Hypertrophic cardiomyopathy (HCM) is a genetic cardiac disease associated with ventricular tachyarrhythmias and sudden death. 1 , 2 , 3 , 4 , 5 In epidemiologic studies, HCM has been reported to occur in about 1:500 individuals in the general population and is therefore the most common genetic cardiovascular disease. 1 , 2 , 3 , 4 , 6 Since its modern description by Teare almost 50 years ago, HCM has been the subject of intense scrutiny and investigation. 7 , 8 A substantial segment of these investigative efforts have focused on recognition of those patients with unacceptably high risk of sudden death, who could benefit from preventive interventions with the implantable cardioverter‐defibrillator (ICD). 8 , 9 , 10 , 11 However, the heterogeneous nature of HCM has presented a significant challenge for clinicians in identifying such patients. Therefore, it is timely to review the spectrum and implications of arrhythmias in HCM with particular focus on the high‐risk patient and strategies for sudden death prevention.
MECHANISMS
The substrate of electrical instability that leads to arrhythmias in HCM lies predominantly in the disordered architecture of left ventricular myocardium in which adjacent myocytes are arranged in a chaotic pattern providing a suitable environment for reentrant ventricular tachyarrhythmias. 12 , 13 In addition, small vessel disease, characterized by abnormal intramural coronary arteries, may importantly contribute to this electrical instability by repetitive bursts of asymptomatic ischemia leading to myocyte death and repair as replacement scarring. 13 In the context of this unstable myocardial substrate ventricular tachycardia/fibrillation may occur in HCM as the usual mechanism of sudden cardiac death. 1 , 2 , 3 , 4 , 5 , 8 Supraventricular tachycardia, particularly atrial fibrillation, commonly occur in HCM (about 25% of patients) and may lead to significant morbidity and mortality by virtue of heart failure and stroke. 14 , 15 However, atrial fibrillation is not tightly linked to sudden cardiac death. Therefore, this review focuses on the profile and prognostic significance of ventricular tachyarrhythmias in HCM.
VENTRICULAR TACHYARRHYTHMIAS ON HOLTER ECG
Numerous clinical studies of 24–72 hour Holter ambulatory ECGs have been reported in HCM patients over the past 25 years (Table 1). 16 , 17 , 18 , 19 , 20 , 21 , 22 Each of these studies has shown a variety of ventricular tachyarrhythmias to occur commonly in HCM, although the precise frequency and pattern of each arrhythmia and their apparent clinical significance has varied among these reports. For example, premature ventricular depolarizations are found in 80%–90% of patients with a broad range in frequency of 1 to >5000. Ventricular couplets occur in 30%–40% and nonsustained ventricular tachycardia (NSVT) in 20%–25% of HCM patients (Table 1; Fig. 1). NSVT bursts on Holter are usually brief (3–5 beats), infrequent (1–3 runs in 24 hours) and unassociated with symptoms. 16 , 17 , 18 , 19 , 20 , 21 , 22 Prevalence of NSVT on Holter ECG increases with the magnitude of left ventricular (LV) hypertrophy, reaching >50% among those HCM patients with extreme increase in wall thickness (≥30 mm) (Fig. 2). 22 , 23 , 24 However, age, gender and presence of LV outflow obstruction do not appear to be associated with NSVT occurrence. 16 , 17 , 18 , 19 , 20 , 21 , 22
Table 1.
Studies in HCM Assessing Ventricular Arrhythmias on Ambulatory Holter ECG
| Author (year) | No. study patients | Mean age | Holter duration (hours) | PVCs | Couplets (% pts.) | NSVT (% pts.) | Follow‐up (months) | Sudden death n (%/year) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| (% pts.) | Range | >30/hour (%) | |||||||||
| Tertiary center based | |||||||||||
| Savage et al. (1979) 16 | 100 | 32 | 24 | 83 | 1–5183 | 20 | 32 | 19 | – | – | |
| Maron et al. (1981) 17 | 84 | 38 | 24 | 82 | – | 20 | – | 20 | 36 | 6 (2.4%) | |
| McKenna et al. (1981) 18 | 86 | 39 | 24–240 (mean 72) | 100 | – | 24 | 37 | 28 | 31 | 7 (3.2%) | |
| Monserrat et al. (2003) 19 | 531 | 39 | 24–48 | – | – | – | – | 20 | 70 | 36 (1.2%) | |
| Community based | |||||||||||
| Spirito et al. (1994) 20 | 151 | 40 | 24–48 | – | – | – | – | 28 | 58 | 6 (0.8%) | |
| Cecchi et al. (1998) 21 | 167 | 41 | 24–48 | – | – | – | – | 46 | 120 | 1 (0.1%) | |
| Adabag et al. (2005) 22 | 178 | 50 | 24 | 88 | 1–5435 | 12 | 42 | 31 | 66 | 11 (1.1%) | |
NSVT = nonsustained ventricular tachycardia; hr = hour; pts = patients; PVCs = premature ventricular complexes.
Figure 1.
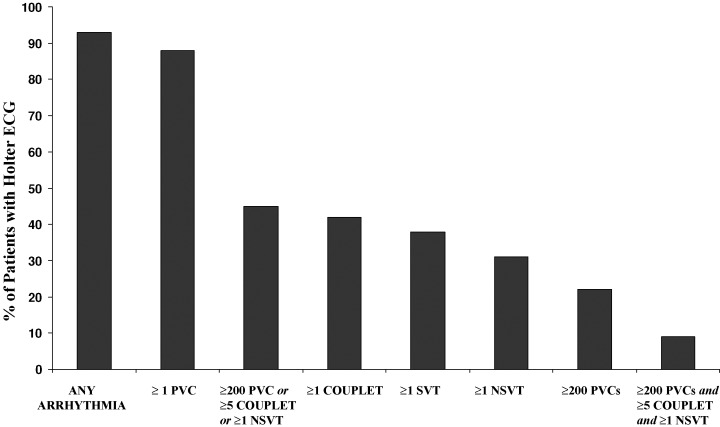
Prevalence of ventricular and supraventricular arrhythmias on 24‐hour ambulatory (Holter) ECG recording in 178 patients with HCM. PVC = premature ventricular complex; NSVT = nonsustained ventricular tachycardia; SVT = supraventricular tachycardia. (Reproduced with permission of American College of Cardiology; from Adabag et al., J Am Coll Cardiol 2005;45:697–704.)
Figure 2.
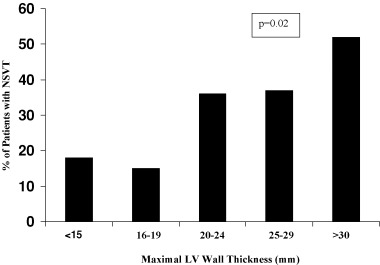
Relation between maximum LV wall thickness and occurrence of NSVT on 24‐hour ambulatory (Holter) ECG recording in 178 HCM patients. Occurrence of NSVT increases in direct relation to maximal LV thickness (p = 0.02 by chi‐square test for trend). LV = left ventricular; NSVT = nonsustained ventricular tachycardia. (Reproduced with permission of American College of Cardiology; from Adabag et al., J Am Coll Cardiol 2005;45:697–704.)
CLINICAL IMPLICATIONS OF ARRHYTHMIAS ON HOLTER ECG
There is no apparent linkage between the number of PVCs or couplets in 24 hours and the risk of sudden death. 16 , 17 , 18 , 22 Indeed, of the various arrhythmias which commonly occur on Holter ECG in HCM, only NSVT has been associated with increased risk for sudden death. 17 , 18 , 19 , 20 , 21 , 22 The studies which support this association come largely from tertiary centers to which patients have been preferentially referred for specialized care, thereby creating cohorts disproportionately skewed to high‐risk profiles 17 , 18 , 19 (Table 1). Two studies in the 1980s from such tertiary centers showed that risk of sudden death was increased up to 10‐fold in HCM patients with NSVT on Holter ECG, compared to those without NSVT. 17 , 18 On the other hand, in studies from less‐selected, community‐based or regional HCM cohorts, NSVT was associated with about a twofold increase in risk for sudden death, which did not achieve statistical significance. 20 , 22
While the frequency of NSVT is similar in a variety of HCM study populations (i.e., tertiary center vs. community based) the strength of the association between NSVT and sudden death differed substantially in accordance with patient selection and risk profile. 16 , 17 , 18 , 19 , 20 , 21 , 22 In high‐risk HCM populations from tertiary care centers, NSVT on Holter ECG proves to be a much stronger marker for sudden death with higher positive predictive value than in lower risk community‐based cohorts.
These principals support the view that short bursts of NSVT on a single Holter ECG should not be considered per se as an indication for ICD implantation. In our clinical practice, in the event that ≥1 short runs of NSVT occur on a random Holter ECG, five additional ambulatory recordings are obtained over an 8–12 week period to expand the monitoring period and assemble an arrhythmia profile that permits prudent clinical decisions. For example, if NSVT is repetitive on sequential Holter ECGs (or prolonged), then such selected patients may be considered for an ICD. On the other hand, should the single NSVT burst which triggered the additional Holter ECGs represent an isolated arrhythmic event over six days, then device therapy would probably not be justified. Conversely, the absence of NSVT on Holter ECG has a high negative predictive value for sudden death in HCM (>90%), which is the basis for a large measure of reassurance to patients with regard to their sudden death risk. 22
VENTRICULAR TACHYARRHYTHMIAS FROM INTERROGATED ICDS
Prior to the ICD era, based on anecdotal evidence from isolated cases, ventricular tachycardia/fibrillation was considered to be the most likely mechanism for sudden death in HCM. 1 , 2 , 3 , 4 , 5 , 25 , 26 More recently, the ICD has afforded access to the arrhythmia sequences which trigger appropriate device interventions, by virtue of stored electrocardiographic recordings. Indeed, ICD studies in high‐risk HCM patients have confirmed the long‐standing hypothesis that primary ventricular tachycardia/fibrillation, presumably emanating from the electrically unstable myocardial substrate are responsible for the unpredictable sudden death events in this disease (Figs. 3 and 4). 27 , 28 , 29 , 30 , 31 , 32 While some investigators have suggested that ventricular tachycardia interrupted by the ICD does not, in fact, represent a potentially fatal arrhythmia (but rather shocks occurring for arrhythmias which would otherwise be self‐limiting) 33 we do not subscribe to this view in HCM, and regard these as life‐saving interventions. Prolonged runs of ventricular tachycardia (8–10 seconds), in the presence of thick hearts with greatly increased LV mass intuitively suggest that such arrhythmias are not likely to terminate spontaneously. It should also be noted that ventricular tachycardia episodes requiring ICD intervention are much longer than those self‐limiting NSVT bursts (3–5 beats) typically evident on Holter ECGs in HCM patients. 11 , 27 , 28 Finally, it has not been possible to conclusively exclude bradycardia‐mediated events in HCM because of the automatically triggered back‐up pacing capability of the ICD. It is therefore possible that more diverse arrhythmic mechanisms are responsible for appropriate device interventions or sudden death in this complex disease.
Figure 3.
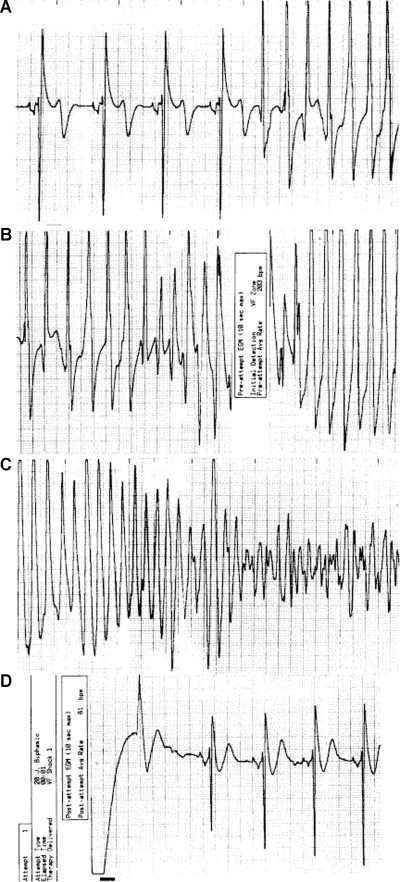
Stored ventricular electrogram from an asymptomatic 35‐year‐old man who received a defibrillator prophylactically because of a family history of sudden death related to hypertrophic cardiomyopathy and marked ventricular septal thickness (31 mm). The electrogram was obtained four years eight months after implantation of the defibrillator. The data were recorded at 1:20 a.m. while the patient was asleep. A continuous recording, at 25 mm/second, is shown in four panels, with the tracing recorded from left to right in each. After 4 beats of sinus rhythm, ventricular tachycardia begins abruptly, at a rate of 200 beats/minute (Panel A). The defibrillator senses ventricular tachycardia and charges (Panel B). Ventricular tachycardia deteriorates into ventricular fibrillation (Panel C). The defibrillator discharges appropriately (a 20‐J shock denoted by the bar, Panel D) during ventricular fibrillation and restores sinus rhythm. (Reproduced with permission of Massachusetts Medical Society; from Maron et al., N Engl J Med 2000;342:365–373.)
Figure 4.
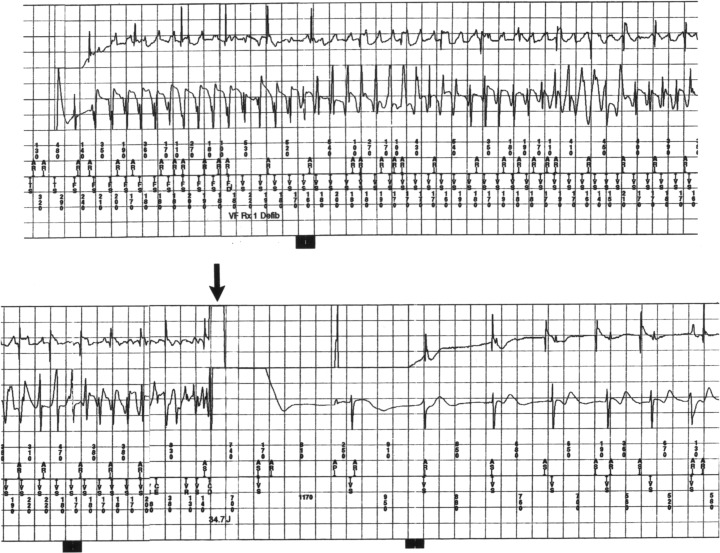
Intracardiac electrogram showing the mechanism of sudden death in a young 28‐year‐old patient with HCM who received a cardioverter‐defibrillator for primary prevention of sudden death. Spontaneous onset of ventricular fibrillation is automatically terminated by a defibrillation shock (arrow) which immediately restores sinus rhythm.
SUDDEN DEATH RISK STRATIFICATION
Arrhythmia‐based sudden death is the most recognized and devastating complication of HCM. 2 , 4 , 5 , 6 , 8 Indeed, HCM is the most common cause of sudden death in young people, including trained athletes. 8 , 34 , 35 Despite a predilection for young people (12–35 years of age), sudden death in HCM may also occur in mid‐life and even beyond, and therefore achieving a particular age does not confer an absolute immunity. 8 , 36 Although only a minority of HCM patients are susceptible to sudden death (perhaps 10%–20%), the unpredictable nature of these events and the fact that most patients who die suddenly do not experience premonitory symptoms creates a sense of vulnerability among the HCM patient population. 2 , 8 , 34 , 35
Recognition of high‐risk HCM patients is a priority and has been the subject of a considerable body of literature as well as persistent controversy. 2 , 8 , 9 , 10 , 11 The frequency of sudden death in HCM has been reported to be as high as 4%‐6%/year in highly selected cohorts from tertiary care referral centers, disproportionately comprised of high‐risk patients. 8 However, in reality sudden death is much less common in HCM (≤1%/year), as demonstrated in community‐based cohorts where selection bias is limited, and which come closest to the true disease presentation. 8 , 36 , 37 , 38
Identification of that minority of HCM patients who are at the highest risk for sudden cardiac death has been challenging due largely to the marked clinical heterogeneity of the disease spectrum. 1 , 2 , 3 , 4 , 5 , 8 , 9 Nevertheless, by virtue of a number of retrospective studies several clinical risk markers have been associated with sudden death risk (Table 2). 6 , 8 , 9 , 24 , 39 For the most part, these markers emphasize arrhythmias (such as sustained or nonsustained ventricular tachycardia and ventricular fibrillation) or variables judged to be associated with or promote arrhythmias (e.g. massive LV hypertrophy or syncope). Other clinical markers such as LV outflow obstruction and atrial fibrillation are not independent strong predictors of sudden death, can be contributing factors in individual patients but not sole indication for prophylactic defibrillator therapy. In addition, selected morphologic subgroups of patients appear to be candidates for primary prevention ICDs due to their propensity for potentially lethal ventricular tachyarrhythmias, including those in the end‐stage phase with systolic dysfunction and extensive fibrosis 40 or with LV apical aneurysm and regional scarring. 41 , 42 Largely due to the low overall rate of sudden death, most clinical risk factors carry low‐positive and high‐negative predictive values, so that the absence of a marker can more easily be used as a source of reassurance.
Table 2.
Risk Markers for Sudden Death in HCM
| Major markers |
| • Previous cardiac arrest |
| • Spontaneous sustained ventricular tachycardia |
| • Family history of sudden death (particularly in a first degree relative and/or multiple in occurrence) |
| • Unexplained syncope (particularly if recurrent, exertional or in the young) |
| • Extreme LV hypertrophy (maximal LV thickness ≥30 mm by echocardiogram) |
| • Abnormal blood pressure response with exercise (fall in pressure or sustained failure to rise >20 mmHg during exercise or recovery, in patients <50 years of age) |
| • NSVT on Holter ECG (≥3 beats and ≥120 beats/minute) |
| Other possible markers |
| • Atrial fibrillation |
| • LV outflow obstruction |
| • Myocardial ischemia |
| • High‐risk mutation |
| • Extensive delayed hyperenhancement by MRI (post‐gadolinium infusion) |
NSVT = nonsustained ventricular tachycardia; LV = left ventricular; ECG = electrocardiogram; MRI = magnetic resonance imaging.
No single available test is capable of solely and accurately assessing risk level in all HCM patients and at least one risk factor can be found in almost 50% of clinically identified HCM patients. Also an undefined but relatively small number of patients without any of the known risk markers nevertheless are subject to the risk of sudden death. 2 , 3 , 4 , 5 , 8 , 9 Consequently, it is apparent that the current risk stratification algorithm for HCM is incomplete and a future challenge in this disease is a more precise identification of those patients who should be targeted for primary prevention. This would include the recognition of new risk markers such as extensive fibrosis evidenced by delayed hyperenhancement on postgadolinium cardiac magnetic resonance imaging. 43
Since HCM is a genetic disease with a variety of mutant genesencoding protein components of the cardiac sarcomere, a role for genetic testing in risk stratification has been proposed based on the concept that mutational analysis could reliably identify benign and malignant genes and in this way predict future events. Indeed, a rapid genetic test is now commercially available which analyzes mutations in the eight most common HCM‐causing genes by direct DNA sequencing (http://www.hpcgg.org/LMM/tests.html). 44 However, it is now evident that due largely to the marked genetic and clinical heterogeneity of HCM (11 genes and >400 individual mutations), this genotyping strategy is not viable in assessing prognosis and sudden death risk and in making clinical management decisions for individual patients.
Noninvasive tests such as signal averaged ECG, heart rate variability, QT dispersion, and T‐wave alternans have either not been studied systematically in HCM or have proven unhelpful for risk stratification. Finally, the practice of electrophysiologic testing with programmed ventricular stimulation and induction of ventricular tachyarrhythmias to identify high‐risk HCM patients has largely been abandoned due to its low specificity for sudden death events (particularly when aggressive induction protocols with three extra stimuli are used), as well as concerns regarding the reliability of a single test result predicting future clinical events over many years. 45
Assessment of high‐risk status in HCM, including those patients who are asymptomatic or only mildly symptomatic, routinely includes personal and family history, physical examination, 12‐lead ECG, 24‐hour Holter ECG, and exercise testing. Subsequent risk analysis should be performed periodically or when a change in clinical status is perceived. Prudent management decisions are currently based on the known risk factors and by integrating all relevant clinical data and individual physician judgment in accord with the risk level acceptable to patient and family.
PREVENTION
Pharmacological treatment
In the pre‐ICD era, management of high‐risk HCM patients had been limited to prophylactic pharmacological treatment with beta‐blockers, verapamil, and antiarrhythmic agents such as procainamide, quinidine and more recently with amiodarone. 2 , 3 , 8 , 46 , 47 However, there are very limited data in HCM supporting the efficacy of such drug treatment in prevention of sudden death. 2 , 3 , 8 , 46 , 47 For example, there have been no controlled studies addressing the protective effects of beta‐blockers or verapamil, while class IA antiarrhythmic agents have been largely abandoned due to potential proarrhythmia. One report, using a retrospective, non‐randomized study design with historical controls 15 years ago, proposed amiodarone as a prophylactic treatment against sudden death in HCM patients with NSVT. 47 However, inexplicably there have been no further reports assessing the long‐term efficacy of amiodarone from those investigators advocating this drug for HCM patients. 45 , 47 Also, the known side effects associated with chronic administration of amiodarone severely limits the prophylactic application of this drug to young HCM patients with characteristically extended periods of risk over many decades. Therefore, due to the paucity of efficacy data, concern for adverse effects, and the risk incurred potentially by patient noncompliance, pharmacological treatment for prevention of sudden death for HCM patients has essentially been abandoned in light of proven efficacy of the ICD.
Implantable cardioverter‐defibrillator
Since its introduction to clinical medicine 25 years ago, 48 the ICD has gained widespread application to the prevention of sudden death in patients with ischemic heart disease and dilated cardiomyopathy. Superiority of the ICD to antiarrhythmic drugs (usually amiodarone) has been documented in several large, prospective, randomized clinical trials over the last decade. 49 , 50 , 51 , 52 , 53 However, application of ICD therapy to relatively young, high‐risk patients with genetic heart diseases (such as HCM) has only more recently received attention over the last five years (Figs. 3 and 4). 27 , 28 , 29 , 30 , 31 , 32 , 54
In a retrospective, multicenter study including 505 HCM patients, ICDs were effective in aborting potentially lethal ventricular tachyarrhythmias in 20% of high‐risk HCM patients over 3.7 years. 28 The average age at first appropriate ICD discharge was 44 ± 19 years and a substantial proportion of these shocks occurred in younger HCM patients (<30 years old). The average rate of appropriate ICD interventions was 11%/year for secondary prevention and 4%/year for primary prevention, largely in otherwise asymptomatic or mildly symptomatic patients. 27 , 28 Notably, among patients who underwent septal reduction interventions to relieve LV outflow obstruction, appropriate ICD discharges were 4‐fold more common following alcohol septal ablation than surgical septal myectomy. 28 In the experience of this registry, only one patient has died of a HCM‐related arrhythmia (at age 21). That event occurred when a defective ICD failed due to short‐circuiting at the time it attempted a defibrillation shock to reverse ventricular fibrillation. 55 , 56
HCM patients with ICDs are younger than those patients with ischemic heart disease and therefore, they are exposed to sudden death risk for much longer periods of time. Furthermore, the time at which high‐risk status is identified in a given HCM patient may not bear a direct relationship to the future timing of a life‐threatening arrhythmia requiring defibrillation. Indeed, the interval between implantation and first appropriate shock has proven to be highly variable and substantial (up to nine years) in some patients (Fig. 5). 27 This observation also underscores the unpredictable nature of the electrically unstable substrate in HCM.
Figure 5.
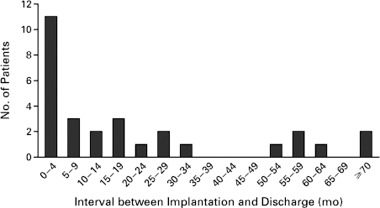
Interval between implantation of the defibrillator and the first appropriate discharge in 29 patients. (Reproduced with permission of Massachusetts Medical Society; from Maron et al., N Engl J Med 2000;342:365–373.)
There is little controversy concerning the appropriateness of ICDs for secondary prevention in HCM patients who have fortuitously survived a cardiac arrest with ventricular fibrillation. 8 On the other hand, there is not yet consensus on the precise selection of HCM patients for primary prevention, i.e., the number and strength of clinical risk markers sufficient to justify an ICD recommendation. While the presence of multiple clinical risk factors makes this decision easier, it is also apparent that a single risk factor may be sufficient to justify offering the option of a prophylactic ICD to some patients. Indeed, some investigators, particularly in the United States, recognizing that the individual risk markers are not equally weighted, favor strong consideration for a primary prevention ICD even in the presence of only one major risk factor (e.g., family history of sudden death in a relative). This was recently substantiated by multicenter data which reported that 40% of HCM patients who experienced an appropriate discharge had been implanted for primary prevention based on recognition of only one risk factor. 28 Other investigators (largely Europeans) are much more conservative and restrictive, usually requiring two or more risk factors before recommending a prophylactic ICD. Of note, the decision to implant an ICD cannot be based solely on an arbitrary number of clinical risk markers, but must be integrated into the overall clinical assessment and profile of a given patient taking into account age, strength of the risk markers identified and the level of uncertainty acceptable to the patient and family.
Also, the potential life‐saving implications of device implantation in patients must be weighed against the relatively uncommon but occasionally serious ICD‐related complications, including inappropriate shocks and other lead‐related problems, as well as the negative psychological impact that can be associated with implants in very young patients. It is also notable that physician and patient attitudes towards ICDs and the access to such devices can vary considerably among countries and cultures, thereby impacting importantly on management decisions. For example, overall ICD implantation rates are about 10 times higher in the United States than in the United Kingdom. 57
Finally, the American College of Cardiology/American Heart Association/North American Society of Pacing and Electrophysiology 2002 guidelines designate the ICD only as a class IIb indication for primary prevention of sudden death in HCM. 58 However, it is unlikely that sufficient data from a randomized trial will ever be available to support a higher classification. Given that HCM is heterogeneous and relatively uncommon, with low annual event rates, a prospective randomized and controlled study would require an extended follow‐up period over many years. Furthermore, the efficacy of ICDs in HCM has already been demonstrated in large retrospective studies. 27 , 28 , 29 Therefore, it would probably be impractical (and possibly unethical) to conduct a randomized trial at this time to document ICD benefit in HCM.
CONCLUSIONS
A variety of ventricular tachyarrhythmias including PVCs, couplets and NSVT occur commonly on ambulatory Holter ECG recordings in patients with HCM. The high frequency of these arrhythmias is disproportionate to the low event rate and relatively uncommon occurrence of sudden death in HCM. In high‐risk HCM populations from tertiary referral centers, NSVT on Holter ECG has been strongly associated with sudden death while in less selected community‐based populations NSVT has proven to be a weaker prognostic marker. While the positive predictive value for NSVT is relatively low (about 10%–20%), its absence has high negative predictive value and may be a source of reassurance to patients concerning their level of risk.
Over the last five years, the ICD has emerged with an important role in both secondary and primary prevention of sudden death for patients with HCM. Arrhythmia data obtained from ICD interrogation has documented primary ventricular tachycardia/fibrillation as the predominant mechanism of sudden death in HCM. While the precise criteria for selection of patients for prophylactic ICDs continues to present challenges, those clinical recommendations may be appropriate for patients with one or more of the acknowledged primary prevention risk factors, guided also by the overall clinical risk profile of the patient.
Acknowledgments
Acknowledgments: Dr. Adabag is supported, in part, by VA Clinical Science R&D Service (Grant no. 04S‐CRCOE‐001).
REFERENCES
- 1. Wigle ED, Rakowski H, Kimball BP, et al Hypertrophic cardiomyopathy. Clinical spectrum and treatment. Circulation 1995;92:1680–1692. [DOI] [PubMed] [Google Scholar]
- 2. Maron BJ. Hypertrophic cardiomyopathy: A systematic review. JAMA 2002;287:1308–1320. [DOI] [PubMed] [Google Scholar]
- 3. Spirito P, Seidman CE, McKenna WJ, et al The management of hypertrophic cardiomyopathy. N Engl J Med 1997;336:775–785. [DOI] [PubMed] [Google Scholar]
- 4. Maron BJ. Hypertrophic cardiomyopathy. Lancet 1997;350:127–133. [DOI] [PubMed] [Google Scholar]
- 5. Watkins H. Sudden death in hypertrophic cardiomyopathy (editorial). N Engl J Med 2000;342:422–444. [DOI] [PubMed] [Google Scholar]
- 6. Maron BJ. Hypertrophic cardiomyopathy: An important global disease (editorial). Am J Med 2004;116:63–65. [DOI] [PubMed] [Google Scholar]
- 7. Teare D. Asymmetrical hypertrophy of the heart in young adults. Br Heart J 1958;20:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Maron BJ, McKenna WJ, Danielson GK, et al American College of Cardiology/European Society of Cardiology Clinical Expert Consensus Document on Hypertrophic Cardiomyopathy. A report of the American college of Cardiology Task Force on Clinical Expert consensus Documents and the European Society of Cardiology Committee for Practice Guidelines Committee to Develop an Expert Consensus Document on Hypertrophic Cardiomyopathy. J Am Coll Cardiol 2003;42:1687–1713. [DOI] [PubMed] [Google Scholar]
- 9. Elliott PM, Poloniecki J, Dickie S, et al Sudden death in hypertrophic cardiomyopathy: identification of high risk patients. J Am Coll Cardiol 2000;36:2212–2218. [DOI] [PubMed] [Google Scholar]
- 10. Maki S, Ikeda H, Muro A, et al Predictors of sudden cardiac death in hypertrophic cardiomyopathy. Am J Cardiol 1998;82:774–778. [DOI] [PubMed] [Google Scholar]
- 11. Cha Y‐M, Rea RF, Brady PA, et al Predictors of ventricular tachyarrhythmic events in patients with hypertrophic cardiomyopathy following defibrillator implantation (abstract). Heart Rhythm. 2004;1(Suppl):S–32. [Google Scholar]
- 12. Shirani J, Pick R, Roberts WC, et al Morphology and significance of left ventricular collagen network in young patients with hypertrophic cardiomyopathy and sudden cardiac death. J Am Coll Cardiol 2000;35:36–44. [DOI] [PubMed] [Google Scholar]
- 13. Basso C, Thiene G, Corrado D, et al Hypertrophic cardiomyopathy and sudden death in the young: Pathologic evidence of myocardial ischemia. Hum Pathol 2000;31:988–998. [DOI] [PubMed] [Google Scholar]
- 14. Olivotto I, Cecchi F, Casey SA, et al Impact of atrial fibrillation on the clinical course of hypertrophic cardiomyopathy. Circulation 2001;104:2517–2524. [DOI] [PubMed] [Google Scholar]
- 15. Maron BJ, Olivotto I, Bellone P, et al Clinical profile of stroke in 900 patients with hypertrophic cardiomyopathy. J Am Coll Cardiol 2002;39:301–307. [DOI] [PubMed] [Google Scholar]
- 16. Savage DD, Seides SF, Maron BJ, et al Prevalence of arrhythmias during 24‐hour electrocardiographic monitoring and exercise testing in patients with obstructive and nonobstructive hypertrophic cardiomyopathy. Circulation 1979;59:866–875. [DOI] [PubMed] [Google Scholar]
- 17. Maron BJ, Savage DD, Wolfson JK, et al Prognostic significance of 24 hour ambulatory electrocardiographic monitoring in patients with hypertrophic cardiomyopathy: A prospective study. Am J Cardiol 1981;48:252–257. [DOI] [PubMed] [Google Scholar]
- 18. McKenna WJ, England D, Doi YL, et al Arrhythmia in hypertrophic cardiomyopathy I: Inluence on prognosis. Br Heart J 1981;46:168–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Monserrat L, Elliott PM, Gimeno JR, et al Non‐sustained ventricular tachycardia in hypertrophic cardiomyopathy: An independent marker of sudden death risk in young patients. J Am Coll Cardiol 2003;42:873–879. [DOI] [PubMed] [Google Scholar]
- 20. Spirito P, Rapezzi C, Autore C, et al Prognosis of asymptomatic patient with hypertrophic cardiomyopathy and nonsustained ventricular tachycardia. Circulation 1994;90:2743–2747. [DOI] [PubMed] [Google Scholar]
- 21. Cecchi F, Olivotto I, Montereggi A, et al Prognostic value of non‐sustained ventricular tachycardia and the potential role of amodarone treatment in hypertrophic cardiomyopathy: Assessment in an unselected non‐referral based patient population. Heart 1998;79:331–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Adabag AS, Casey SA, Kuskowski MA, et al Spectrum and prognostic significance of arrhythmias on ambulatory Holter electrocardiogram in hypertrophic cardiomyopathy. J Am Coll Cardiol. 2005;45:697–704. [DOI] [PubMed] [Google Scholar]
- 23. Spirito P, Watson RM, Maron BJ. Relation between extent of left ventricular hypertrophy and occurrence of ventricular tachycardia in hypertrophic cardiomyopathy. Am J Cardiol 1987;60:1137–1142. [DOI] [PubMed] [Google Scholar]
- 24. Spirito P, Bellone P, Harris KM, et al Magnitude of left ventricular hypertrophy and risk of sudden death in hypertrophic cardiomyopathy. N Engl J Med 2000;342:1778–1785. [DOI] [PubMed] [Google Scholar]
- 25. Nicod P, Polikar R, Peterson KL. Hypertrophic cardiomyopathy and sudden death. N Engl J Med 1988;18:1255–1256. [DOI] [PubMed] [Google Scholar]
- 26. Maron BJ, Bonow RO, Cannon RO, et al Hypertrophic cardiomyopathy: Interrelation of clinical manifestations, pathophysiologoy and therapy (Parts I and II). N Engl J Med 1987;316:780–789. and 844–852 [DOI] [PubMed] [Google Scholar]
- 27. Maron BJ, Shen W‐K, Link MS, et al Efficacy of implantable cardioverter‐defibrillators for the prevention of sudden death in patients with hypertrophic cardiomyopathy. N Engl J Med 2000;342:365–373. [DOI] [PubMed] [Google Scholar]
- 28. Maron BJ, Spirito P, Haas TS, for the ICD in HCM Investigators . Efficacy of the implantable defibrillators for prevention of sudden death in hypertrophic cardiomyopathy: Data from the International Registry of 506 high risk patients (abstract). Circulation 2005;112(suppl II):II–531. [Google Scholar]
- 29. Jayatilleke I, Doolan A, Ingles J, et al Long‐term follow‐up of implantable cardioverter defibrillator therapy for hypertrophic cardiomyopathy. Am J Cardiol 2004;93:1192–1194. [DOI] [PubMed] [Google Scholar]
- 30. Maron BJ, Estes NA, III, Maron MS, et al Primary prevention of sudden death as a novel treatment strategy in hypertrophic cardiomyopathy. Circulation 2003;107:2872–2875. [DOI] [PubMed] [Google Scholar]
- 31. Maron BJ. Contemporary considerations for risk stratification, sudden death and prevention in hypertrophic cardiomyopathy. Heart 2003;89:977–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Almquist AK, Montgomery JV, Haas TS, et al Cardioverter‐defibrillator implantation in high‐risk patients with hypertrophic cardiomyopathy. Heart Rhythm 2005;2:814–819. [DOI] [PubMed] [Google Scholar]
- 33. Germano JJ, Reynolds M, Essebag V, et al Frequency and Causes of Implantable Cardioverter‐Defibrillator Therapies: Is Device Therapy Proarrhythmic? Am J Cardiol 2006;97:1255–1261. [DOI] [PubMed] [Google Scholar]
- 34. Maron BJ, Shirani J, Poliac LC, et al Sudden death in young competitive athletes. Clinical, demographic, and pathological profiles. JAMA 1996;276:199–204. [PubMed] [Google Scholar]
- 35. Maron BJ. Sudden death in young athletes. N Engl J Med 2003;349:1064–1075. [DOI] [PubMed] [Google Scholar]
- 36. Maron BJ, Olivotto I, Spirito P, et al Epidemiology of hypertrophic cardiomyopathy‐related death: revisited in a large non‐referral‐based population. Circulation 2000;102:858–864. [DOI] [PubMed] [Google Scholar]
- 37. Maron BJ, Casey SA, Poliac LC, et al Clinical course of hypertrophic cardiomyopathy in a regional United States cohort. JAMA 1999;281:650–655. [DOI] [PubMed] [Google Scholar]
- 38. Maron BJ, Mathenge R, Casey SA, et al Clinical profile of hypertrophic cardiomyopathy identified de novo in rural communities. J Am Coll Cardiol 1999;33:1590–1595. [DOI] [PubMed] [Google Scholar]
- 39. Spirito P, Maron BJ. Relation between extent of left ventricular hypertrophy and occurrence of sudden cardiac death in hypertrophic cardiomyopathy. J Am Coll Cardiol 1990;15:1521–1526. [DOI] [PubMed] [Google Scholar]
- 40. Harris KM, Spirito P, Maron MS, et al Prevalence, clinical profile and significance of left ventricular remodeling in the end‐stage phase of hypertrophic cardiomyopathy. Circulation 2006. (in press). [DOI] [PubMed] [Google Scholar]
- 41. Matsubara K, Nakamura T, Kuribayashi T, et al Sustained cavity obliteration and apical aneurysm formation in apical hypertrophic cardiomyopathy. J Am Coll Cardiol 2003;42:288–295. [DOI] [PubMed] [Google Scholar]
- 42. Zenovich AG, Lesser JR, Hanna CA, et al Identical twins with hypertrophic cardiomyopathy and apical aneurysm. Am J Cardiol 2006;97:1109. [DOI] [PubMed] [Google Scholar]
- 43. Rickers C. Wilke NM, Jerosch‐Herold M, et al Utility of cardiac magnetic resonance imaging in the diagnosis of hypertrophic cardiomyopathy. Circulation 2005;112:855–861. [DOI] [PubMed] [Google Scholar]
- 44. Maron BJ, Seidman JG, Seidman CE. Proposal for contemporary screening strategies in families with hypertrophic cardiomyopathy. J Am Coll Cardiol 2004;44:2125–2132. [DOI] [PubMed] [Google Scholar]
- 45. Behr ER, Elliott P, McKenna WJ. Role of invasive EP testing in the evaluation and management of hypertrophic cardiomyopathy. Card Electrophysiol Rev 2002;6:482–486. [DOI] [PubMed] [Google Scholar]
- 46. Sherrid MV, Barac I, McKenna WJ, et al Multicenter study of the efficacy and safety of disopyramide in obstructive hypertrophic cardiomyopathy. J Am Coll Cardiol 2005;45:1251–1258. [DOI] [PubMed] [Google Scholar]
- 47. McKenna WJ, Oakley CM, Krikler DM, et al Improved survival with amiodarone in patients with hypertrophic cardiomyopathy and ventricular tachycardia. Br Heart J 1985;53:412–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mirowski M, Reid PR, Mower MM, et al Termination of malignant ventricular arrhythmias with an implanted automatic defibrillator in human beings. N Engl J Med 1980;303:322–324. [DOI] [PubMed] [Google Scholar]
- 49. Moss AJ, Hall WJ, Cannom DS, et al Improved survival with an implantaed defibrillator in patients with coronary disease at high risk for ventricular arrhythmia: Multicenter Automatic Defibrillator Implantation Trial Investigators. N Engl J Med 1996;335:1933–1940. [DOI] [PubMed] [Google Scholar]
- 50. Buxton AE, Lee KL, Fisher JD, et al A randomized study of the prevention of sudden death in patients with coronary artery disease: Multicenter Unsustained Tachycardia Trial Investigators. N Engl J Med 1999;341:1882–1890. [DOI] [PubMed] [Google Scholar]
- 51. The Antiarrhythmics vs Implantable Defibrillators (AVID) Investigators . A comparison of antiarrhythmic‐drug therapy with implantable defibrillators in patients resuscitated from near‐fatal ventricular arrhythmias. N Engl J Med 1997;37:1576–1583. [DOI] [PubMed] [Google Scholar]
- 52. Moss AJ, Zareba W, Hall WJ, et al Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med 2002;346:877–883. [DOI] [PubMed] [Google Scholar]
- 53. Bardy GH, Lee KL, Mark DB, et al Amiodarone or an implantable cardiverter‐defibrillator for congestive heart failure. N Engl J Med 2005;352:225–237. [DOI] [PubMed] [Google Scholar]
- 54. Goldenberg I, Moss AJ, Maron BJ, et al Cost‐effectiveness of implanted defibrillators in young people with inherited cardiac arrhythmias. Ann Noninvasive Electrocardiol 2005;10(4 Suppl):67–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Gornick CC, Hauser RG, Almquist AK, et al Unpredictable implantable cardioverter‐defibrillator pulse generator failure due to electrical overstress causing sudden death in a young high‐risk patient with hypertrophic cardiomyopathy. Heart Rhythm 2005;2:681–683. [DOI] [PubMed] [Google Scholar]
- 56. Hauser RG, Maron BJ. Lessons from the failure and recall of an implantable cardioverter‐defibrillator. Circulation 2005;112:2040–2042. [DOI] [PubMed] [Google Scholar]
- 57. Maron BJ, Shen W‐K, Spirito P. Implantable Defibrillator for Prevention of Sudden Death in Hypertrophic cardiomyopathy In Maron BJ. (ed.): Diagnosis and Management of Hypertrophic Cardiomyopathy. Malden , MA , Futura Blackwell Publishing Co, 2004, pp. 327–344. [Google Scholar]
- 58. Gregoratos G, Abrams J, Epstein AE, et al ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices. A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/NASPE Committee to Update the 1998 Pacemaker Guidelines). Circulation 2002;106:2145–2161. [DOI] [PubMed] [Google Scholar]


