Abstract
Background: The effects of active and passive mental stress (PMS) on the QT interval were studied by using an intraindividual regression method of QT‐interval correction for heart rate.
Methods: Thirty healthy males (age 21.2 ± 1.8 years) performed a mental arithmetic for 1 minute, which was considered as active mental stress (AMS) because of the performance requirement. A 1‐minute unpleasant video clip was used for PMS. Two baseline and two (an early and a late) ECGs were prepared in both mental stress periods. The individual QT–RR relationship was assessed by linear regression analysis of 7–15 (11.0 ± 1.9) controlled QT–RR data pairs, also obtained from ECGs gained during a successive set of 9 isometric stretching exercises.
Results: Heart rate has increased significantly at both measurements in response to AMS (P < 0.0001), but not in response to passive stress. QTc significantly prolonged early in AMS (P = 0.0004), then normalized by the end of the period. During PMS, no significant QTc changes were observed. The evolution of bifid T waves was noted in 14 subjects: 8 presented bifid T waves during both AMS and exercise, and 6 during only exercise.
Conclusions: AMS and PMS elicit different cardiovascular reactions. Our results indicate that changes in the autonomic tone, probably abrupt sympathetic predominance, may cause QTc prolongation and bifid T waves. This suggests that besides stress quality and intensity, the dynamics of stress application and perception also influence repolarization.
Keywords: autonomic modulation, mental stress, sympathetic activity, QT interval, T wave
The QT interval reflects depolarization and repolarization of the myocardium. The heart rate, and particularly, the duration of the cardiac cycle are the primary sources of the changes in QT interval. The autonomic nervous system, which can act directly at the cellular level or indirectly through modulation of heart rate, is another important source of QT changes. 1 The role of the autonomic nervous system was demonstrated by the prolongation of the QT interval during sleep, independent of heart rate. This observation was attributed to circadian changes in sympathovagal balance. 2 The QT interval is prolonged in patients with diabetic autonomic neuropathy 3 and in patients with familial dysautonomia. 4 Similarly, QT interval is increased in patients with primary autonomic failure due to pure autonomic failure or multiple system atrophy. 5
Both chronic and acute mental stresses induce cardiovascular and neuroendocrine responses. Stress‐induced autonomic nervous system activation might also trigger lethal arrhythmias through alterations of the neural transmissions to the heart. 6 Epidemiologic evidence suggests that there is a relationship between stress and cardiac morbidity and mortality in susceptible individuals. 7
The prolonged QT interval is regarded as a marker of imbalanced distribution of sympathetic nervous system activity on the heart; moreover, QT‐interval prolongation has been associated with a lowered ventricular fibrillation threshold and with the occurrence of sudden cardiac death. 8 However, the effect of psychological stress on the QT interval is subject to speculation. Previous published reports provided conflicting data on the effect of mental stress on the QT‐interval duration. For example, it was observed that the QT interval of physicians prolonged when they got alarm calls. 9 Also, being awakened with bad news in the night was associated with marked QT prolongation. 10 Conversely, laboratory‐based studies reported QT‐interval shortening as an effect of mental stress. 11 , 12 , 13 , 14 , 16 Huang et al. 11 reported that during “stressful interviews” the QT intervals shortened. Similarly, QT shortening was observed by Insulander et al. 12 during Stroop color‐word test and by Haapalahti et al. 13 during mental arithmetic. In these three studies, QT intervals were not corrected for heart rate, so almost certainly the physiologic decrease of QT time was related to increases in heart rate. In addition, Paavonen et al. 14 measured heart rate and QT‐interval duration at peak heart rate during a combination of Stroop color‐word test and mental arithmetic, and reported QTc‐interval shortening. However, they used the Bazett and Fridericia methods to correct QT intervals for heart rate, but this approach has been severely criticized. 15 It has been shown that no fixed correction formula is suitable to compare QT intervals at different heart rates because the QT–RR relationship exhibits a substantial intersubject variability. Consequently, individualized correction should always be considered when comparison of the QT interval at the same heart rate is not possible. 15 Finally, in one published study, it was attempted to overcome the shortcomings inherent in the use of fixed correction formulae. Hedman et al. 16 applied fixed rate ventricular pacing on 10 subjects with high‐degree atrioventricular block. Stroop color‐word task was presented, while the ventricular rate was kept constant by ventricular pacing. QT measurements were performed at “peak stress” defined as the maximal atrial rate. With this method a minor QT‐interval shortening was detected. But, since it has been shown that asynchronous VVI pacing triggers both sympathetic overactivity and vagal withdrawal, 17 the net effect of mental stress on the QT interval remained still unclear.
One major problem in this area of research is the use of inconsistent test protocols to induce mental stress. Different methods have previously been used, with studies assuming that the mental stress response is generic. Conversely, it has been shown that different psychological stressors produce specific cardiovascular responses. 18 In addition, it has been demonstrated that conditions of active mental stress (AMS) and passive mental stress (PMS) promote different levels of sympathetic and parasympathetic stimulation to the heart, thereby producing different heart rate and blood pressure responses. 19 Despite these known differences in the cardiovascular response to these two forms of mental stress, to date, no attempt was made to separately assess the QT responses to AMS and PMS. Therefore, to date it is unknown how the QT interval is affected by these two forms of mental stress.
The purpose of this research was to examine electrocardiographic QT interval in response to AMS and PMS in a laboratory setting using a within‐subjects design. To overcome past controversy about the use of fixed equations for correcting the QT interval for heart rate, we have adopted a recently recommended and more valid individualized QT correction method. 15
METHODS
Participants and Materials
Thirty nonsmoking male university students (mean age 21.2 ± 1.8 years), with no history of cardiac disease and normal resting 12‐lead ECGs, volunteered for the study. Participants were instructed not to consume any alcohol or caffeine, or to engage in strenuous physical activities for 12 hours prior to testing. According to self‐reports, no drugs or medication were taken by any the participants for at least 2 weeks before the study. The research was approved by an internal research ethics committee at Nottingham Trent University in the United Kingdom. All participants signed a written consent form.
The ECGs were recorded and printed at a paper speed of 25 mm/s and amplifier gain of 10 mm/mV on a Cardiomax FX‐3010 Electrocardiograph (Fukuda Denshi Co. Ltd., Tokyo, Japan) using Nutrode‐P20M0 pregelled ECG electrodes (GE Medical Systems Co. Inc., Waukesha, WI). Trauma depicting photographs from various media resources were edited into a 1‐minute video clip at Nottingham Trent University Audiovisual Suite. This video clip, consisting of 16 rotating shocking images accompanied by a soundtrack of loud weather noises, was used as the passive mental stressor (PMS). On the basis of the two pilot studies, it was confirmed that the video clip was shocking and capable of inducing high subjective ratings of psychological stress. The stressor was presented to the participants on a combined television/VHS player (Philips, Model PV235/07, Koninklijke Philips Electronics Ltd., Eindhoven, The Netherlands), while the accompanying soundtrack was presented via a cordless headphone (Goodmans, Model Pro‐CD 9007, Goodmans Industries Ltd., Portsmouth, U.K.). Participants' blood pressure was measured with a Hem‐405 C digital blood pressure monitor (OMRON Corporation Ltd., Kyoto, Japan).
Stress Protocols
Active psychological stress
Active psychological stress is defined in the literature as stress in which the subject is required to actively cope (do something) or perform in a challenging situation. In this study, participants were asked to perform a 1‐minute mental arithmetic task, which has been shown to induce psychological stress. 19 This task results in parasympathetic withdrawal and a mixed alpha and beta‐adrenergic effect, although most subjects respond more with a beta pattern. The task involved fast and correct serial subtraction by 7 from 700. To increase the perceived importance and stressfulness of the task, as well as to assure active involvement, participants were told that the number of correct answers would be recorded.
Passive psychological stress
Passive psychological stress is defined in the literature as stress in which the subject is unable to actively cope (do something) about an unpleasant or distressing situation. It is characterized by the lack of control over the source of stress. In this study, participants watched the 1‐minute video clip of distressing images. Past research confirms that this form of stress triggers a stress response, which implies affective engagement and results in activation of the parasympathetic nervous system. 19 Consequently, for a manipulation check purpose, immediately after exposure, participants were asked to rate the perceived level of stress on a 7‐point Likert scale: 1 = not stressed at all, 7 = extremely stressed. 20 Stress‐exposure periods were followed by a 5‐minute recovery period.
Isometric exercises
The third stage of testing composed of each participant performing a series of nine physical exercises in conditions free of psychological stress. These physical exercises were designed to elicit different levels of sympathetic and vagal responses, so that conditions of gravitational and isometric stress, and a combination of both, were involved. Briefly, these exercises entailed sitting, skier's squat and standing (on one or two legs and balancing on tip‐toes), with arms by side or raised above head at 180° or held at 90° with 2‐kg weights. The purpose of these exercises was to collect additional QT and RR interval data at different heart rates for the subject‐specific QT‐interval correction. Each exercise was performed for 3 minutes to allow for QT hysteresis, because previously, it was described that 90% of QT‐interval adaptation to an abrupt change in heart rate takes approximately 2 minutes. 21
Experimental design
Tests were conducted between 9:00 a.m. and 4:00 p.m. in the Human Performance Laboratory at Nottingham Trent University. The arrival of the participants was followed by a 5‐minute briefing session in which the nature and purpose of the study was fully explained. Volunteers were then seated, blood pressure was measured, and ECG electrodes were attached to their bodies. To account for the “muscle noise,” the Mason‐Likar method of electrode placement was used. 22 After the leads have been connected and a good trace was observed, participants sat quietly until a steady heart rate was measured for more than 3 minutes.
The experimental design is shown in Figure 1. First, a 10‐second baseline ECG was recorded at rest. The participants were then exposed to isolated, 1‐minute conditions of AMS and PMS in a counterbalanced order. Between the two mental stress periods, the participants had a 5‐minute rest. Two 10‐second ECG measurements were recorded during each mental stress period: the first between 0 and 10 seconds, and the second between 30 and 40 seconds. After 5‐minute stress recovery, a second resting ECG (a second baseline) was recorded. Subsequently, a series of nine 3‐minute isometric stretches were performed, and a 10‐second ECG trace was obtained in the last 10 seconds of each period. This way, a total of 15 ECGs were obtained from each participant.
Figure 1.
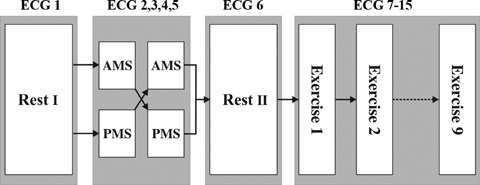
Experimental design. Participants were exposed to active and passive mental stress (AMS and PMS) in a counterbalanced order. A set of isometric exercises yielded 9 additional ECGs, so that a total of 15 ECGs were gained from each subject.
Participant‐specific heart rate correction
Visual checks verified the automatic QT interval and RR measurements in all records. ECGs with poor quality data were excluded. To study the QT–RR relationship, the data of QT and RR intervals of each participant were studied separately, because recently, the concept of a subject‐specific (i.e., individualized or intraindividual) correction method was proposed in order to minimize the correction error. 15 This technique is based on multiple ECG samples at different heart rates obtained within the same individual that are further used to determine the QT–RR relationship for that person. QT values from the 15 ECG records obtained during the study were plotted against their corresponding RR values producing a QT–RR plot with a total of 15 data points for each person. Although the best‐fit mathematical form that describes the pattern of the QT–RR relationship varies among individuals, the added value of nonlinear mathematical modeling appears to be limited. 23 Therefore, linear regression analysis of the QT–RR data pairs was used to determine the slope of the regression line in each participant, which is the value of the parameter α in the generic linear heart rate correction formula: QTc = QT +α× (1 – RR).
Statistical Analysis
All continuous variables are reported as mean ± SD. Repeated measures of analysis of variance was used to compare means of measurements among the 6 study periods. The Likert scores were evaluated by using nonparametric Mann–Whitney U test and Wilcoxon signed‐rank test when appropriate. Correlations between heart rate and QT interval were calculated using Pearson product–moment correlation. P <0.05 was considered significant for all tests. All analyses were performed using GraphPad Prism version 4.00 for Windows (GraphPad Software, San Diego, CA).
RESULTS
Subject‐Specific QT‐Interval Correction
A total of 450 ECGs were prepared during the study. ECGs obtained at rest and during mental stress were all suitable for analysis, but 37 exercise ECGs had to be discarded because of inadequate quality due to muscle noise. Consequently, the participant‐specific QT–RR relationship could be evaluated using 11.0 ± 1.9 (minimum = 7) ECGs per subject. Heart rate changes induced during the study were sufficient for regression analysis in each subject (44.1 ± 12.0 bpm, range 23.3–71.1 bpm). The linear regression model was a good fit for the QT–RR data (r2= 0.63 ± 0.18), and the slope was highly subject‐specific (α= 0.1136 ± 0.0584, range = 0.0229–0.1879). This adjustment of QT for heart rate successfully abolished the correlation between uncorrected QT and heart rate (Fig. 2).
Figure 2.
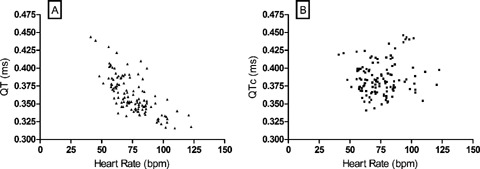
Subject‐specific QT‐interval correction. Uncorrected QT and corrected QTc data of 30 subjects obtained in four mental stress periods are plotted against heart rate. In panel A, a highly significant correlation is present between the uncorrected QT time and heart rate (r =–0.7132, P < 0.0001). In panel B, as a result of the subject‐specific correction method, QTc and heart rate do not correlate (r = 0.1144, P = 0.2136, ns).
The Effect of Mental Stress
Participants' blood pressure was in the normal range (systolic 118 ± 11 mmHg, diastolic 82 ± 10 mmHg) except for one subject who had a slightly increased systolic value (150/85 mmHg). Heart rate and QTc values in the six study periods are shown in Table 1. Heart rate was significantly increased during both periods of AMS (P < 0.0001), PMS did not elicit significant heart rate changes. During AMS, an early significant QTc prolongation could be observed (AMS I, P = 0.0004), but QTc measured between 30 and 40 seconds (AMS II) was not significantly changed. During PMS, no significant QTc changes could be detected.
Table 1.
Heart Rate and Individually Corrected QT Intervals in Six Study Periods
| Rest I | AMS I | AMS II | PMS I | PMS II | Rest II | |
|---|---|---|---|---|---|---|
| Heart rate (bpm) | 66.6 ± 11.9 | 87.9 ± 15.9* | 79.8 ± 11.1* | 67.2 ± 11.9 | 66.7 ± 11.7 | 67.7 ± 12.0 |
| QTc (ms) | 379.5 ± 26.4 | 390.0 ± 24.4† | 385.9 ± 25.3 | 381.8 ± 22.1 | 384.1 ± 21.1 | 382.0 ± 22.3 |
AMS = active mental stress; PMS = passive mental stress. Data are means ± SD. *P < 0.0001; †P = 0.0004.
Each participant presented normal T waves at rest; however, 14 subjects developed bifid (or notched) T waves during AMS and during one or more exercise conditions. From this 14 subjects, 8 presented bifid T waves during both AMS and exercise, and 6 subjects developed bifid T waves only during exercise. The number of ECGs with bifid T waves varied from 1 to 6 in affected participants, and bifid T waves seemed to appear more frequently at higher heart rates (Fig. 3). Conditions associated with multiple cases of bifid T waves were as follows: standing, arms by side (nine cases), AMS I (eight cases), standing, balanced on tip‐toes (eight cases), skiers' squat (six cases), and AMS II (three cases). During PMS, no T‐wave changes could be observed. Representative ECGs for QT‐interval prolongation and bifid T waves are shown in Figures 4 and 5.
Figure 3.
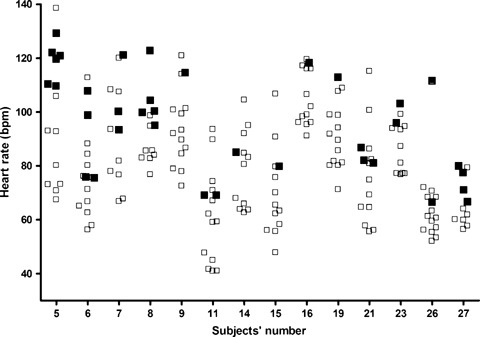
This graph illustrates the heart rates for 14 (out of the 30) participants who have developed bifid T waves during at least one study period. Open squares indicate normal T waves; filled squares indicate bifid T waves. Although bifid T waves seem to appear at higher heart rates, this observation cannot be the only explanation.
Figure 4.
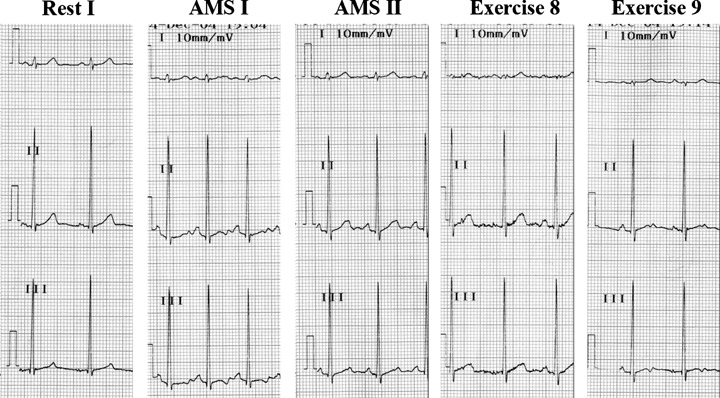
Five representative ECGs from subject no. 8. QT‐interval prolongation and T‐wave notching is present early in AMS (AMS I). Both QT duration and T wave normalize later in AMS (AMS II). Heart rate is nearly identical in exercise 8 and 9, but QT duration and the shape of the T waves are distinct: T waves become bifid again in exercise 9.
Figure 5.
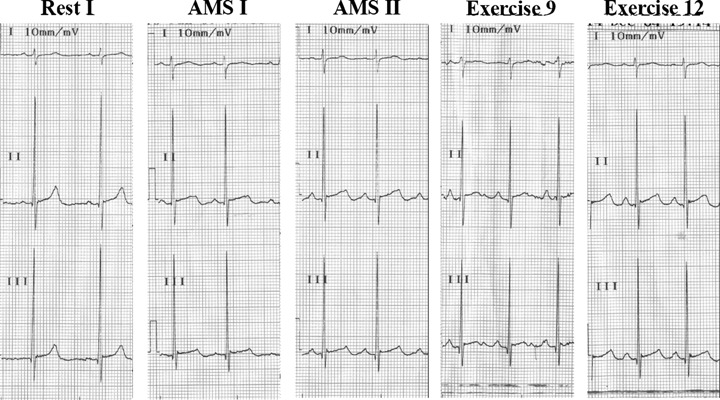
Subject no. 21 presents a substantial QT‐interval prolongation in AMS I that persists through AMS II. QT prolongation is also present in exercises 9 and 12. The incipient T‐wave notching in AMS II becomes clearly visible in exercise 9.
Subjects found the perceived level of stress during AMS higher than that during PMS, as indicated by the difference in Likert scale scores that showed marginal significance (3.7 ± 1.1 vs 3.1 ± 1.2, P = 0.06).
DISCUSSION
New Findings
This is the first report where the effect of AMS and PMS on the QT interval is compared. The most important contribution of this study is that a significant QT‐interval prolongation presents early in AMS (in the first 10 seconds). Another important finding of this research is that AMS and certain exercise conditions may elicit notched (bifid) T waves in healthy individuals with normal baseline ECGs. In our study, PMS had no significant effect on the QT‐interval duration and T waves.
The perceived level of stress was below the midpoint of 4 on the 7‐point Likert scale, so the stressors were not strong in terms of subjective perception. Yet, we still observed significant effects of AMS, indicating that even mild AMS may induce changes in cardiac repolarization. Also, it cannot be excluded, that a more aggressive passive stressor might have induced significant cardiac changes.
Mental Stress–Induced QT‐Interval Changes
Our study was different from past research from several aspects. We designed very brisk mental stress periods and measured heart rate and QT‐interval duration twice in each stress session. The first QT measurement was carried out just at the launch of the stress periods and such instant sampling has not been reported yet. With this method, we successfully detected the initial QT‐interval prolongation at the onset of AMS that other investigators have failed to observe. More important, in this study, QT intervals were individually corrected, and currently only this method is considered suitable for trials where QT‐interval durations are compared at different heart rates. 15
Adjustment of QT to changing heart rate is a dynamic phenomenon consisting of fast adaptation phase and slow adaptation phase. Franz et al. 24 showed that after rapid change in heart rate, fast adaptation phase of repolarization usually lasts 30–60 seconds followed by a 2‐minute slow adaptation. We propose that a part of the QT prolongation we noted was due to a delay in heart rate adaptation and only the remaining fraction was caused directly by the AMS, but the setting up of this study did not allow the quantification of these proportions.
Bifid (Notched) T Wave
Bifid T wave (i.e., notched or bifurcated) has been traditionally considered as one of the diagnostic signs of long QT syndrome (LQTS). Exercise or pharmacological testing of subjects with suspected LQTS has been suggested to induce morphological changes of the T wave to pick up silent gene carriers or to discriminate between the main genotypes. 25 , 26 On the other hand, the presence of bifid T waves has been also noted in a minority of the general population, with a prevalence of 2.8% among 4000 consecutive tracings analyzed by Watanabe et al. 27 and 3.0% of 3980 normal subjects reported by Ishikawa and Ohnuma. 28 In addition, it was shown that epinephrine infusion elicited T‐wave notching in one‐third of normal subjects, 26 and approximately half of the male and all female healthy volunteers developed bifid or biphasic T waves on isoproterenol infusion. 29 Exercise testing has also been used to “unmask” LQTS in suspected patients with normal resting ECG. During exercise test QT prolongation and T‐wave changes have been reported in LQTS patients, but not in healthy controls. 25 Bifid T waves were found characteristic mainly in LQTS2 patients. 25 Moreover, it has been shown that such T‐wave abnormalities in healthy subjects are prone to normalize during exercise test. 30
Recent studies on myocardial wedge preparations indicated the transmural dispersion of repolarization as one possible cause of bifid T waves. 31 Consequently, the appearance of bifid T waves in pathological conditions has been speculated to provide a substrate for reentry and life‐threatening arrhythmias. 32 As both local (nondipolar) and global (dipolar) repolarization components may contribute to the genesis of T waves on the body surface ECG, 33 the relative contribution of each of these two repolarization forces to T‐wave aberrations may vary among different clinical conditions.
We observed with surprise that almost half of our subjects developed bifid T waves in one or more study periods. To best of our knowledge, neither mental stress nor exercise conditions were reported to elicit bifid T waves so far. The setup of this study does not allow a comprehensive interpretation of this phenomenon, only some comments can be added. Bifid T waves seemed to appear at higher heart rates, but high heart rate is unlikely to explain the evolution of bifid T waves by itself, as bifid T waves also occurred at lower heart rates (Fig. 3). We hypothesize that the overt sympathetic predominance characterizing the onset of AMS and the final phase of the 3‐minute isometric physical exercises might have played a role in inducing the T‐wave notching.
Clinical Implications
Most episodes of tachyarrhythmias leading to sudden death are preceded by sinus tachycardia 34 and the relationship between sudden arousal and life‐threatening cardiac arrhythmias is clinically and experimentally well documented. 6 , 7 , 8 This study successfully demonstrates that an abrupt, approximately 30% increase in heart rate accompanied by a significant QT‐interval prolongation can be elicited by the onset of AMS. The observed QT prolongation was not excessive, but clearly reflects sympatho‐adrenergic effects on myocardial repolarization, thus may help to understand the association between sudden stress and malignant rhythm disturbances.
In addition, our observation that 14 out of 30 subjects developed bifid (notched) T waves during at least one study period suggests that the evolution of bifid T waves in stress conditions in healthy subjects likely represents a normal and innocent variant rather than a T‐wave profile pathognomic of LQTS or heralding arrhythmogenic substrate. This issue needs further research in patients with heart disease, in whom this phenomenon may be of clinical importance.
Study Limitations
The use of automatic measurement of the QT intervals has raised questions, and supplementary manual reading has been recommended. 35 In our study, each ECG was visually inspected by a trained cardiologist (G.A.), and ECGs with suboptimal quality or inaccurate QT data were discarded. The adequate number of QT–RR‐interval data points and the sufficiently wide range of recorded heart rates are crucial for the correct approximation of the individual QT–RR‐interval pattern. 15 In our study, by the means of the isometric exercises a considerably wide range of heart rates were provided that likely compensate, to some extent, for the limited number of QT–RR‐interval data.
CONCLUSIONS
Our findings are in accord with those clinical observations, where QT‐interval prolongation was noticed on sudden stress. 9 , 10 Also, our results indicate that changes in the autonomic tone, probably sympathetic predominance, may cause bifid T waves. These findings suggest that besides stress quality and intensity, the dynamics of stress application and perception also crucially influence QT responses.
Acknowledgments
Acknowledgment: Robert Spooner is thanked for his assistance in data collection.
The authors declare that they had no financial or other kind of interest in conducting this trial.
REFERENCES
- 1. Browne KF, Zipes DP, Heger JJ, et al Influence of the autonomic nervous system on the QT‐interval. Am J Cardiol 1982;50:1099–1103. [DOI] [PubMed] [Google Scholar]
- 2. Bexton RS, Vallin HO, Camm AJ. Diurnal variation of the QT‐interval‐influence of the autonomic nervous system. Br Heart J 1986;55:253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bellavere F, Ferri M, Guarini L, et al Prolonged QT period in diabetic autonomic neuropathy: A possible role in sudden cardiac death? Br Heart J 1988;59:379–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Glickstein JS, Schwartzman D, Friedman D, et al Abnormalities of the corrected QT‐interval in familial dysautonomia: An indicator of autonomic dysfunction. J Pediatr 1993;122:925–928. [DOI] [PubMed] [Google Scholar]
- 5. Lo SS, Mathias CJ, Sutton MS. QT‐interval and dispersion in primary autonomic failure. Heart 1996;75:498–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Eliot RS, Buell JC. Role of emotions and stress in the genesis of sudden death. J Am Coll Cardiol 1985;6:95B–98B. [DOI] [PubMed] [Google Scholar]
- 7. Kamarck T, Jennings JR. Biobehavioral factors in sudden death. Psychol Bull 1999;109:42–75. [DOI] [PubMed] [Google Scholar]
- 8. Algra A, Tijssen JGP, Roeland JRTC, et al QTc prolongation measured by standard 12‐lead electrocardiography is an independent risk factor for sudden death due to cardiac arrest. Circulation 1991;83:188–194. [DOI] [PubMed] [Google Scholar]
- 9. Toivonen L, Helenius K, Viitasalo M. Electrocardiographic repolarization during stress from awakening on alarm call. J Am Coll Cardiol 1997;30:774–779. [DOI] [PubMed] [Google Scholar]
- 10. Merz CN, Pardo Y. Mental versus physical stress, QT prolongation, and the autonomic nervous system. Circulation 2000;101:e213–e214. [DOI] [PubMed] [Google Scholar]
- 11. Huang MH, Ebey J, Wolf S. Responses of the QT‐interval of the electrocardiogram during emotional stress. Psychosom Med 1989;51:419–427. [DOI] [PubMed] [Google Scholar]
- 12. Insulander P, Freyschuss U, Juhlin‐Dannfelt A, et al Electrophysiological effects of mental stress in healthy subjects: A comparison with epinephrine infusion. J Electrocardiol 2003;36:301–309. [DOI] [PubMed] [Google Scholar]
- 13. Haapalahti P, Makijarvi M, Montonen J, et al Effects of cardiovascular autonomic function tests on QT dispersion in the 12‐lead electrocardiogram of healthy patients. J Electrocardiol 2000;33:321–327. [DOI] [PubMed] [Google Scholar]
- 14. Paavonen KJ, Swan H, Piipo K, et al Response of the QT‐interval to mental and physical stress in types LQT1 and LQT2 of the long QT syndrome. Heart 2001;86:39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Malik M, Farbom P, Batchvarov V, et al Relation between QT and RR intervals is highly individual among healthy subjects: Implications for heart rate correction of the QT‐interval. Heart 2002;87:220–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hedman A, Nordlander R. Changes in QT and Q‐aT intervals induced by mental and physical stress with a fixed rate and atrial triggered ventricular inhibited cardiac pacing. PACE 1988;11:1426–1431. [DOI] [PubMed] [Google Scholar]
- 17. Chiladakis JA, Kalogeropoulos A, Manolis AS. Autonomic responses to single‐ and dual‐chamber pacing. Am J Cardiol 2004;93:985–989. [DOI] [PubMed] [Google Scholar]
- 18. Kasprowicz AL, Manuck SB, Malkoff SB, et al Individual differences in behaviorally evoked cardiovascular response: Temporal stability and hemodynamic patterning. Psychophysiology 1990;27:605–619. [DOI] [PubMed] [Google Scholar]
- 19. Steptoe A, Vogele C. Methodology of mental stress testing in cardiovascular research. Circulation 1991;83:SII14–SII23. [PubMed] [Google Scholar]
- 20. Likert A. A technique for the measurement of attitudes. Methods Psychol (Frankfurt) 1932;140:44–53. [Google Scholar]
- 21. Pueyo E, Smetana P, Laguna P, et al Estimation of the QT/RR hysteresis lag. J Electrocardiol 2003;36(Suppl.):187–190. [DOI] [PubMed] [Google Scholar]
- 22. Mason RE, Likar A. A new system of multiple‐lead exercise electrocardiography. Am Heart J 1966;71:196–204. [DOI] [PubMed] [Google Scholar]
- 23. Extramiana F, Maison‐Blanche P, Cabanis MJ, et al Individual QT‐R‐R relationship: Average stability over time does not rule out an individual residual variability: Implication for the assessment of drug effect on the QT‐interval. Ann Noninvasive Electrocardiol 2005;10:169–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Franz MR, Swerdlow CD, Liem LB, et al Cycle length dependence of human action potential duration in vivo. Effects of single extrastimuli, sudden sustained rate acceleration and deceleration, and different steady‐state frequencies. J Clin Invest 1988;82:972–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Takenaka K, Ai T, Shimizu W, et al Exercise stress test amplifies genotype‐phenotype correlation in the LQT1 and LQT2 forms of the long‐QT syndrome. Circulation 2003;107:838–844. [DOI] [PubMed] [Google Scholar]
- 26. Khositseth A, Hejlik J, Shen WK, et al Epinephrine‐induced T‐wave notching in congenital long QT syndrome. Heart Rhythm 2005;2:141–146. [DOI] [PubMed] [Google Scholar]
- 27. Watanabe Y, Toda H, Nishimura M. Clinical electrocardiographic studies of bifid T waves. Br Heart J 1984;52:207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ishikawa K, Ohnuma H. The significance of a notch on the T wave. Jpn Circ J 1979;43:539–546. [Google Scholar]
- 29. Nakagawa M, Ooie T, Ou B, et al Gender differences in autonomic modulation of ventricular repolarization in humans. J Cardiovasc Electrophysiol 2005;16:278–284. [DOI] [PubMed] [Google Scholar]
- 30. Aravindakshan V, Surawicz B, Allen RD. Electrocardiographic exercise test in patients with abnormal T waves at rest. Am Heart J 1977;93:706–714. [DOI] [PubMed] [Google Scholar]
- 31. Yan GX, Antzelevitch C. Cellular basis for the normal T wave and the electrocardiographic manifestations of the long QT syndrome. Circulation 1998;98:1928–1936. [DOI] [PubMed] [Google Scholar]
- 32. Shimizu W, Antzelevitch C. Cellular basis for the ECG features of the LQT1 form of the long‐QT syndrome: Effects of beta‐adrenergic agonists and antagonists and sodium channel blockers on transmural dispersion of repolarization and torsade de pointes. Circulation 1998;98:2314–2322. [DOI] [PubMed] [Google Scholar]
- 33. Zabel M, Franz MR. The electrophysiological basis of QT dispersion: Global or local repolarization? Circulation 2000;101:E235–E236. [DOI] [PubMed] [Google Scholar]
- 34. Bayes de Luna, Coumel P, Leclercq JF. Ambulatory sudden death: Mechanisms of production of fatal arrhythmia on the basis of data from 157 cases. Am Heart J 1989;117:151–159. [DOI] [PubMed] [Google Scholar]
- 35. Goldenberg I, Moss AJ, Zareba W. QT‐interval: How to measure it and what is “normal.” J Cardiovasc Electrophysiol 2006;17:333–336. [DOI] [PubMed] [Google Scholar]


