Abstract
Background: Myocardial ischemia during coronary spasm may generate malignant ventricular arrhythmias. The J‐wave pattern was suggested to be a marker of a disorder associated with life‐threatening arrhythmias. Results: We report the case of a patient with vasospastic angina and J‐wave pattern in inferior and lateral leads associated with polymorphic ventricular tachycardia which was effectively treated only with quinidine—vasodilating drugs were not able to prevent the arrhythmia although they were effective in preventing ischemic events. Conclusion: The J‐wave pattern in inferolateral leads may be a sign of electrical vulnerability to lethal ventricular arrhythmia in patients suffering from vasospastic angina—quinidine can effectively prevent such arrhythmias in these patients.
Keywords: J wave, Prinzmetal angina, vasospastic angina, polymorphic ventricular tachycardia, sudden cardiac death, quinidine
Myocardial ischemia during coronary spasm may generate the malignant ventricular arrhythmia which increases risk of sudden cardiac death. 1 The J‐wave pattern in inferior and lateral leads was suggested to be a marker of a disorder associated with life‐threatening arrhythmias. 2 We report the case of a patient with vasospastic angina and J‐wave pattern associated with polymorphic ventricular tachycardia (PVT) which was effectively treated only with quinidine.
CASE REPORT
A 53‐year‐old man with a 2‐year history of syncope and angina was admitted for elective cardiac evaluation. Some of the syncope episodes were preceded with angina and two of them resulted in serious head injuries. Angina usually appeared at rest and occasionally during exertion and resolved spontaneously or after taking nitroglycerin. Previously, two coronary angiographies revealed no significant coronary stenosis. On admission, results of physical examination, an echocardiogram and hematological and blood chemistry analyses were all normal, a resting electrocardiogram (ECG) showed the early repolarization pattern with J waves in inferior and lateral leads (Fig. 1A). However, during an exercise test the J waves became more prominent and the upsloping ST‐segment depressions appeared in precordial leads and then the PVT occurred which was followed by the second‐degree atrioventricular block and ST‐segment elevations in inferolateral leads (Figs. 1B–D)—the patient reported a chest pain. The immediate coronary angiography showed no significant organic stenosis but global spasm of a dominant left coronary artery which resolved after nitroglycerin intracoronary administration.
Figure 1.
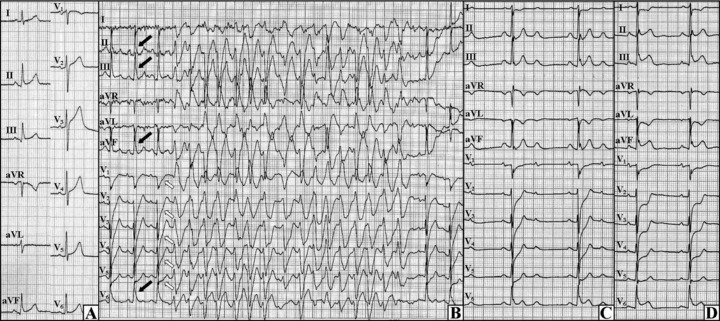
Initial ECG and the first exercise test. (A) The resting ECG exhibits J waves in inferior and lateral leads. (B) The first exercise test, the J wave increased in leads II, III, aVF, and V6 (black arrows) and the upsloping ST‐segment depressions emerged in V1–V5 (open arrows) and then the polymorphic ventricular tachycardia occurred. (C) The atrioventricular block (2:1) appeared at the end of the test. (D): The ST‐segment elevations in inferolateral leads with the reciprocal ST‐segment depressions in anterior leads were observed in the recovery phase.
Vasospastic angina was diagnosed and the treatment with calcium channel blocker (slow‐release 240 mg verapamil o.d.) and nitrates (100 mg isosorbide mononitrate and 8 mg molsidomine) was implemented. Due to the risk of sudden cardiac death (i.e., history of syncope and confirmed PVT) an implantable cardioverter‐defibrillator (ICD) was implanted. Six days later, a Holter monitoring revealed episodes of atrial fibrillation and flutter and asymptomatic incidents of ST‐segment elevations in inferior leads occasionally triggering short series of PVT. Treatment with amiodarone was initiated and the dose of verapamil was augmented to 240 mg b.d. After the next 12 days, during the second exercise test the patient did not report angina, however the upslopping ST‐segment depressions appeared in precordial leads and the J wave increased in lead V6 followed by PVT—the arrhythmia was ceased by ICD shock and then the J waves emerged in leads II, III, and aVF (Figs. 2A and B). Considering the above facts, amiodarone was recognized as ineffective and replaced with Cordihin 1 tablet t.i.d. (a 160 mg quinidine and 80 mg verapamil combination). In the following days, the episodes of ST‐segment elevation without ventricular arrhythmias were observed (Figs. 2C and D). After the intensification of vasodilating treatment with verapamil up to 320 mg t.i.d., no other ischemic events happened.
Figure 2.
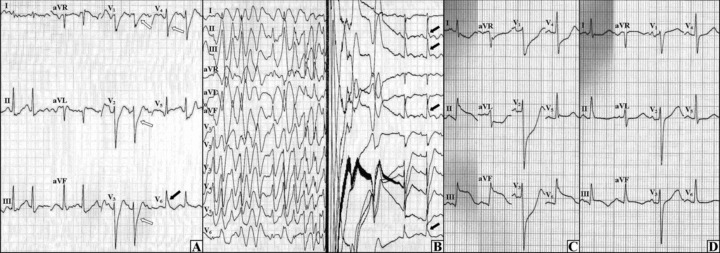
The second exercise test and the resting ECGs on the other day. (A) During the exercise test, the J wave was initially seen in lead V6 (black arrow) with the upsloping ST‐segment depressions in V1–V4 (open arrows). (B) As the exercise test progressed, the polymorphic ventricular tachycardia occurred which was ceased by ICD shock—after the shock the J waves were observed in leads II, III, aVF, and V6 (black arrows). (C) The ECG during a resting chest pain, ST‐segment elevations in inferolateral leads with reciprocal changes in anterior leads can be seen. (D) Immediately after the nitroglycerin administration, the ST‐segment elevations (from panel C) and the chest pain disappeared.
Seven days later during the third exercise test neither arrhythmia nor angina appeared, nevertheless the J waves in inferolater leads and upsloping ST‐segment depressions in anterior leads were observed (Fig. 3A). After 7 minutes of the exercise, the patient's blood pressure collapsed and the cardiac arrest occurred due to pulseless electrical activity (Fig. 3B)—cardiopulmonary resuscitation and administration of adrenaline and atropine restored the circulation. The cause of this electromechanical dissociation was ascribed to the cardiodepressive effects of high doses of verapamil—the drug was replaced with 20 mg nitrendipine o.d. and Cordihin was reduced to 1 tablet b.d. In other days, the patient was stable without any arrhythmic and ischemic events. The fourth exercise test (13 days after resuscitation) revealed neither arrhythmic nor ischemic abnormalities—the J wave was not observed either (Fig. 3C). The treatment was recognized as an effective one and the patient was discharged home. After 2 months he was admitted again for an inadequate ICD therapy triggered by the atrial flutter with fast ventricular rate. Thanks to successful ablation, the atrial flutter was eliminated and no other events occurred during the next 4 months.
Figure 3.
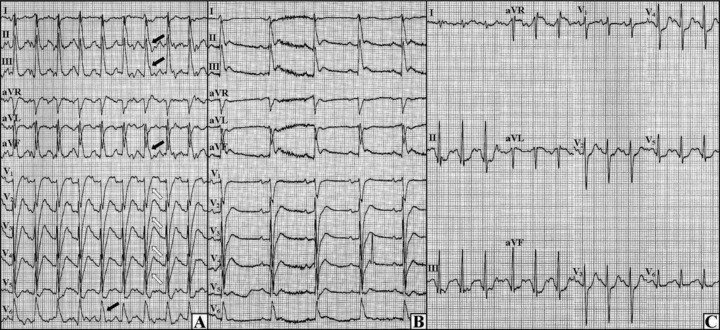
The third and fourth exercise tests. (A) Final phase of the third test, the J waves in inferolateral leads (black arrows) with upsloping ST‐segment depressions in anterior leads (open arrows) can be seen. (B) The third test, the ECG during pulseless electrical activity is shown. (C) Final phase of the fourth test, no J wave can be observed.
DISCUSSION
In the present case, the vasospastic angina with J‐wave pattern on ECG was associated with episodes of PVT. Most of the serious arrhythmic events appeared during exertion and they were preceded by the accentuation of J wave in inferior and lateral leads and upslopping ST‐segment depressions in anterior leads. High doses of verapamil and nitrates were not able to prevent PVT although they were effective in preventing the ischemic events, only quinidine could effectively treat ventricular arrhythmia in this patient.
It has been suggested that the J‐wave pattern recorded in inferolateral leads may be a sign of underlying electrical vulnerability that increases the risk of lethal ventricular arrhythmias under favorable pathological conditions. 2 The acute myocardial ischemia is the condition which can induce prominent J waves and fatal ventricular arrhythmias in vulnerable patients. 3 However, the myocardial ischemia was not the only cause of PVT in our case because antianginal agents were ineffective in preventing the arrhythmia and on the other hand a severe ischemia during the cardiac arrest did not provoke PVT when the patient was treated with quinidine (Fig. 3B). Each PVT episode was preceded by the J‐wave augmentation and only quinidine was able to eliminate the arrhythmia.
Experimental studies showed that the J wave on ECG results from transmural differences in the early repolarization phase of the action potential, i.e., from a prominent Ito‐mediated action potential notch in ventricular epicardium. 4 , 5 An outward shift in repolarizing current due to a decrease in sodium or calcium channel currents or an increase in Ito or other outward currents lead to the J‐wave augmentation or appearance of ST‐segment elevation and causes a dispersion of repolarization within myocardium—this creates favorable circumstances for the phase 2 reentrant extrasystoles and PVT. 6 , 7 Quinidine which inhibits Ito, reduces the magnitude of the J wave and normalizes ST‐segment elevation in patients with J‐wave pattern. 8 , 9 This drug was very effective in preventing PVT in our case, moreover it eliminated the J wave during the last exercise test (Fig. 3C). However, it is also possible that the verapamil dose reduction contributed to the J‐wave elimination in the last test—there is some evidence that the depression of inward calcium channel current may lead to the J‐wave augmentation. 6 Thus, nondihydropiridine calcium channel blockers (i.e., verapamil and diltiazem) should be probably omitted in patients with J‐wave pattern associated with malignant ventricular arrhythmia. Nevertheless, further studies are needed to identify factors that modulate arrhythmogenicity in patients with J‐wave pattern.
In the present case, we found some more peculiarities. First, contrary to the experimental bases and clinical observations of other early repolarization cases, 7 , 10 the J wave was increasing during an exercise. Second, the ST‐segment changes in precordial leads (i.e., upsloping ST‐segment depressions) were even more pronounced than J‐wave augmentation just before VT occurrences (Figs. 1B and 2A). These changes in anterior leads may reflect ischemic changes in myocardium, however it is also possible that they represent the mirror image of J waves coming from the posterior region—see Figure 4.
Figure 4.
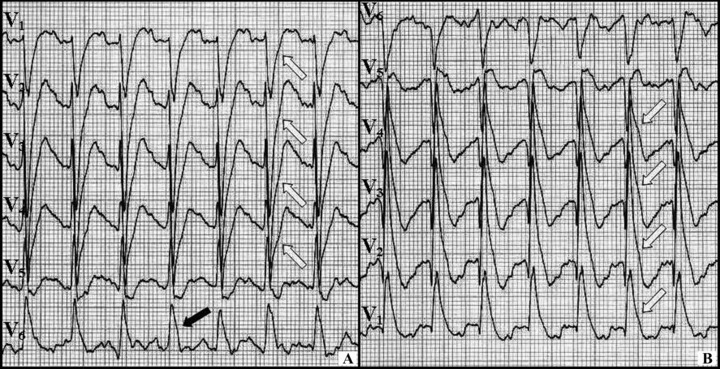
The ECG from the third exercise test. (A) The upsloping ST‐segment depressions in V1–V4 (open arrows) accompanying the J wave in V6 (black arrow) can be seen. (B) After reversing the ECG, the J‐wave pattern can be observed in V1–V4 (open arrows)—thus the changes in the precordial leads may be the mirror image of J waves coming from the posterior region (similarly to the ST‐segment depressions in anterior leads during the posterior wall myocardial infarction).
In conclusion: (1) J‐wave pattern in inferolateral leads may be a sign of electrical vulnerability to lethal ventricular arrhythmia in patients suffering from vasospastic angina, (2) quinidine can effectively prevent PVT in such patients, (3) nondihydropiridine calcium channel blockers should be used with caution in such instances, and (4) upsloping ST‐segment depressions in anterior leads may represent reciprocal J waves coming from the posterior region (i.e., the mirror image of J waves).
REFERENCES
- 1. Myerburg RJ, Kessler KM, Mallon SM, et al Life‐threatening ventricular arrhythmias in patients with silent myocardial ischemia due to coronary‐artery spasm. N Engl J Med 1992;326:1451–1455. [DOI] [PubMed] [Google Scholar]
- 2. Haissaguerre M, Derval N, Sacher F, et al Sudden cardiac death associated with early repolarization. N Engl J Med 2008;358:2016–2023. [DOI] [PubMed] [Google Scholar]
- 3. Jastrzebski M, Kukla P. Ischemic J wave: Novel risk marker for ventricular fibrillation? Heart Rhythm 2009;6:829–835. [DOI] [PubMed] [Google Scholar]
- 4. Litovsky SH, Antzelevitch C. Transient outward current prominent in canine ventricular epicardium but not endocardium. Circ Res 1988;62:116–126. [DOI] [PubMed] [Google Scholar]
- 5. Antzelevitch C, Sicouri S, Litovsky SH, et al Heterogeneity within the ventricular wall. Electrophysiology and pharmacology of epicardial, endocardial, and M cells. Circ Res 1991;69:1427–1449. [DOI] [PubMed] [Google Scholar]
- 6. Fish JM, Antzelevitch C. Role of sodium and calcium channel block in unmasking the Brugada syndrome. Heart Rhythm 2004;1:210–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Antzelevitch C, Yan GX. J wave syndromes. Heart Rhythm 2010;7:549–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yan GX, Antzelevitch C. Cellular basis for the Brugada syndrome and other mechanisms of arrhythmogenesis associated with ST‐segment elevation. Circulation 1999;100:1660–1666. [DOI] [PubMed] [Google Scholar]
- 9. Gussak I, Antzelevitch C, Bjerregaard P, et al The Brugada syndrome: Clinical, electrophysiologic and genetic aspects. J Am Coll Cardiol 1999;33:5–15. [DOI] [PubMed] [Google Scholar]
- 10. Antzelevitch C, Yan GX. Cellular and ionic mechanisms responsible for the Brugada syndrome. J Electrocardiol 2000;33(Suppl):33–39. [DOI] [PubMed] [Google Scholar]


