Abstract
Background: All family members of patients with Brugada syndrome (BS) should be screened. Fluctuations between diagnostic and nondiagnostic electrocardiogram (ECG) patterns in patients with BS are recognized, but systematic studies are lacking. The objective of this work was to prospectively evaluate the spontaneous changes between diagnostic and nondiagnostic ECG patterns in a family screened for BS.
Methods: One hundred twenty‐nine family members were possibly affected plus the index case were screened with two ECGs with an interval of 6 months. Only coved‐type ECG pattern was defined as diagnostic; type 2 and 3 ECGs were considered suggestive.
Results: The first ECG series made six diagnostics and the second 11, but only three patients maintained the diagnostic ECG. Patients with basal diagnostic ECG were older and more frequently symptomatic. Body mass index (BMI) was significantly lower in adults with diagnostic plus suggestive ECG when compared with the others. No significant gender difference was found among relatives with or without diagnostic ECG.
Conclusion: Spontaneous phenotypic manifestation of BS was more frequent in older symptomatic patients, absent in children, and related with low BMI. ECG manifestations were intermittent in more than 3/4 of the affected patients. Fluctuations between diagnostic and nondiagnostic ECGs may have an implication on the correct phenotyping in family screening so several ECGs with drug challenging are mandatory.
Ann Noninvasive Electrocardiol 2010;15(4):337‐343
Keywords: Brugada syndrome, family screening, ECG, sudden cardiac death
In 1992, Brugada and Brugada described a new syndrome associated with increased risk of sudden cardiac death (SCD) in patients with no structural heart disease, characterized by an electrocardiographic phenotype of right bundle branch block and ST‐segment elevation in the right precordial leads. 1
The worldwide prevalence of Brugada syndrome (BS) is difficult to calculate since the electrocardiogram (ECG) changes are dynamic and often not spontaneous, but it is estimated to be 5/10,000 inhabitants. BS is thought to be responsible for 4–12% of all SCD and for 20–50% of SCD in subjects without structural cardiopathy. 2
Three different patterns of ST elevation have been described (Fig. 1) to detect this entity. Risk stratification of SCD in Brugada patients is a hot debate and presently is based on the spontaneous ECG, the history of syncope or aborted SCD and the inducibility of ventricular tachyarrhythmias during electrophysiological study. The presence of a spontaneous diagnostic ECG has been documented as a strong predictor of events. 3 However, it is well known that the ECG pattern compatible with a Brugada diagnostic may only be transient. 4 , 5 ECG manifestations can be modulated or unmasked by sodium channel blockers, vagotonic agents, α‐adrenergic agonists, β‐adrenergic blockers, tricyclic or tetracyclic antidepressants, first generation antihistaminics, alcohol, cocaine, combination of glucose and insulin, fever, hyperkalemia, hypokalemia, or hypercalcemia. 2 Possible fluctuations between diagnostic and nondiagnostic ECGs have major implications for correct phenotyping and for the risk stratification of patients diagnosed. In the present study, we prospectively evaluated the spontaneous changes between diagnostic and nondiagnostic ECG patterns in a family screened for BS.
Figure 1.
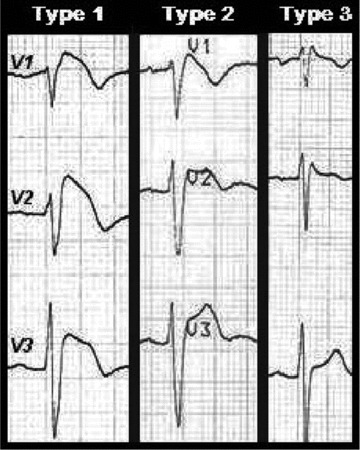
In the type 1 ECG, the elevated ST segment (≥2 mm) descends with an upward convexity to an inverted T wave. This is referred to as the coved‐type Brugada pattern. The type 2 and 3 patterns have a saddle‐back ST‐T wave configuration, in which the elevated ST segment descends toward the baseline, then rises again to an upright or biphasic T wave. The ST segment is elevated ≥1 mm in type 2 and <1 mm in type 3.
CASE REPORT
Male, 34‐year‐old, personal history of syncope, spontaneous ECG with characteristic phenotype of type 1 Brugada (Fig. 2) and family history of five known cases of SCD under the age of 45. Initial investigation of the patient's genealogical tree revealed an extensive family because his maternal grandmother had five siblings and his mother had ten siblings and twenty one cousins, making a total of 160 possibly affected individuals.
Figure 2.
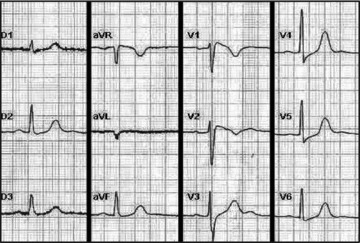
Spontaneous ECG of the index case: coved ST‐segment elevation ≥2 mm (0.2 mV) followed by a negative T wave.
METHODS
All available family members (130) possibly affected were screened with two ECGs with an interval of 6 months. The ECGs were analyzed for the presence of coved type (type 1), saddle‐back type (types 2 and 3), or no changes (normal). Only coved‐type ECG pattern, in at least two right precordial leads, was defined as diagnostic, and type 2 or 3 was only considered suggestive. Underlying structural cardiac abnormalities were excluded in all patients by noninvasive methods (biochemical analysis, transthoracic echocardiography, and exercise testing) used at our discretion.
ECG recordings were performed in the absence of antiarrhythmic drugs and at normal electrolyte levels. All ECGs were recorded in a supine position at normal body temperatures (under 37.5°C). Recording speed was 25 mm/s. The right precordial leads (V1 and V2) were carefully placed in the fourth intercostal space parasternally (using anatomic references). All ECGs were analyzed by two experienced physicians.
STATISTICS
A univariate statistical analysis was carried out, via logistic regression, Fisher's exact test, and Mann‐Whitney test. Associations were also assessed through multiple logistic regression. A value of P < 0.05 was considered statistically significant. Results for age and BMI are given as mean ± standard deviation. The SPSS version 15 software package (SPSS Inc., Chicago, IL, USA) was used.
RESULTS
A total of 260 12‐lead surface ECGs were recorded, two ECGs were obtained per patient. Minimal time interval between two ECGs was 5 months and the maximal interval was 7 months. There was no discrepancy in the diagnosis between the two blinded physicians who analyzed the ECGs.
The first ECG (Fig. 3) made 6 (4.6%) diagnostics and the second (Fig. 4) 11 (8.5%), but only 3 patients maintained the diagnostic pattern in both ECGs. Ten (7.7%) family members had a suggestive repolarization pattern in the first ECG series and seven (5.4%) in the second ECG. The two separated ECGs revealed 14 (10.8%) patients (eight males) with a coved type, five with type 2, and three with type 3 Brugada repolarization pattern.
Figure 3.
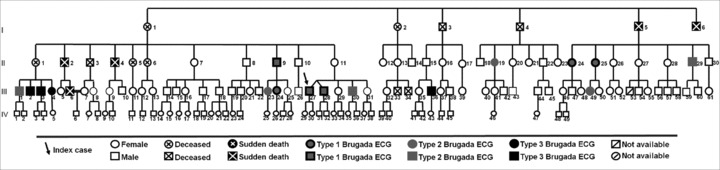
1st ECG: genealogical tree.
Figure 4.
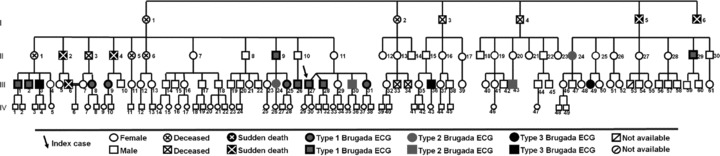
2nd ECG: genealogical tree.
Three analyses were then conducted comparing groups of cases: the first analysis (A), in which cases with BS diagnosis (one diagnostic ECG), was compared with nondiagnostic cases; the second analysis (B) compare the cases with at least one diagnostic or suggestive ECG against the ones with normal ECGs, and a third analysis (C), opposing cases with a diagnostic and stable ECG against cases with a diagnostic but dynamic ECG.
Patients with basal diagnostic ECG were significantly older than patients with nonspontaneous diagnostic ECG (Table 1) and patients with basal diagnostic or suggestive ECG were also significantly older than patients with normal ECG. Patients with diagnostic and stable ECG tend to be older than those with a diagnostic but dynamic ECG, but this difference isn't statistically significant. All 43 relatives aged under 16 years had normal basal ECG, and the youngest patient with a type 1 ECG was found on an 18‐year‐old asymptomatic boy. Also, univariable logistic regression analysis showed that age is a significant predictor of diagnostic and/or suggestive ECG pattern.
Table 1.
Analysis Conducted Comparing Groups of Cases against Age and Body Mass Index
| Age | Body Mass Index | ||||||
|---|---|---|---|---|---|---|---|
| n | Mean ± SD | Mann‐Whitney Test | n | Mean ± SD | Mann‐Whitney Test | ||
| A | Diagnostic ECG | 14 | 37.43 ± 11.07 | P < 0.05 | 11 | 219 ± 2.8 | P > 0.05 |
| Nondiagnostic ECG | 116 | 23.74 ± 16.63 | 35 | 23.3 ± 3.1 | |||
| B | Diagnostic plus suggestive ECG | 22 | 35.05 ± 11.15 | P < 0.05 | 16 | 21.3 ± 2.7 | P < 0.05 |
| Normal ECG | 108 | 23.21 ± 16.9 | 30 | 23.9 ± 2.9 | |||
| C | Diagnostic and stable ECG | 3 | 43.67 ± 15.57 | P > 0.05 | 2 | 24.1 ± 0.5 | P > 0.05 |
| Diagnostic and dynamic ECG | 11 | 35.73 ± 9.8 | 9 | 21.4 ± 2.8 | |||
Body mass index (BMI) was assessed in 46 family members more than 16 years old and was significantly lower in those with diagnostic plus suggestive ECG when compared with others (Table 1). Patients with basal diagnostic ECG had a lower BMI against patients with nonspontaneous diagnostic ECG, and patients with diagnostic and stable ECG tend to be thinner than those with a diagnostic but dynamic ECG, but these differences could not be considered as statistically significant.
Additionally, multivariable logistic regression revealed that age and BMI are significant predictors of diagnostic ECG pattern, the odds of BS diagnosis being estimated to increase by about 9.4% for every 1‐year increase of age (adjusted odds ratio = 1.094; P < 0.05) and to decrease by about 32% for every 1‐unit increase of BMI (adjusted odds ratio = 0.681; P < 0.05).
Furthermore, the odds of BS diagnostic or suggestive pattern in an ECG is estimated to increase by about 7.3% for every 1‐year increase of age (adjusted odds ratio = 1.073; P < 0.05) and to decrease by about 38.3% for every 1‐unit increase of BMI (adjusted odds ratio = 0.617; P < 0.05).
Surprisingly, no significant difference was found in gender among relatives with or without diagnostic ECG (Table 2). Even though male patients had more diagnostic ECG, more diagnostic plus suggestive ECGs, and more diagnostic and stable ECGs, these differences could not be considered as statistically significant.
Table 2.
Analysis Conducted Comparing Groups of Cases against Gender, Symptoms and Electrophysiological Study
| Gender | Symptoms | Electrophysiological Study Test | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | Fisher's Exact Test | Yes | No | Fisher's Exact Test | Positive | Negative | Fisher's Exact Test | ||
| A | Diagnostic ECG | 8 | 6 | P > 0.05 | 2 | 12 | P < 0.05 | 3 | 6 | P > 0.05 |
| Nondiagnostic ECG | 60 | 56 | 1 | 115 | 0 | 6 | ||||
| B | Diagnostic plus suggestive ECG | 14 | 8 | P > 0.05 | 2 | 20 | P > 0.05 | |||
| Normal ECG | 54 | 54 | 1 | 107 | ||||||
| C | Diagnostic and stable ECG | 2 | 1 | P > 0.05 | 1 | 2 | P > 0.05 | 2 | 1 | P > 0.05 |
| Diagnostic and dynamic ECG | 6 | 5 | 1 | 10 | 1 | 10 | ||||
Brugada ECG was statistically related with symptoms (Table 2). In fact, symptoms were significantly more frequent in those with diagnostic ECG when compared with the ones with nondiagnostic ECG. However, there was no significant difference between patients with diagnostic and stable ECGs against those with a diagnostic but dynamic ECG, with respect to symptoms.
All inducible patients (Table 2) in the electrophysiological study had a spontaneous diagnostic Brugada ECG and patients with diagnostic and stable ECG tend to be more inducible than those with a diagnostic but dynamic ECG, but these differences are not statistically significant.
We found significant difference between the percentages of diagnoses with only the first ECG (4.6%) against two serial ECGs (10.8%) (McNemar's test, P < 0.05). Among the 14 relatives with a diagnostic ECG, 11 (79%) exhibited one nondiagnostic ECG and 6 (43%) one normal ECG. In fact, among diagnostic cases, according to Cohen's Kappa coefficient, there is no agreement between the first and second ECG (K < 0). Furthermore, of the 22 patients with a suggestive or a diagnostic ECG, 10 (45%) had the other ECG classified as normal. For these 22 patients, the low value of Cohen's Kappa coefficient (K < 0.1) is indicative of just slight agreement between the results of the 1st and 2ndECGs.
DISCUSSION
From our study, the onset of a diagnostic Brugada ECG was at 18 years, and the suggestive ECG at 16 years old. ECG changes tend to occur more often later in life around the third decade of life and patients with basal diagnostic and/or suggestive ECG were older. Even though patients with diagnostic and stable ECG tended to be older, this difference wasn't significant.
Our findings don't corroborate others that show a higher incidence of BS in males. 6 , 7 Studies suggest that sex‐related intrinsic differences in ionic currents and a testosterone‐dependent transient outward potassium current, favoring the characteristic electrical manifestation of BS, is responsible for a 8–10 for 1 ratio favoring males. 8 The gender‐specific findings, in this family, could be explained by individual hormonal levels (higher levels of testosterone in females or lower in males) or an uneven distribution of the disease‐favoring females in this family leading to a false perception of an equal distribution between genders.
We observed significant fluctuations between diagnostic and nondiagnostic ECGs within 6 months. Seventy‐nine percent of the patients diagnosed with BS presented fluctuations between diagnostic and nondiagnostic ECGs and 43% had a normal ECG. Twenty‐one percent of the patients presented a continuously diagnostic coved‐type ECG. Possible explanations for dynamic ECG changes in Brugada patients are time of the examination, relation with meals, body temperature, drugs, autonomic balance, especially, vagal tone, hormonal status, and lead position. 2 All can be excluded in our patients except slight nonpathological body temperature differences, an eventual relation with meals and millimetric differences in lead position. BS electrical manifestations are linked with the right ventricular outflow tract, anatomically related with the third and second intercostal space. Placement of the right precordial leads, in a slightly upper position than the fourth intercostal space, can change the repolarization pattern and manifest the Brugada phenotype. In fact, it is consensual that raising the positions of the electrodes from the conventional positions increases the number of type 1 ECG patterns, that is, increases the sensitivity of the ECG. Another study about this topic assured similar position of the right precordial leads in the different surface ECGs by similar R‐wave amplitude, with a maximal deviation of 0.1 mV. 9 We believe that our method (precise placing of the right precordial leads (V1 and V2) in the fourth intercostal space parasternally) represents more accuracy in “real life” examinations and inevitable differences between examinations.
Obviously, the pertinent question is: how often should we recommend repeating the ECG to these 130 potential patients? Weekly? Monthly? Twice a year? All potential Brugada patients from this family should do pharmacological challenge with sodium blockers but drug challenge is acknowledged to have less than 100% sensitivity, specificity, and reproducibility. Evidence has been reported that false positive and false negative responses to sodium channel blockers may occur. 10 So, in this particular family, can a negative provocative test exclude the disease? In this context, genetic study may play an important role if the mutation involved can been identified, so that noncarriers can be excluded. But if we don't have access to genetic results, we think that every potential Brugada patient should do an ECG at least twice a year regardless the provocative test and in every febrile event.
Regarding risk stratification of patients with BS, the diagnostic ECG is one of the significant parameters in predicting risk of events. In 2001, Brugada et al. published that 14% of the asymptomatic patients with a diagnostic ECG experienced a malignant ventricular arrhythmia within a follow‐up of 2.5 years. 11 More recently, the same group showed again that a spontaneous diagnostic ECG predicts a higher risk in experiencing SCD within the mean follow‐up of 2 years than a diagnostic ECG induced by drugs. 3 Because 79% of the diagnostic cases in this family showed fluctuations between coved and noncoved ECGs, only one ECG could cause a potential underdiagnosis or incorrect risk stratification to the majority of patients. Due to repetitive ECG recordings, diagnostic ECGs could be obtained and a correct estimation of SCD risk could be done. In this family we had two patients with history of syncope, one classified as “high risk” because the first ECG was diagnostic and the other classified as “low risk” because the first ECG was negative. After the second ECG (diagnostic in both), we realized that their individual risk was the same.
Currently, there are no differences regarding risk stratification between patients with “stable” Brugada ECG against “dynamic” Brugada ECG (shifting diagnostic to nondiagnostic ECG over time) because there are no data that prove that these patients have a different risk of events. However, the Brugada phenotypic manifestation and the arrhythmic events result from an ionic imbalance between the inward and outward currents during phase 1 of the action potential. Loss of the action potential dome in right ventricular epicardium but not endocardium underlies the ST‐segment elevation seen in the BS and that electrical heterogeneity within right ventricular epicardium leads to the development of closely coupled premature ventricular contractions via a phase 2 reentrant mechanism that then precipitates ventricular tachycardia/ventricular fibrillation. 12 From a theoretical standpoint, we could then presume that patients with a chronic and stable imbalance, and electrical heterogeneity may be a subgroup with a higher risk of events, but in our study we lack patients and events to prove it.
CONCLUSION
Age and BMI were identified as significant predictors of the manifestation of Brugada patterns. Not only type 1 (diagnostic pattern) but also types 2 and 3 pattern manifestations tend to occur in older subjects and with low BMI. No significant relation between gender and Brugada pattern manifestations was found. Gender, age, and BMI do not seem to be significantly related to fluctuations in ECG results of BS patients. We found a significant increase in the percentage of diagnosis with only one ECG against two serial ECGs. Besides that, Cohen's Kappa coefficient is indicative of a low degree of agreement between the first and the second ECGs within subjects that have had a Brugada pattern ECG. So, with only one ECG, a BS patient may well not be identified, leading the physician to a misleading prognosis. We must conclude that screening for BS with only one ECG is not enough and several ECGs with drug challenging are mandatory.
Finally, no conclusions with respect to a prognostic significance of ECG fluctuations in patients with at least one Brugada ECG can be done based on this data. Future studies with a longer follow‐up and a large patient population are needed to assess the prognostic significance of fluctuations in BS.
LIMITATIONS
There are some limitations in this study. First, this analysis was performed on a single family, so extrapolations to the general population of Brugada patients must be done carefully.
The leads V1 and V2 were placed in the conventional position (fourth intercostal space). Over 6 months, an exactly identical position of the right precordial leads cannot be guaranteed. Some minor deviation of the position of the ECG electrodes between the different ECG recordings may have occurred.
Acknowledgments
Acknowledgement: The authors would like to thank all the professionals from de Cardiology Department from São Teotónio Hospital.
Conflict of Interest: None declared.
REFERENCES
- 1. Brugada P, Brugada J. Right bundle branch block, persistent ST segment elevation and sudden cardiac death: A distinct clinical and electrocardiographic syndrome. A multicenter report. J Am Coll Cardiol 1992;20:1391–1396. [DOI] [PubMed] [Google Scholar]
- 2. Antzelevitch C. Brugada syndrome. Pacing Clin Electrophysiol 2006;29:1130–1159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brugada J, Brugada R, Brugada P. Determinants of sudden cardiac death in individuals with the electrocardiographic pattern of Brugada syndrome and no previous cardiac arrest. Circulation 2003;108:3092–3096. [DOI] [PubMed] [Google Scholar]
- 4. Priori SG, Napolitano C, Gasparini M, et al Natural history of Brugada syndrome: Insights for risk stratification and management. Circulation 2002;105:1342–1347. [DOI] [PubMed] [Google Scholar]
- 5. Mizumaki K, Fujiki A, Tsuneda T, et al Vagal activity modulates spontaneous augmentation of ST elevation in the daily life of patients with Brugada syndrome. J Cardiovasc Electrophysiol 2004;15:667–673. [DOI] [PubMed] [Google Scholar]
- 6. Antzelevitch C. Androgens and male predominance of the Brugada syndrome phenotype. Pacing Clin Electrophysiol 2003;26:1429–1431. [DOI] [PubMed] [Google Scholar]
- 7. Wilde AA, Antzelevitch C, Borggrefe M, et al Proposed diagnostic criteria for the Brugada syndrome: Consensus report. Circulation 2002;106:2514–2519. [DOI] [PubMed] [Google Scholar]
- 8. Fish JM, Antzelevitch C. Cellular and ionic basis for the sex‐related difference in the manifestation of the Brugada syndrome and progressive conduction disease phenotypes. J Electrocardiol 2003;36(Suppl.):173–179. [DOI] [PubMed] [Google Scholar]
- 9. Veltmann C, Schimpf R, Echternach C, et al A prospective study on spontaneous fluctuations between diagnostic and non‐diagnostic ECGs in Brugada syndrome: Implications for correct phenotyping and risk stratification. Eur Heart J 2006;27:2544–52 [DOI] [PubMed] [Google Scholar]
- 10. Priori SG, Napolitano C, Gasparini M, et al Clinical and genetic heterogeneity of right bundle branch block and ST‐segment elevation syndrome: A prospective evaluation of 52 families. Circulation 2000;102:2509–2515. [DOI] [PubMed] [Google Scholar]
- 11. Brugada J, Brugada R, Antzelevitch C, et al Long‐term follow‐up of individuals with the electrocardiographic pattern of right bundle‐branch block and ST‐segment elevation in precordial leads V1 to V3. Circulation 2002;105:73–78. [DOI] [PubMed] [Google Scholar]
- 12. Gussak, I , Antzelevitch, C , Bjerregaard, P , et al The Brugada syndrome: Clinical, electrophysiologic and genetic aspects. J Am Coll Cardiol 1999;33:5. [DOI] [PubMed] [Google Scholar]


