Abstract
Objective: Despite the progress that has been reached in emergency medical systems and resuscitation, sudden cardiac death (SCD) continues to be the major cause of the death, and remains a significant public health problem. In this publication we are reporting our Latin American experience in the secondary prevention of SCD, by means of an ongoing registry involving seven Latin American countries and 770 patients.
Methods: Every individual within the present registry to date has presented with antecedents of aborted sudden death or cardiac arrest due to ventricular tachycardia or ventricular fibrillation. Patients included have fulfilled the Class I indication for implantable cardioverter defibrillator (ICD) and they were implanted with a Biotronik ICD (all models). The study was not sponsored by Biotronik, nor did they have access to the data. A specific protocol was designed for implantation and follow‐up of patients. The database was completely registered through the Internet and a personal password was assigned to each group of investigators. The primary end point was death from all causes. Secondary end points were SCD and death due to congestive heart failure (CHF).
Results: The etiology of cardiac disease was found to be predominantly coronary artery disease (CAD) 39.7% (306 patients), followed by Chagas disease (ChD), 26.1% (201 patients), and idiopathic dilated cardiomyopathy (DCM), 17% (131 patients). Any remaining pathologies were included as miscellaneous 13.2% (101 patients). In 31 patients (4%) the etiology was unknown. The age did not differ within the principal pathologies, but was significantly older than the miscellaneous group (62.0 ± 11.3 years vs 48.2 ± 18.9 years, P < 0.0001). The follow‐up period was 27 ± 25 months (1–113 months) for the whole group. The mortality in functional classes I–II was significantly lower than mortality for functional classes III–IV (relative risk 1.46, CI 95%, P < 0.0001). Mean left ventricular ejection fraction (LVEF) for the whole group was 37.7 ± 14.3%. Male LVEF was 36.1 ± 14.1% and female LVEF was 42.2 ± 13.8% P < 0.0001. During the follow‐up period, 130 deaths were reported (global mortality 16.9 ± 9.7%), out of which 84 (64.6%) were attributed to cardiac causes (10.9 ± 5.1% of the total population). The annual adjusted cardiac mortality was 5.2 ± 1.72% (range 3.5–7.0%). Among cardiac deaths the most common cause was progressive heart failure, 48 patients (57%) including 3 patients with pulmonary embolism. The second main cause of cardiac death was SCD, 36 patients (43%), including 4 patients with electrical storm and 3 patients with electromechanical dissociation after multiple shock therapy treatments.
Conclusions: Despite the differences in terms of pathologies between the ICD‐LABOR (Latin American bioelectronic ongoing registry) and randomized ICD trials, a parallel evolution in all cause mortality and cardiac mortality was observed. Independent risk factors for mortality included age >70 years, male gender, NYHA III/IV, and ejection fraction <0.30. The etiology of heart disease (Chagas vs Coronary Disease) was not found to be a risk factor.
Keywords: ICD, sudden death, Chagas disease, Latin American registry
Sudden cardiac death (SCD) is a major cause of death and most often is due to ventricular tachyarrhythmias. Three classic randomized clinical trials performed in developed countries have provided considerable evidence of increased survival in patients with resuscitated ventricular tachycardia or ventricular fibrillation, treated with an implantable cardioverter defibrillator (ICD). 1 , 2 , 3 However, these results should not be directly extrapolated to general practice in other populations of the world due to different local conditions and regional pathologies that could modify the results. 4 , 5 , 6 Based on this hypothesis, an assessment of ICDs performance in Latin America was developed. The ICD‐LABOR (Latin American bioelectronic ongoing registry) is a nonrandomized, prospective registry, based on observational data of which the main end point is total mortality. This ongoing registry began in January 1995 and includes data collected as of May 2004 for the purpose of this publication. A total of 770 patients from seven different countries (Argentina, Uruguay, Brazil, Mexico, Chile, Cuba, and Venezuela), 94 Medical Centers and 134 investigators have participated.
MATERIALS AND METHODS
All the individuals included in the present registry had presented with antecedents of aborted sudden death or cardiac arrest due to ventricular tachycardia/ventricular fibrillation and the ICD implant indication was considered as “secondary prevention.” Patients were eligible for enrollment to this registry if they fulfilled the Class I indication for ICD according to the “Consensus Statement on Indications, Guidelines for Use, and Recommendations for Follow‐up of Implantable Cardioverter‐Defibrillators” from NASPE 7 and were implanted with a Biotronik ICD (all models). The study was not sponsored by Biotronik, nor did they have access to the data.
A specific protocol was designed for all the Medical Centers involved in the registry and patients were informed about their inclusion. Every patient was required to complete information according to predetermined instructions for the implanting procedure and for the follow‐up. The database was completely registered through the Internet and a personal password was assigned to each group of investigators, so they could manage their own center information freely. The patients' follow‐up and new implantations could be registered instantaneously through the web. At least three times per year, routine follow‐up was required for the whole population. An evaluation committee controlled all of the information received from every implantation and follow‐up procedure for each patient. At the time of implantation, all of the patients were classified according to the NYHA functional classification.
The left ventricular ejection fraction (LVEF) at the time of implantation was determined by means of bidimensional echocardiography, scintigraphy, or invasive methods (hemodynamic study). Two kinds of models were implanted, a single‐ or dual‐chamber device.
To establish the shock energy to control ventricular arrhythmias, two different methods were used during the implantation procedures: (1) “true defibrillation threshold” performed by inducing multiple ventricular fibrillation episodes and testing different energy shock levels (biphasic shock) to rescue the heart rhythm and (2) predefined energy shock.
Cointervention
Since this is an ongoing registry, antiarrhythmic drugs and/or any other cardiac drug could be used in patients and were included as variables in the results.
Endpoints
The primary end point included death from all causes. Secondary end points were SCD and death due to congestive heart failure (CHF).
An Event Committee (five members) reviewed the information about deaths obtained from first‐hand reports of witnesses, hospital records, physician reports, or police reports. Information about the condition and activities of subjects immediately before their deaths was obtained by interviews with family members, private physicians, and from our own investigators. The Event Committee categorized the mode of death in each case by consensus or vote.
Definitions of fatal events: 8 All cause mortality included death from any cause. SCD was defined as a death within 1 hour after onset of acute symptoms. Unwitnessed death, which is unexpected and without other apparent cause, including death during sleep, was considered as SCD. Non‐SCD: included all cardiac deaths not classified as sudden deaths. Noncardiac death: was defined as all deaths not classified as cardiac deaths.
Statistical Methods
The cumulative mortality experience of the group was summarized as a survival curve, which was estimated using the Kaplan–Meier method. Statistical analysis of metric data was performed using the two‐sided unpaired t‐test or one‐way ANOVA test for more than two groups and a dichotomous proportion analysis was done using Fisher's test. Hazards regression model was used in multiple covariate analyses. A P value ≤0.05 was considered statistically significant.
RESULTS
Overall Results
The study population consisted of 770 patients (Table 1), of which only 761 patients (98.8%) completed the protocol as of the cutoff date for this publication. The whole group consisted of 581 males with an average age of 60.1 ± 13.9 years (range 6–88), median 62; and 189 females, 58.4 ± 13.9 years (range 15–85), median 61. The ages in both sexes were similar (P = 0.14). The etiology of cardiac disease was predominantly coronary artery disease (CAD) 39.7% (306 patients), followed by Chagas disease (ChD), 26.1% (201 patients), and dilated cardiomyopathy (DCM), 17% (131 patients). Any remaining pathologies were included as miscellaneous 13.2% (101 patients). In 31 patients (4%) the etiology was unknown. The age did not differ within the principal pathologies (CAD, ChD, DCM), but this group was significantly older than the miscellaneous group (62.0 ± 11.3 years vs 48.2 ± 18.9 years, P < 0.0001). The follow‐up period was 27 ± 25 months (1–113 months) for the whole group.
Table 1.
Patients Characteristics (n = 770 patients)
| Age (years, mean ± SD) | 60 ± 13 |
| Male sex (%) | 75 |
| Congestive heart failure (%) | |
| NYHA class I or II | 81 |
| NYHA class III or IV | 19 |
| LVEF (mean ± SD) | 37.7 ± 14.3 |
| Primary cardiac diagnosis (%) | |
| CAD | 39.7 |
| ChD | 26.1 |
| DCM | 17.0 |
| Unknown | 4.0 |
| Miscellaneous (%) | |
| Hypertrophic cardiomyopathy | 4.7 |
| Brugada syndrome | 2.1 |
| Valvular heart disease | 1.7 |
| Long QT syndrome | 1.7 |
| No heart disease | 1.7 |
| ARVD | 0.6 |
| Systemic hypertension | 0.5 |
| Congenital disease | 0.2 |
| Concomitant antiarrhythmic medications (%) | |
| Amiodarone | 56.8 |
| Amiodarone + Other antiarrhythmic drugs | 26.1 |
| Beta‐blockers | 9.6 |
| Other antiarrhythmic drugs | 1.3 |
| Without antiarrhythmic treatment | 6.2 |
| Unknown | 17.9 |
Functional Class
The population was divided into two groups: New York Classes I–II (81%) and New York Classes III–IV (19%). When the proportion of patients in Classes I–II versus III–IV were compared, women presented a greater proportion of patients in Classes I–II (P = 0.051).
The mortality in functional classes I–II was significantly lower than mortality for functional classes III–IV (CI 95%, P < 0.0001).
Ventricular Function
The mean LVEF for the whole group was 37.7 ± 14.3% (range 11–90%, median 35%). Male LVEF was 36.1 ± 14.1% (range 11–90%, median 35%) and female LVEF was 42.2 ± 13.8% (range 18–79%, median 40%) P < 0.0001. Within the different etiologies, LVEF was as follows: CAD 33.9 ± 11.4%, ChD 37.5 ± 11.2%, DCM 31.7 ± 11.1% and miscellaneous 53.6 ± 16.6%. The statistical relationship of LVEF values within the different pathologies are displayed in Table 2.
Table 2.
Relationship Between the EF in the Different Pathologies
| Pathology | Ejection Fraction | P Value |
|---|---|---|
| CAD vs Chagas | 33.9 ± 11.4% vs 37.5 ± 11.2% | 0.05 |
| CAD vs DCM | 33.9 ± 11.4% vs 31.7 ± 11.1% | NS |
| CAD vs Misc. | 33.9 ± 11.4% vs 53.6 ± 16.6% | 0.001 |
| Chagas vs DCM | 37.5 ± 11.2% vs 31.7 ± 11.1% | 0.001 |
| Chagas vs Misc. | 37.5 ± 11.2% vs 53.6 ± 16.6% | 0.001 |
| DCM vs Misc. | 31.7 ± 11.1% vs 53.6 ± 16.6% | 0.001 |
CAD = coronary artery disease; DCM = dilated cardiomyopathy; Misc. = miscellaneous.
Concomitant Medications
Concomitant antiarrhythmic drugs were indicated in 593 pts (93.8%). Amiodarone was the most common treatment, as single drug therapy (56.8%), or combined with beta‐blockers (22.5%) or other antiarrhythmic drugs (3.6%). Beta‐blockers alone were given to 9.6% of the patients. Other antiarrhythmic agents (mexiletine, verapamil etc.) were prescribed as a sole antiarrhythmic therapy in 1.3% of the population. In 6.2% of the patients, no drug therapy was prescribed, and in the end, antiarrhythmic treatment could not be confirmed in 138 pts (17.9%).
Cardioverter Defibrillator Models
Although the number of dual‐chamber ICDs implanted have been increasing progressively during the course of this registry, the single‐chamber ICD was the most common model utilized and had been implanted in 72.3% of all procedures (Fig. 1).
Figure 1.
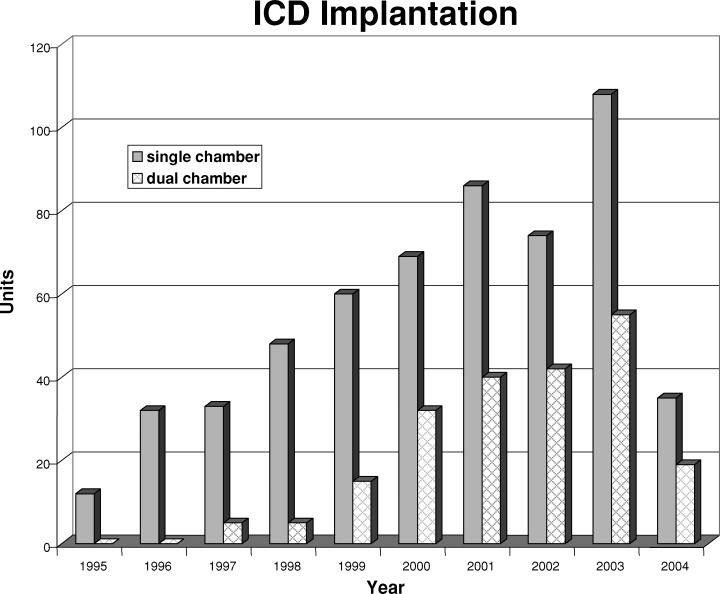
Distribution of single‐ and dual‐chamber ICD implants. The cutoff date corresponded to May 2004.
Defibrillation Threshold
During the implantation procedure, the “true defibrillation threshold” was measured by inducing multiple ventricular fibrillation episodes and testing different energy shock levels in 174 pts (22.6%): average 15.7 ± 6.7 J, (range 4.5–30 J). In the remaining 596 pts (77.4%), a predefined energy shock was used. In this group, the average energy shock level was 14.9 ± 3.9 J, (range 7–30 J). The comparison between both groups did not reach statistical significance (P = 0.08).
Mortality
During the follow‐up period (27 ± 25 months), 130 deaths were reported (global mortality 16.9 ± 9.7%), of which 84 (64.6%) were attributed to cardiac causes (10.9 ± 5.1% of the total population). The annual adjusted cardiac mortality was 5.2 ± 1.72% (range 3.5–7.0%).
In comparison with the survivors, those who died during the follow‐up were older, had lower EF, higher NYHA class, and were more likely to be males (Table 3).
Table 3.
Univariate Factors Related to Mortality
| Patients | Alive (n = 640) | Dead (n = 130) | P Value |
|---|---|---|---|
| LVEF (%) | 39 ± 14.6 | 31.6 ± 10.4 | 0.0001 |
| Age (years) | 58.9 ± 14.3 | 63.3 ± 10.7 | 0.0009 |
| Male sex (%) | 471 (73%) | 110 (85%) | 0.0048 |
| FC I–II/III–IV | 92%/73% | 8%/27% | 0.0001 |
| Follow‐up (months) | 28.6 ± 26.7 | 19.4 ± 19.3 | 0.0002 |
LVEF = left ventricular ejection fraction; FC = functional classes.
There were 36 noncardiac deaths. The most common cause was sepsis/infection (only one case related with the implantation procedure). There were 10 deaths not classifiable.
Cumulative Probability of Survival and Cause of Death
The Kaplan–Meier analysis of cumulative probability of survival was similar among the four clinical groups (Fig. 2).
Figure 2.
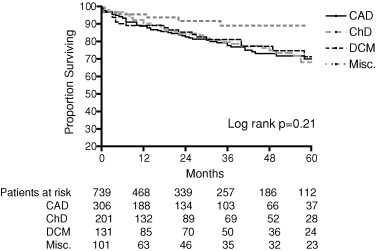
Kaplan–Meier analysis of cumulative probability of survival among different pathologies. CAD = coronary artery disease; ChD = Chagas disease; DCM = dilated cardiomyopathy; Misc. = Miscellaneous.
Among cardiac deaths the most common cause was progressive heart failure, 48 pts (57%) including 3 pts with acute right ventricular failure due to pulmonary embolism. The second main cause of cardiac death was SCD, 36 pts (43%), including 4 pts with electrical storm (in 1 patient the device was deactivated), and 3 pts with electromechanical dissociation after multiple shock therapy treatments (Table 4).
Table 4.
Outcome Event Rate Summary (n = 770 patients)
| Events | No. of Events | Rate |
|---|---|---|
| All‐cause mortality | 130 | 16.9% |
| Nonsudden cardiac death | 48 | 6.2% |
| Sudden cardiac death | 36 | 4.7% |
| Cause of noncardiac death | ||
| Sepsis/Infection | 13 | 1.8% |
| Pulmonary | 7 | 0.9% |
| Stroke/Embolism | 5 | 0.6% |
| Cancer | 4 | 0.5% |
| Others | 7 | 0.9% |
| Unknown | 10 | 1.3% |
Influence of Covariates on Survival
A Cox regression model was used to investigate the influence of covariates on total mortality (age, gender, LVEF, NYHA class, underlying disease, stimulation threshold, defibrillation threshold, antiarrhythmic treatment). Some of these variables failed to reach significance in a multivariate model. Nevertheless, four parameters were statistically significant as predictors of total and cardiac mortality: age, gender, LVEF, and NYHA class (Table 5, Fig. 3).
Table 5.
Age, Male Gender, NYHA Functional Class, and LVEF Were the Independent Risk Factors for Mortality
| Variable | Hazard Ratio | 95% CI | P Value |
|---|---|---|---|
| NYHA 3–4 | 2.9 | 1.8–4.7 | <0.001 |
| EF < 30% | 1.6 | 1.1–2.3 | <0.05 |
| Age >70 | 2.1 | 1.5–3.1 | <0.001 |
| Male sex | 2.1 | 1.3–3.5 | <0.01 |
| ChD | 1.2 | 0.8–1.8 | >0.05 |
| DCM | 0.9 | 0.5–1.4 | >0.05 |
| Misc. | 0.8 | 0.4–1.5 | >0.05 |
CAD = coronary artery disease; ChD = Chagas disease; DCM = dilated cardiomyopathy; Misc. = miscellaneous.
Figure 3.
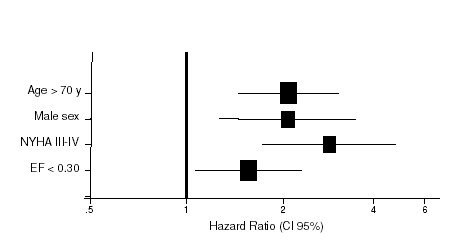
Hazard ratio (and 95% confidence limits) for predictors of total mortality.
DISCUSSION
Evidence‐based medicine has increased in importance during the last decade. Some randomized trials 1 , 2 , 3 have assisted in establishing new guidelines in the therapy of SCD and ICD indications. Despite its utility, 9 the improbability of applying their conclusions to other populations in the world is becoming more evident, especially when publications from different regions emphasize several clinical features being found in their ICD local populations distinct from those described in developed countries. 4 , 5 , 6
This has prompted the international medical community in recent years to develop local research investigations, most of which represent so‐called “experience‐based medicine.” This practice fortunately includes many parallels to “evidence‐based medicine,” particularly when large cohorts of individuals are recruited. To that end, the development of a registry for patients treated with ICD was undertaken in Latin America to offer a snapshot representing the diversity of this region. 10 Latin America is a large community with similar cultural habits, economic problems, scarce resources, and particular endemic pathologies, such as ChD. Within this region, an increasing number of new patients are receiving ICD therapy every year, which is creating new challenges related to indications, implantation procedures, and clinical follow‐up.
Some findings within the analysis of the ICD‐LABOR data must be emphasized, namely, the close correlation in terms of global mortality and cardiac mortality between the ICD‐LABOR and the most well‐known randomized trials. This association has occurred despite certain differences in the general features between this registry and the classic trials (Table 6), out of which the presence of a large number of patients with ChD and the markedly lower rate of CAD are the most relevant.
Table 6.
Comparison Between ICD‐LABOR and Major ICD Secondary Prevention Trials
| AVID | CIDS | CASH | ICD‐LABOR | |
|---|---|---|---|---|
| Patients with | N = 507 | N = 328 | n = 99 | n = 770 |
| ICD | ||||
| Age (years) | 65 ± 11 | 63 ± 9 | 58 ± 11 | 60 ± 13a |
| Male (%) | 79 | 85 | 79 | 75a |
| CAD (%) | 82 | 83 | 73 | 40a |
| Nonischemic | 15 | 10 | 11 | 43a |
| CM (%) | ||||
| LVEF (%) | 32 ± 13 | 34 ± 15 | 46 ± 19 | 38 ± 14a |
| Amiodarone (%) | 26 | 16 | 0 | 85a |
Nonischemic CM = nonischemic cardiomyopathy; AVID = antiarrhythmic versus implantable defibrillators; CIDS = Canadian implantable defibrillator study; CASH = cardiac arrest study Hamburg.
aStatistical significance between ICD‐LABOR and AVID, CIDS, and CASH trials.
In addition, a significantly high proportion of women was found in our population compared with those found in the classic trials and diverse publications. 11 , 12 This observation must be attributed to a large number of patients with ChD (26.1%), whereby the distribution of the infection was similar in both sexes. Yet another outstanding feature in comparing ICD‐LABOR to the classic trials is that in the AVID and CIDS trials, only a few patients without CAD or DCM were included in the protocol (3% and 7%, respectively), whereas, the ICD‐LABOR protocol included 13.6% of the patients in the miscellaneous group.
There are several possible explanations for these results. First and foremost, these studies were conducted at different times (AVID and CIDS in the 1990s), when Brugada syndrome, long QT syndrome and other entities were just starting to be considered for ICD implantation. And second, the Latin American region clearly has a different incidence of pathologies as compared to other developed countries, as has also been described by several local communities throughout the rest of the world. 4 , 5 , 6
Four independent predictors of risk of death were found in the present study: age, male sex, advanced NYHA functional class, and low LVEF. The strongest predictors of total mortality were those related to the severity of the underlying disease (LVEF and NYHA class). Among the mortality causes, CHF, as other authors have previously reported, appeared as the most common cause of death. 13 , 14 , 15 , 16 In elderly patients with low ejection fraction, a high proportion of deaths due to CHF were observed within the first year postimplantation. 17 These high‐risk patients must be under close clinical surveillance and medical treatment optimization. The indication of ICD plus resynchronization therapy in this population should now be considered. 18
Due to the small difference found between the true defibrillation threshold and an empiric value, we concluded that, in our experience, it is not necessary to induce multiple ventricular fibrillation episodes. We found that with modern devices and biphasic shocks, a successful test using 50% of the maximal energy should be considered safe enough for patients. Nevertheless, the concept of “upper limit of vulnerability,” 19 , 20 , 21 , 22 that is a shock strength above which ventricular fibrillation cannot be induced, was used in several patients in order to obtain a “true defibrillation threshold” without subjecting the patient to repeated episodes of circulatory arrest.
Limitations of the Study
A registry signifies the participation of a large group of patients, as well as a large number of medical centers, which often use different criteria and techniques. We cannot exclude a selection bias, taking into account that neither availability of the devices was homogeneous among the different countries, nor that ChD has similar distribution within the Latin American region.
CONCLUSION
Despite the differences in terms of pathologies between the ICD‐LABOR and the reported randomized trials, a parallel evolution of all cause mortality and cardiac mortality was observed. In the ICD‐LABOR study, a proportional regression model established the independent predictor value of risk of death as LVEF and NYHA class, as well as male gender and age >70 years old. CHF was the most common mode of death. According to our observations, in patients with antecedents of SCD with advanced age and severe ventricular dysfunction, the indication of an ICD with resynchronization capabilities should now be considered.
The development of modern technology related to devices and shock coils has also facilitated the ICD implantation procedure and in some respect has minimized the need to determine certain values, such as the true defibrillation threshold. This, in turn, allows for a more focused approach.
Abud Marcelo: Sanatorio San Gerónimo, Santa Fé, Argentina; Aguinaga Luis: Centro Privado de Cardiología, Tucumán, Argentina; Alba Ricardo: IMECC, Capital, Argentina; Alimenti Hugo: Hospital Italiano, Bahía Blanca, Argentina; Amezaga Bingen, Manuel Patete Ayala: Hospital Miguel Pérez Carreño, Caracas, Venezuela; Arabia Luis: Clínica del Salvador, Córdoba, Argentina; Arregui Víctor: Hospital San Juan de Dios, Buenos Aires, Argentina; Asenjo René: Hospital Clínico, Universidad de Chile, Santiago, Chile; Avila Esteban: ITEC, Tucumán, Argentina; Azara Daniel, Ruffa Horacio, Roquinotti Mónica, Bolaños Alberto: Hospital Militar, Capital, Argentina; Balado Roberto: Clínica María Auxiliadora, Olavarría, Argentina; Bassani Carlos: Sanatorio Romagosa, Córdoba, Argentina; Berenstein César: Hospital Regional de Ushuaia, Ushuaia, Argentina; Boccardo Daniel, Coll Marcelo, Tibaldi Miguel A: Instituto Modelo, Córdoba, Argentina; Buenfil Medina José Carlos: Hospital Naval, DF, México; Caccavo Alberto: Clínica Coronel Suárez, Coronel Suárez, Argentina; Caeiro Andrés: Hospital Privado, Córdoba, Argentina; Cardona Marcelo: Hospital Español, Rosario, Argentina; Castellanos Ramiro, González Sergio: Instituto de Cardiología, Tucumán, Argentina; Castoldi Florencio: Instituto del Diagnóstico, Santa Fe, Argentina; Chambó Marcelo: CEMICO, Neuquén, Argentina; Chavez Carlos: Clínica del Comahue, Buenos Aires, Argentina; Cipolleti Luis: Sanatorio Pasteur, Catamarca, Argentina; Cohn José Luis: S. Sta Rosa de Lima, Capital, Argentina; Conejeros Kindel Carlos: Hospital Dr. Barros Luco, Santiago, Chile; Danoviz Julio: Sanatorio Boratti, Posadas, Argentina; De Dios Fernando: Policlínico del Docente, Capital, Argentina; De la Fuente Roberto: Sanatorio Evangelista, Montevideo, Uruguay; de Zuloaga Claudio, Pérez Mayo Osvaldo: Hospital Posadas, Buenos Aires, Argentina; Defeo Magdalena: Hospital Rossi, La Plata, Argentina; Del Río Alfredo: Sanatorio Parque, Rosario, Argentina; Demozzi Angel: Hospital Aeronáutico, Capital, Argentina; Dorticós Francisco: Instituto de Cardiología, La Habana, Cuba; Dubner Sergio: Sanatorio Suizo, Capital, Argentina; Dussaut Eduardo: Clínica San Carlos, Escobar, Argentina; Elencwajg Benjamín: Hospital Eva Perón, Buenos Aires, Argentina; Esteban Alejandro, Constantini Sonia: Instituto del Corazón, General Roca, Argentina; Estebanez María José: Hospital Privado del Sur, Bahía Blanca, Argentina; Estepo José, Cáceres Monié César: Hospital Británico, Capital, Argentina; Fernández Eduardo, Di Tomaso Fernando: Hospital Rivadavia, Capital Federal, Argentina; Fernández Gonzalo: Hospital Militar, Centro Cardiovascular, Montevideo, Uruguay; Freire Diego, Lujambio Mariela, Rivara Alvaro: Hospital de Clínicas, Montevideo, Uruguay; Fuenmayor Arocha Abdel: Clínica Albarregas, Mérida, Venezuela; Fuganti Claudio: Irmandade Santa Casa de Londrina, Londrina, Brazil; Galizio Néstor, González José L: Fundación Favaloro, Capital, Argentina; Galvao Silas: Sociedad de Beneficencia Portuguesa, Sao Paulo, Brazil; Garillo Raúl: Universidad del Salvador, Capital, Argentina; Garro Hugo, Pastori Julio: Hospital Ramos Mejía, Capital, Argentina; Gil Silvina: Sanatorio Mayo, Córdoba, Argentina; González Zuelgaray Jorge, Scazzuso Fernando, Goyeneche R: Hospital Argerich, Capital, Argentina; Greco Oswaldo Tadeu: Instituto de Molestias Cardiovasculares, Sao José do Rio Preto, Brazil; Guillén Horacio: Clínica San Nicolás, Rosario, Argentina; Helguera Marcelo, De Elizalde Guillermo, Muratore Claudio: Hospital Italiano, Capital, Argentina; Kogan César: Sanatorio de la Esperanza, Capital, Argentina; Labadet Carlos: Institutos Antártida, Capital, Argentina; Lamarca Silvia: Sacre Coeur, Capital, Argentina; Lanzotti Marcelo, Norberto Cittá: Hospital Británico, Rosario, Argentina; Ledesma Raúl: Hospital de Clínicas, Córdoba, Argentina; Lucchese Fernando: Santa Casa, Porto Alegre, Brazil; Martellotto Ricardo, Velarde Mariscal José L: Hospital Italiano, Córdoba, Argentina; Martínez Marcelo: Hospital San Roque, Córdoba, Argentina; Montenegro José L, Vidal Luis, Vanerio Gabriel, Fernández Pablo: CASMU, Montevideo, Uruguay; Oseroff Oscar, Retyk Enrique, Suárez Jorge: Hospital Castex, Buenos Aires, Argentina; Pachón M José C, Pachón M Juan C, Pachón M Enrique, Albornoz Remy: Instituto Dante Pazzanese, Sao Paulo, Brazil; Panini Julio, Bruno Martin: Hospital de San Isidro, San Isidro, Argentina; Pardo Gutiérrez José: Hospítal Militar, Santiago, Chile; Parra Pavich Miguel Angel: Instituto Cardiovascular del Chaco, Resistencia, Argentina; Pellinzón Oscar: Univ Nac Rosario, Rosario, Argentina; Pérez América: Sanatorio Mendez, Capital, Argentina; Pesce Ricardo, Valero Elina: Fleni, Capital, Argentina; Poliserpi Claudio: Hospital Policial Churruca, Capital, Argentina; Pozzer Luis, Reyes Ignacio: Instituto de Cardiología de Corrientes, Corrientes, Argentina; Pugliese Eduardo: Clínica El Rosario, Jujuy, Argentina; Rabinovich Rafael: Hospital Israelita, Capital Federal, Argentina; Ramos José Luis, Sanziani Laura: Hospital Italiano, Rosario, Argentina; Repetto Horacio: Hospital de Clínicas, Capital, Argentina; Reyes Oscar: Clínica Modelo, Paraná, Argentina; Reyes Walter, Calleriza Fernando: Casa de Galicia, Montevideo, Uruguay; Rivero Paz Roberto: Sanatorio Quintar, Jujuy, Argentina; Romero Horacio: Hospital Privado Regional, Río Negro, Argentina; Sánchez Jorge: Sanatorio El Carmen, Salta, Argentina; Sánchez Osvaldo: Hospital Regional, San Juan, Argentina; Sansalone Rodolfo: Sanatorio Güemes, Capital Federal, Argentina; Sendra Vicente: Hospital Italiano, Mendoza, Argentina; Senesi Máximo, Cueto Alejandro: Hospital Durand, Capital, Argentina; Seoane Claudio: Sanatorio Colegiales, Capital, Argentina; Serra José Luis: Sanatorio Allende, Córdoba, Argentina; Sgarlatta Horacio, Martínez Darío, Sgarlatta Héctor: Centro Privado Vélez Sarsfield, Córdoba, Argentina; Sirena Juan José: Instituto de Cardiología, Santiago del Estero, Argentina; Solá Miguel: Instituto de Cardiología, Salta, Argentina; Tentori Cristina, Mazzetti Héctor, Dasso Daniel, Mascheroni Osvaldo: Hospital Fernández, Capital, Argentina; Toledo Mónica: Hospital Italiano, Santa Fe, Argentina; Treggia Alberto: Sanatorio Plaza, Rosario, Argentina; Valentino Mariana: Hospital Héroes de Malvinas, Merlo, Argentina; Ventura Alejandro: Cordis, Resistencia, Argentina; Vieyra Gustavo: Hospital Vicente López, Buenos Aires, Argentina; Villamil Alejandro: Hospital Santojanni, Capital, Argentina; Vital Martínez: Hospital Naval, Bahía Blanca, Argentina; Yanguas Marcelo: Clínica Bernal, Bernal, Argentina; Ylarri Ernesto: Hospital Héctor Cura, Olavarría, Argentina.
REFERENCES
- 1. Kuck KH, Cappato R, Siebels J, et al Randomized comparison of antiarrhythmic drug therapy with implantable defibrillators in patients resuscitated from cardiac arrest. The cardiac arrest study Hamburg (CASH). Circulation 2000;102: 748–754. [DOI] [PubMed] [Google Scholar]
- 2. Connolly S, Gent M, Roberts R, et al Canadian implantable defibrillator study (CIDS). A randomized trial of the implantable cardioverter defibrillator against amiodarone. Circulation 2000;101: 1297–1302. [DOI] [PubMed] [Google Scholar]
- 3. The Antiarrhythmic versus Implantable Defibrillators (AVID) Investigators . A comparison of antiarrhythmic‐drug therapy with implantable defibrillators in patients resuscitated from near‐fatal ventricular arrhythmias. N Engl J Med 1997;337: 1576–1583. [DOI] [PubMed] [Google Scholar]
- 4. Tsai Ch, Huang SK, Lin JL, et al Distinct clinical features in the recipients of the implantable cardioverter defibrillator in Taiwan: A multicenter registry study. Pacing Clin Electrophysiol 2003;26: 2083–2090. [DOI] [PubMed] [Google Scholar]
- 5. Behdin K. Survey of implantable cardioverter‐defibrillator in Iran 2000–2002. (Abstract) Pacing Clin Electrophysiol 2003;26(II):S20. [Google Scholar]
- 6. Cho JG, Park HW, Rhew JY, et al Clinical characteristics of unexplained sudden cardiac death in Korea. Jpn Circ J 2001;65: 18–22. [DOI] [PubMed] [Google Scholar]
- 7. Winters SL, Packer DL, Marchlinski F, et al Consensus statement on indications, guidelines for use, and recommendations for follow‐up of implantable cardioverter defibrillators. Pacing Clin Electrophysiol 2001;24: 262–266. [DOI] [PubMed] [Google Scholar]
- 8. Kim SG, Fogoros RN, Furman S, et al Standardized reporting of ICD patient outcome: The report of a North American Society of Pacing and Electrophysiology policy Conference, February 9–10, 1993. Pacing Clin Electrophysiol 1993;16(Part I):1358–1362. [DOI] [PubMed] [Google Scholar]
- 9. Ezekowitz JA, Armstrong PW, McAlister FA. Implantable cardioverter defibrillators in primary and secondary prevention: A systematic review of randomized controlled trials. Ann Intern Med 2003;138: 445–452. [DOI] [PubMed] [Google Scholar]
- 10. Garillo R, Pachon M JC, Dubner S, et al Analysis of mortality in patients treated with implantable cardioverter‐defibrillator. A Latin‐American cooperative registry: The ICD‐LABOR. (Abstract) Pacing Clin Electrophysiol 2003;26(II):S42. [Google Scholar]
- 11. Horton HL, Marinchack RA, Rials SJ, et al Gender differences in device therapy for malignant ventricular arrhythmias. (Abstract) Arch Intern Med 1995;155(21):2342. [PubMed] [Google Scholar]
- 12. Albert ChM, Chae CU, Grodstein F, et al Prospective study of sudden cardiac death among women in the United States. (Abstract) Circulation 2003;107(16):2096. [DOI] [PubMed] [Google Scholar]
- 13. Sheldon R, Connolly S, Krahn A, et al Identification of patients most likely to benefit from implantable cardioverter defibrillator therapy. Circulation 2000;101: 1660–1664. [DOI] [PubMed] [Google Scholar]
- 14. Domanski MJ, Sakseena S, Epstein AE, et al Relative effectiveness of the implantable cardioverter defibrillator and antiarrhythmic drugs in patients with varying degrees of left ventricular dysfunction who have survived malignant ventricular arrhythmias. J Am Coll Cardiol 1999;34: 1090–1095. [DOI] [PubMed] [Google Scholar]
- 15. Moss AJ. Implantable cardioverter defibrillator therapy. The sickest patients benefit the most. Circulation 2000;101: 1638–1640. [DOI] [PubMed] [Google Scholar]
- 16. Stevenson WG, Stevenson LW, Middlekauff HR, et al Sudden death prevention in patients with advanced ventricular dysfunction. Circulation 1993;88: 2953–2961. [DOI] [PubMed] [Google Scholar]
- 17. Oseroff O, Rabinovich R, Garillo R, et al Congestive heart failure as the early death cause in elderly patients with implantable cardioverter defibrillator. (Abstract) Europace 2002;3(Suppl. A):A24. [Google Scholar]
- 18. Bristow MR, Saxon LA, Boehmer J, et al Cardiac‐resynchronization therapy with or without an implantable defibrillator in advance chronic heart failure. N Engl J Med 2004;350: 2140–2150. [DOI] [PubMed] [Google Scholar]
- 19. Glickson M, Friedman P. Routine arrhythmia inductions for ICD: Are they obsolete? Pacing Clin Electrophysiol 2001;24: 915–919. [DOI] [PubMed] [Google Scholar]
- 20. Dillon SM, Kwaku KF. Progressive depolarization: A unified hypothesis for defibrillation and fibrillation induction by shocks. J Cardiovasc Electrophysiol 1998;9: 529–552. [DOI] [PubMed] [Google Scholar]
- 21. Hwang C, Swerdlow ChD, Kass RM, et al Upper limit of vulnerability reliably predicts the defibrillation threshold in humans. Circulation 1994;90: 2308–2314. [DOI] [PubMed] [Google Scholar]
- 22. Peng‐Shen C, Swerdlow ChD, Hwang Ch. Current concepts of ventricular defibrillation. J Cardiovasc Electrophysiol 1998;9: 553–562. [DOI] [PubMed] [Google Scholar]


