Abstract
Background: Heart rate variability (HRV) is an accepted and reliable means for assessing autonomic nervous system dysfunction. A 5‐minute measurement of HRV is considered methodologically adequate. Several studies have attempted to use shorter recordings of 1–2 minutes or 10 seconds. The aim of this study was to determine the reliability of HRV parameters calculated from ultra‐short electrocardiogram recordings.
Methods: Seventy healthy volunteers were recruited for the study. HRV was evaluated for 5 minutes according to accepted procedures. Thereafter, HRV parameters were recalculated from randomly selected 1‐minute and 10‐second intervals. The standard and ultra‐short measurements were correlated using intraclass correlation coefficients.
Results: Good correlations between the 5‐minute electrocardiograms (ECGs) and both the 1‐minute and 10‐second ECGs were noted for average RR interval, and root mean square of successive differences in RR intervals (RMSSD). No correlation was noted for standard deviation of the RR interval (SDNN) and several other HRV parameters.
Conclusions: RMSSD, but not SDNN, seem a reliable parameter for assessing HRV from ultra‐short (1 minute or 10 seconds) resting electrocardiographic recordings. Power spectral analysis and evaluation of other HRV parameters require longer recording periods. Further research is required to evaluate the importance of ultra‐short RMSSD for cardiovascular risk stratification.
Ann Noninvasive Electrocardiol 2011;16(2):117–122
Keywords: heart rate variability, electrocardiography, autonomic nervous system, ultra‐short HRV
Heart rate variability (HRV) is a reliable clinical tool for evaluating autonomic nervous system (ANS) function. 1 Abnormal HRV has been found to be associated with an adverse prognosis in various clinical conditions. 2 Using 24‐hour Holter monitoring, clinicians can calculate ultra–low frequency (ULF) components (0.003–0.0001 Hz) as well as standard deviation (SD) of the 5‐minute average NN intervals (SDANN). 2 However, this method is less practical than in‐clinic short‐term measurements. Five‐minute electrocardiographic (ECG) recordings are currently considered acceptable in evaluating HRV. 3
Several researchers have attempted to apply measurements made from even shorter recordings for evaluating cardiovascular risk stratification, evaluating sinoatrial node function, monitoring mental stress, and evaluating anesthesia outcome. 4 , 5 , 6 , 7 , 8
Some studies have reported that the SD of the NN intervals (SDNN), calculated from 10‐second ECGs recorded in healthy humans 9 and in patients during and after myocardial infarction, 10 was associated with mortality risk. Few studies have shown that HRV measurements were reproducible, 11 , 12 although 20‐second recordings were too short to provide reproducibility. 13 In general, there is lack of consensus as to the clinical value and reliability of ultra‐short HRV measurement.
Therefore, the aim of the present study was to further asses the validity of HRV parameters evaluated from ultra‐short recordings, given the growing interest in this methodology 7 , 9 , 10 and the lack of standardization for very short term measurements. Evaluation of electrocardiographic parameters from shorter time periods may provide a more accessible clinical tool for the medical practitioner.
We hypothesized that several ultra‐short HRV parameters would prove to be more reliable than others.
METHODS
Study Design
A consecutive case series observational study design was used. Data were collected prospectively to answer the study's hypothesis. The research protocol was approved by the institutional review board. All participants gave written informed consent.
Study Subjects
Seventy healthy volunteers were included in the study and were recruited after attending the outpatient clinic for a routine health checkup. Health status was determined following screening of medical records, an interview, and a complete physical examination. None had an acute or chronic condition, and no one regularly used drugs known to affect heart rate or ECG parameters.
Procedure
Participants were asked not to smoke, drink caffeinated beverages, or take other stimulants 3 hours prior to the test, and to avoid strenuous exercise for 24 hours prior to the test. The test was conducted between 9:00 a.m. and noon to avoid the circadian influence on heart rate and ANS function. To prevent sympathetic overactivity, subjects were requested to empty their bladder before the test. Room temperature was maintained at 21–23°C. Before starting the test, participants were asked to lie motionless in a supine position for 10 minutes. A standard ECG device was used, at a sampling rate of 2000 Hz. The ECG electrodes were placed in anatomical positions according to standard procedure, and recordings were made from the limb leads for 5 minutes. Recordings of inadequate quality were repeated. The data were saved in a binary format and processed with custom‐made computer software, validated, and tested for reproducibility according to accepted standards. 2
RR intervals were measured between two consecutive beats. To quantify the HRV time domain, the following variables were calculated: SDNN, reflecting the cyclic variability of the heart rate during the recording period; RMSSD, the root mean square of successive differences of RR intervals; NN50, the number of intervals differing by 50 msec from the preceding interval; pNN50, calculated by dividing NN50 by the total number of RR intervals; and HRV triangular index, which is the integral of the density distribution divided by the maximum of the density distribution. RMSSD reflects the average change in RR interval between beats. Power spectral analysis was conducted using the fast Fourier transform. Integral calculations of the area beneath the power spectral density curve for frequency range were made in absolute values of power (ms2). The spectral components were divided into very low frequency (VLF 0.003–0.04 Hz), LF (0.04–0.15 Hz), and high frequency (HF 0.15–0.4 Hz). The total power was computed.
A repeated analysis of HRV parameters was conducted from a randomly chosen 1‐minute‐long series and another randomly chosen 10‐second series. None of the recordings included premature beats.
Statistical Analysis
Results were expressed as mean and SD. Agreement between measurements obtained from the entire 5‐minute recording with the 1‐minute and 10‐second interval recordings was determined by calculating the intraclass correlation coefficient (ICC). An ICC significantly (P < 0.05) above 0.7 was considered a finding of a good correlation, and an acceptable measure of reliability. 14 Analyses were performed using SPSS 15 for Windows software (SPSS Inc., Chicago, IL) and JMP version 7.0 (SAS Institute, Cary, NC).
RESULTS
Mean (±SD) subject age was 41.5 ± 16.1 years; 52.3% were male. Mean height was 1.7 ± 0.09 meters and mean weight, 68.8 ± 13.1 kg. Mean body mass index (BMI) was 23.5 ± 2.8 kg/m2.
The HRV parameters calculated from the 5‐minute, 1‐minute, and 10‐second recordings and the ICCs between them are shown in Tables 1 and 2. Figure 1 illustrates a bivariate fit of 1 minute/10 seconds average RR, RMSSD, and SDNN by 5 minutes calculated values.
Table 1.
Correlation of Electrocardiographic Measurements of HRV over 1 and 5 Minutes
| 1 Minute | 5 Minutes | ICC | 95% CI | ||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||||
| Maximal RR duration (ms) | 986.80 | 145.25 | 1047.83 | 157.43 | 0.831 | 0.401 | 0.932 |
| Minimal RR duration (ms) | 797.13 | 101.15 | 723.30 | 113.33 | 0.582 | 0.087 | 0.797 |
| Average RR duration (ms) | 889.69 | 112.43 | 889.89 | 113.88 | 0.983* | 0.972 | 0.989 |
| SDNN (ms) | 42.02 | 26.05 | 51.40 | 25.72 | 0.863 | 0.463 | 0.946 |
| RMSSD (ms) | 40.68 | 34.99 | 42.75 | 32.32 | 0.963* | 0.941 | 0.977 |
| HRV triangular index | 10.13 | 4.00 | 15.04 | 4.96 | 0.430 | −0.094 | 0.733 |
| NN50 | 4.96 | 6.20 | 27.61 | 30.05 | 0.247 | −0.047 | 0.493 |
| pNN50 | 2.81 | 3.73 | 8.41 | 9.53 | 0.499 | 0.036 | 0.739 |
| VLF (ms2) | 143.00 | 97.58 | 218.06 | 98.60 | 0.524 | 0.034 | 0.762 |
| LF (ms2) | 200.98 | 101.49 | 159.39 | 65.92 | 0.544 | 0.277 | 0.716 |
| HF (ms2) | 193.44 | 107.94 | 142.65 | 91.28 | 0.710 | 0.287 | 0.864 |
| Total power (ms2) | 549.52 | 86.06 | 570.27 | 81.51 | 0.725 | 0.592 | 0.820 |
HF = high‐frequency components; HRV = heart rate variability; ICC = intraclass correlation; LF = low‐frequency components; NN50 = number of intervals differing by >50 ms from preceding interval; pNN50 = NN50 divided by total number of intervals; RMSSD = root mean square of successive differences in RR intervals; SDNN = standard deviation of RR interval; VLF = very low frequency components.
* Good correlation.
Table 2.
Correlation of Electrocardiographic Measurements of HRV over 10 Seconds and 5 Minutes
| 10 Seconds | 5 Minutes | ICC | 95% CI | ||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||||
| Maximal RR duration (ms) | 952.01 | 135.83 | 1047.83 | 157.43 | 0.695* | 0.032 | 0.883 |
| Minimal RR duration (ms) | 844.86 | 104.59 | 723.30 | 113.33 | 0.423* | −0.096 | 0.723 |
| Average RR duration (ms) | 898.24 | 116.88 | 889.89 | 113.88 | 0.958* | 0.933 | 0.974 |
| SDNN (ms) | 34.49 | 24.54 | 51.40 | 25.72 | 0.676* | 0.025 | 0.872 |
| RMSSD (ms) | 38.70 | 33.27 | 42.75 | 32.32 | 0.909* | 0.853 | 0.944 |
| HRV triangular index | 5.26 | 1.99 | 15.04 | 4.96 | 0.068* | −0.053 | 0.238 |
| NN50 | 0.69 | 1.20 | 27.61 | 30.05 | 0.038* | −0.089 | 0.190 |
| pNN50 | 0.56 | 0.98 | 8.41 | 9.53 | 0.108* | −0.068 | 0.296 |
| LF (ms2) | 171.86 | 116.76 | 159.39 | 65.92 | 0.363* | 0.142 | 0.549 |
| HF (ms2) | 276.33 | 116.58 | 142.65 | 91.28 | 0.247* | −0.084 | 0.526 |
| Total power (ms2) | 487.79 | 100.73 | 570.27 | 81.51 | 0.482* | −0.045 | 0.750 |
HF = high‐frequency components; HRV = heart rate variability; ICC = intraclass correlation; LF = low‐frequency components; NN50 = number of intervals differing by >50 ms from preceding interval; pNN50 = NN50 divided by total number of intervals; RMSSD = root mean square of successive differences in RR intervals; SDNN = standard deviation of RR interval; VLF = very low frequency components.
*Good correlation.
Figure 1.
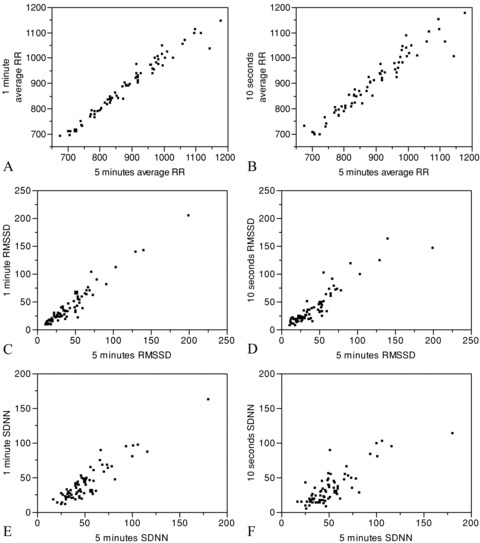
Bivariate fit of 1‐minute average RR by 5‐minute average RR (A), 10‐second average RR by 5‐minute average RR (B), 1‐minute RMSSD by 5‐minute RMSSD (C), 10‐second RMSSD by 5‐ minute RMSSD (D), 1‐minute SDNN by 5‐minute SDNN (E), and 10‐second SDNN by 5‐minute SDNN (F).
A good correlation between the 5‐ and 1‐minute recordings was found for the following parameters: average RR duration (95% CI 0.972–0.989), and RMSSD (95% confidence interval [CI] 0.941–0.977). A good correlation was found between the 5‐minute and 10‐second recordings for average RR duration (95% CI 0.933–0.974), and RMSSD (95% CI 0.853–0.944). There was no statistically significant correlation between the 5‐minute and short‐term recordings for maximal and minimal RR duration, SDNN, HRV triangular index, NN50, pNN50, VLF, LF, and HF.
DISCUSSION
Application of ultra–short‐term and reliable evaluation of HRV is desirable in order to increase the applicability of HRV to the common practitioner.
In an attempt to verify the reliability of 5‐minute recordings compared with prolonged recordings (24 hours), researchers noted a modest correlation between a 24‐hour SDANN and a 5‐minute SDNN, with the same notes for frequency domains. 15 The correlation was stronger between the 8‐hour and 5‐minute recordings, 14 probably because of the known differences between daytime and nighttime HRV parameters, which affects the 24‐hour HRV results. 2 A good correlation HF spectra was also found between 24‐hour and 5‐minute ECGs. 15 Schroeder et al. 12 reported a good correlation between 10‐second ECGs and 2‐ and 6‐minute ECGs; however, all HRV parameters were not included.
Five‐minute ECG recordings are the accepted standard for the in‐clinic evaluation of heart rate dynamics. 3 Although 10‐second, 12‐lead recordings, are commonly used to detect resting abnormalities in interval length, wave morphology, and segment elevations \depressions, their reliability for evaluating HRV is still unclear.
RMSSD, NN50, and pNN50 are primarily affected by vagal influence during respiration 2 , 3 . A dynamic ANS function will be evident by an increase in SDNN, NN50, and pNN50 values.
Values of the HF component in the supine position are highly associated with vagal activity and reflect the respiratory influence on sinus arrhythmia. 16 Supine LF is a more controversial parameter, which may reflect both sympathetic and parasympathetic balance. 2 , 3 The LF component is believed to be influenced by the baroreceptor system. 17
In the present study, values of average RR interval, and RMSSD calculated from the 1‐minute and 10‐second recordings were found to be as reliable as those calculated from the 5‐minute recordings when evaluating HRV. However, SDNN was highly dependent on the length of recording, as were minimal and maximal RR intervals, HRV triangular index, NN50, and pNN50.
According to the Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology, a minimum recording of 1 minute is required to estimate the HF component, and at least 2 minutes, to estimate the LF component. 3 Accordingly, in the present study, there was no correlation of these parameters between measurements made from the 5‐minute and 10‐second recordings. In disagreement with the earlier reports, 3 we found no correlation between HF calculated from a 1‐ and a 5‐minute recording.
Similar to Schroeder et al.'s findings, 12 our results supported the reliability of RMSDD measurements from ultra‐short ECG recordings. However, the lack of correlation shown here for SDNN between the 5‐minute measurement, the 10‐second (95% CI 0.025–0.872), and 1‐minute (95% CI 0.463–0.946) measurements disagrees with the earlier study. Schroeder et al. 12 reported a quite high correlation between the 6‐minute measurement, the 2‐minute, and 10‐second measurements, and a high ICC for both (95% CI 0.89–0.95 and 0.68–0.82, respectively). This discrepancy is hard to explain given the similarities between the studies in the number of participants (63 and 70, respectively), sex distribution (51% vs 52.3% males), and mean resting heart rate measured from 10‐second ECGs (60 ± 7 vs 67.4 ± 8.9 bpm, respectively). However, our patients were younger (mean age 41.5 years vs 52 years) and thinner (mean BMI 23.5 kg/m2 vs 27 kg/m2). Further studies are needed to determine if subject characteristics affect the reliability of some ultra‐short HRV parameters.
Importantly, both Dekker et al. 9 and Karp et al. 10 evaluated SDNN from a 10‐second ECG recording, but for purposes of mortality risk stratification, not quantification of autonomic nervous dysfunction. Based on the present findings, we suggest that RMSSD rather than SDNN provides more valid estimation of HRV on ultra‐short ECG recordings compared with standard measurement techniques. Our findings provide the practitioner with a feasible and accessible tool for in‐clinic short‐term HRV evaluation. The clinical values of RMSSD for cardiovascular risk stratification warrant further research.
Study Limitations
HRV measurements were conducted without metronomic breathing to stimulate a typical ECG recording, and in accordance with the accepted methodological guidelines. Nevertheless, we can not predict whether paced breathing would have altered our results. In addition, HRV measurements were conducted under strict conditions that may not reflect the conditions in which ECG are regularly conducted, therefore limiting the application of the current results. Only healthy individuals were included in the study. Thereby, the results are applicable to healthy subjects. Further research should focus on assessing the reliability of ultra‐short HRV evaluation in patients with autonomic dysfunction and high‐risk cardiac patients.
Acknowledgments
Acknowledgments: We thank Gloria Ginzach and Phyllis Curchack Kornspan for their editorial assistance. This study is dedicated to the memory of Haim Gueron.
There are no conflicts of interest or sources of funding.
REFERENCES
- 1. Nussinovitch N, Livneh A, Katz K, et al Heart rate variability in familial Mediterranean fever. Rheumatol Int 2011;31:39–43. [DOI] [PubMed] [Google Scholar]
- 2. Heart rate variability . Standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Eur Heart J 1996;17:354–381. [PubMed] [Google Scholar]
- 3. Heart rate variability: Standards of measurement, physiological interpretation and clinical use . Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation 1996;93:1043–1065. [PubMed] [Google Scholar]
- 4. Carnethon MR, Liao D, Evans GW, et al Does the cardiac autonomic response to postural change predict incident coronary heart disease and mortality? The Atherosclerosis Risk in Communities Study. Am J Epidemiol 2002;155:48–56. [DOI] [PubMed] [Google Scholar]
- 5. Carnethon MR, Golden SH, Folsom AR, et al Prospective investigation of autonomic nervous system function and the development of type 2 diabetes: The Atherosclerosis Risk in Communities study, 1987–1998. Circulation 2003;107:2190–2195. [DOI] [PubMed] [Google Scholar]
- 6. Sosnowski M, Petelenz T. Heart rate variability. Is it influenced by disturbed sinoatrial node function? J Electrocardiol 1995;28:245–251. [DOI] [PubMed] [Google Scholar]
- 7. Salahuddin L, Cho J, Jeong MG, et al Ultra short term analysis of heart rate variability for monitoring mental stress in mobile settings. Conf Proc IEEE Eng Med Biol Soc 2007;2007:4656–4659. [DOI] [PubMed] [Google Scholar]
- 8. Fujiwara Y, Ito H, Asakura Y, et al Preoperative ultra short‐term entropy predicts arterial blood pressure fluctuation during the induction of anesthesia. Anesth Analg 2007;104:853–856. [DOI] [PubMed] [Google Scholar]
- 9. Dekker JM, Schouten EG, Klootwijk P, et al Heart rate variability from short electrocardiographic recordings predicts mortality from all causes in middle‐aged and elderly men. The Zutphen Study. Am J Epidemiol 1997;145:899–908. [DOI] [PubMed] [Google Scholar]
- 10. Karp E, Shiyovich A, Zahger D, et al Ultra‐short‐term heart rate variability for early risk stratification following acute ST‐elevation myocardial infarction. Cardiology 2009;114:275–283. [DOI] [PubMed] [Google Scholar]
- 11. Marks BL, Lightfoot JT. Reproducibility of resting heart rate variability with short sampling periods. Can J Appl Physiol 1999;24:337–348. [DOI] [PubMed] [Google Scholar]
- 12. Schroeder EB, Whitsel EA, Evans GW, et al Repeatability of heart rate variability measures. J Electrocardiol 2004;37:163–172. [DOI] [PubMed] [Google Scholar]
- 13. Dekker JM, de Vries EL, Lengton RR, et al Reproducibility and comparability of short‐ and long‐term heart rate variability measures in healthy young men. Ann Noninvasive Electrocardiol 1996;1:287–292. [Google Scholar]
- 14. Baumgartner TA, Chung H. Confidence limits for intraclass reliability coefficients. Meas Phys Educ Exerc Sci 2001;5:179–188. [Google Scholar]
- 15. Min KB, Min JY, Paek D, et al Is 5‐minute heart rate variability a useful measure for monitoring the autonomic nervous system of workers? Int Heart J 2008;49:175–181. [DOI] [PubMed] [Google Scholar]
- 16. Kleiger RE, Stein PK, Bigger JT, Jr . Heart rate variability: Measurement and clinical utility. Ann Noninvasive Electrocardiol 2005;10:88–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hilz MJ, Dutsch M. Quantitative studies of autonomic function. Muscle Nerve 2006;33:6–20. [DOI] [PubMed] [Google Scholar]


