0.245 | 0.061 | −0.207 | 0.115 | −0.072 | 0.590 |
| QT‐HF | −0.592 | <0.0001 | 0.621 | <0.0001 | 0.438 | 0.0005 |
| QT‐LF | −0.476 | <0.0001 | 0.515 | <0.0001 | 0.439 | 0.0005 |
| QT‐TP | −0.564 | <0.0001 | 0.632 | <0.0001 | 0.549 | <0.0001 |
| QT‐LF/HF | 0.062 | 0.643 | −0.103 | 0.436 | −0.026 | 0.844 |
RR‐HF = high‐frequency of RR interval; RR‐LF = low‐frequency of RR interval; RR‐TP = total power of RR interval; RR‐LF/HF = the ratio of low‐frequency to high‐frequency power of RR interval; QT‐HF = high‐frequency of QT interval; QT‐LF = low‐frequency of QT interval; QT‐TP = total power of QT interval; QT‐LF/HF = the ratio of low‐frequency to high‐frequency power of QT interval.
The other abbreviations as in Table 2.
At aVF lead, which did not correspond with the infarction site, all frequency‐domain measures of the QT interval did not correlate significantly with LVG parameters, though we did not show detailed data in table.
Reproducibility of RR and QT Intervals with Computer‐Assisted Method
The differences between the first and second ECG recordings in mean of RR intervals, QT intervals, and QTVI were −13.93–6.86, −1.98–3.10, and −0.16–0.24, respectively. These differences fell within the mean ± 2SD (Fig. 2).
Figure 2.
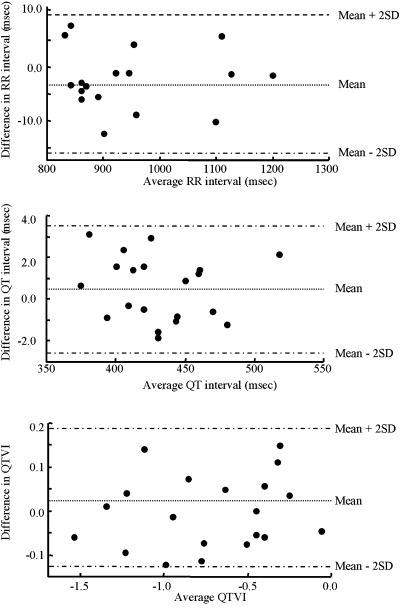
Difference against mean of RR interval, QT interval, and QTVI data. Difference in RR Interval. Mean =−3.03, Mean + 2SD = 9.33, Mean − 2SD =−15.83. Difference in QT Interval. Mean = 0.51, Mean + 2SD = 3.59, Mean – 2SD =−2.57. Difference in QTVI. Mean = 0.035, Mean + 2SD = 0.193, Mean – 2SD =−0.123.
DISCUSSION
The present study demonstrates that patients with previous anterior MI exhibited increased QTVI that reflected the infarction site with increase in LVESVI, while RR interval indices did not correlate with LV function.
It was previously reported that QTV increases with worsening functional class but is independent of EF in patients with dilated cardiomyopathy. 3 However, the present study showed good correlation between QTV and LV function, particularly between QTVI and LVESVI. A mechanistic explanation for this observation involves the reduction of current densities of the inward rectifier K+ current and transient outward K+ current in the failing human heart that contribute to action potential prolongation. 6 We speculate that the temporal dispersion of action potential duration increased as QT interval prolonged, and that QT variability increased as the temporal dispersion of action potential duration increased. Recently, Serneri et al. showed that cardiac angiotensin II generation increased with progression of heart failure and that end‐systolic wall stress was the only independent predictor of angiotensin II formation. 7 In addition, Akiyama et al. demonstrated that coronary occlusion increased myocardial interstitial noradrenaline levels in the ischemic region, but not in the nonischemic region. 8 Therefore, we speculated that increased cardiac angiotensin II via the angiotensin type 1 receptor results in sympathetic nervous system activation. In other words, LVESVI increased as cardiac angiotensin II levels increased and, thus, sympathetic nervous system activation progressed as LVESVI increased.
Our results indicate that temporal dispersion of QT interval that reflected the infarction site was increased secondary to increased interstitial noradrenaline levels in patients with MI. Therefore, the highest correlations were found between QTVI that reflected the infarction site and LVESVI in this study. We speculate that greater sympathetic activation, as reflected by increased LVESVI, produce greater QTV in patients with previous anterior MI.
Atiga et al. demonstrated that QTVI could be used to identify patients with sudden cardiac death and to predict arrhythmia‐free survival. They further demonstrated that QTVI ≥ 0.1 is associated with higher risk of arrhythmic events. 1 In this study, only one patient had QTVI ≥ 0.1, but that patient did not experience life‐threatening arrhythmias. Two patients that experienced ventricular arrhythmias had QTVI < 0.1. Therefore, we did not observe a relationship between QTVI and sudden cardiac death.
The autonomic nervous system has differential effects on the electrophysiologic properties of the sinus node, AV node, and ventricular muscle. 9 , 10 , 11 , 12 , 13 Recently, many studies investigated the significance of frequency‐domain indices of the RR interval, but not frequency‐domain indices of the QT interval. Spectral analysis was used to noninvasively estimate the autonomic modulation of the RR and QT intervals in this study. Previous studies demonstrated that dipyridamole, which induces ischemia, caused a loss of autonomic coupling between heart rate and ventricular repolarization for sympathetic and parasympathetic activities. 14 Our measurements in patients with previous anterior MI show that the indices of the QT interval that reflected the infarction site in frequency domain correlated with LV function, but the indices of the RR intervals in the frequency domain did not correlate with LV function. These data indicate that the indices of the QT interval that reflected the infarction site in frequency domain are better reflections of sympathetic nervous system activity than those of the RR interval. This phenomenon may occur as a result of autonomic imbalance toward sympathetic over activity or vagal withdrawal. 15
LIMITATIONS
The present study has several limitations. First, patients with frequent extrasystoles were excluded from this study. However, patients with previous anterior MI exhibited increased frequency of ventricular ectopies as EF decreased, and ventricular ectopy is associated with arrhythmic events. Therefore, these data do not apply to patients with frequent ventricular ectopies. Second, only one lead was used in calculating the data. Third, the method for measuring the RR interval indices is a broad‐based measure of autonomic function. However, measurements of the QT interval indices are not broad‐based measures of temporal dispersion of ventricular repolarization. A prospective study in a large population is required to overcome the limitations of the current study.
CONCLUSION
The autonomic nervous system had differential effects on electrophysiologic properties of the sinus node and ventricular muscle. QTV showed variation from lead to lead in ECG. Properties of the ventricular myocardium may be altered by autonomic nervous system changes observed with MI and may be associated with increased ventricular arrhythmias. There was variability in temporal dispersion of QT interval and a strong correlation between QTV corresponded with the infarcted site and LV function in patients with anterior MI.
REFERENCES
- 1. Atiga WL, Calkins H, Lawrence JH, et al. Beat‐to‐beat repolarization lability identifies patients at risk for sudden cardiac death. J Cardiovasc Electrophysiol 1998;9:899–908. [DOI] [PubMed] [Google Scholar]
- 2. Sarma JS, Singh N, Schoenbaum MP, et al. Circadian and power spectral changes of RR and QT intervals during treatment of patients with angina pectoris with nadolol providing evidence for differential autonomic modulation of heart rate and ventricular repolarization. Am J Cardiol 1994;74:131–136. [DOI] [PubMed] [Google Scholar]
- 3. Berger RD, Kasper EK, Baughman KL, et al. Beat‐to‐beat QT interval variability: Novel evidence for repolarization lability in ischemic and nonischemic dilated cardiomyopathy. Circulation 1997;96:1557–1565. [DOI] [PubMed] [Google Scholar]
- 4. Tsuji H, Venditti FJ Jr, Manders ES, et al. Reduced heart rate variability and mortality risk in an elderly cohort. The Framingham Heart Study. Circulation 1994;90:878–883. [DOI] [PubMed] [Google Scholar]
- 5. Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986;1:307–310. [PubMed] [Google Scholar]
- 6. Beuckelmann DJ, Nabauer M, Erdmann E. Alterations of K+ currents in isolated human ventricular myocytes from patients with terminal heart failure. Circ Res 1993;73:379–385. [DOI] [PubMed] [Google Scholar]
- 7. Serneri GG, Boddi M, Cecioni I, et al. Cardiac angiotensin II formation in the clinical course of heart failure and its relationship with left ventricular function. Circ Res 2001;88:961–968. [DOI] [PubMed] [Google Scholar]
- 8. Akiyama T, Yamazaki T, Ninomiya I. Differential regional responses of myocardial interstitial noradrenaline levels to coronary occlusion. Cardiovasc Res 1993;27:817–822. [DOI] [PubMed] [Google Scholar]
- 9. Warner MR, DeTarnowsky JM, Whitson CC, et al. Beat‐by‐beat modulation of AV conduction. II. Autonomic neural mechanisms. Am J Physiol 1986;251:H1134–H1142. [DOI] [PubMed] [Google Scholar]
- 10. Urthaler F, Neely BH, Hageman GR, et al. Differential sympathetic parasympathetic interactions in sinus node and AV junction. Am J Physiol 1986;250:H43–H51. [DOI] [PubMed] [Google Scholar]
- 11. Inoue H, Zipes DP. Changes in atrial and ventricular refractoriness and in atrioventricular nodal conduction produced by combinations of vagal and sympathetic stimulation that result in a constant spontaneous sinus cycle length. Circ Res 1987;60:942–951. [DOI] [PubMed] [Google Scholar]
- 12. Ardell JL, Randall WC, Cannon WJ, et al. Differential sympathetic regulation of automatic, conductile, and contractile tissue in dog heart. Am J Physiol 1988;255:H1050–H1059. [DOI] [PubMed] [Google Scholar]
- 13. Kowallik P, Braun C, Meesmann M. Independent autonomic modulation of sinus node and ventricular myocardium in healthy young men during sleep. J Cardiovasc Electrophysiol 2000;11:1063–1070. [DOI] [PubMed] [Google Scholar]
- 14. Theres H, Romberg D, Leuthold T, et al. Autonomic effects of dipyridamole stress testing on frequency distribution of RR and QT interval variability. Pacing Clin Electrophysiol 1998;21(Pt. II):2401–2406. [DOI] [PubMed] [Google Scholar]
- 15. Ong JJ, Sarma JS, Venkataraman K, et al. Circadian rhythmicity of heart rate and QTc interval in diabetic autonomic neuropathy: Implications for the mechanism of sudden death. Am Heart J 1993;125:744–752. [DOI] [PubMed] [Google Scholar]


