Abstract
Background: P‐wave dispersion (Pd) is an appealing marker for predicting the risk of developing atrial fibrillation. At present, no definitive cutoff value has been determined as to the diagnosis of high‐risk patients. Our aims were to evaluate P‐wave parameters of healthy subjects published in the literature, determine normal range and weighted means of Pd and P‐wave parameters, and investigate the influences of gender, age, and BMI on the weighted results.
Methods: A systematic search of studies published in PubMed was conducted. Only studies which included control groups of healthy individuals were included.
Results: Of the 657 studies initially identified, 80 were eligible for inclusion. The total number of participants was 6,827. The highest reported Pd values were 58.56 ± 16.24 ms; the lowest were 7 ± 2.7 ms. The weighted mean was 33.46 ± 9.65 ms; weighted median was 32.2 ms. Gender and age were not found to be associated with significant influences on P‐wave parameter values. High‐normal BMI was not found to be associated with increased P‐wave parameter values.
Conclusions: Pd, Pmax, and Pmin span a wide range of values in healthy individuals. Seemingly, abnormal values were often reported in healthy adults. The high variability of P‐wave parameters in healthy individuals, and overlapping of the results with those reported for patients with increased risk for atrial fibrillation, might suggest that this technique has limited sensitivity and specificity. The variability between studies may stem from methodological issues and, therefore, there is a definite need for methodological standardization of Pd measurements.
Ann Noninvasive Electrocardiol 2012;17(1):28–35
Keywords: atrial fibrillation, P‐wave dispersion, electrocardiography, supraventricular arrhythmia
INTRODUCTION
Atrial fibrillation (AF) is the most common form of arrhythmia causing severe medical complications, as well as early death. 1 AF may be encountered in patients with cardiac disease, although in others no underlying clinical condition was found. The traditional risk factors for AF involvement are advanced age, male gender, heart failure, valvular disease, and hypertension. 2 Scientific attention has been focused on attempting to identify patients with an increased risk for AF evolvement.
P‐wave dispersion (Pd) is calculated by subtracting the minimal P wave (Pmin) duration from the maximal P wave (Pmax) duration, as measured by multiple surface ECG leads, from a single beat. Measurement of Pd is based on an assumption that surface ECG represents partially regional changes in myocardial activation. Nevertheless, it remains unknown whether Pd is determined only by heterogeneity of atrial conduction or by other factors as well. 3 , 4 Pd values of >40 ms were found to be correlated with AF, with a sensitivity of 74–83% and specificity of 81–85%. 5
Nevertheless, a consensus regarding Pd values, distinguishing normal patients from high‐risk patients, is lacking. Various research groups have included control subjects with different clinical characteristics. Cardiovascular risk factors, like obesity, hypertension, coronary artery disease, valvular disease, and diastolic dysfunction, were all suggested to influence the Pd values. Nonetheless, the influences of demographic parameters such as gender, age, and body mass index (BMI) on P‐wave parameters remain to be determined. Therefore, our aim was to conduct a meta‐analysis of P wave parameters in healthy study groups, in an attempt to estimate their normal range.
METHODS
The meta‐analysis was conducted in accordance with the “preferred reporting items for systematic reviews and meta‐analyses.” 6
Eligibility Criteria
Only articles published in English were included. The control group was completely healthy. Control group studies which included patients with diabetes, hypertension, coronary artery disease or other congenital or acquired heart condition, lung diseases, collagen vascular diseases, thyroid dysfunction, rhythms other than sinus, or use of any type of medication were excluded. Studies with an insufficient amount of data regarding the health status of the participants were also excluded from analysis. Patients who underwent coronary angiography prior to the study, due to suspected coronary disease, regardless of the results were excluded, as well as patients with a history of coronary artery disease associated with morbidity. Patients with a history of supraventricular or ventricular arrhythmias, obstructive sleep apnea, or alcoholism were also excluded. Smoking was not an exclusion criterion. Control patients enrolled prior to surgery were also excluded.
Information Source
A PubMed‐based search was conducted on Jan 15, 2011.
Search Strategy
The following key words were used “P‐wave dispersion” (202 results), “P dispersion” (36 results), “p‐disp” (5 results), “dispersion AND P[Title] AND wave[Title]” (185 results), ““P wave” AND dispersion” (262 results). Overall, 657 unsorted results were initially found.
Data Synthesis
Studies were selected in which Pd was calculated according to the following equation: Pd equals Pmax minus Pmin. When a study included two healthy groups of patients who were exposed to influences not affecting Pd (such as high altitude), 7 both groups were included in the analysis. Studies where measurements were repeated several times a day, morning values were chosen for the analysis. However, it is noteworthy that values in the controls were similar throughout the day. 8 Also, when healthy individuals were evaluated for Pd on several occasions (i.e., during different seasons), 9 yearly results were averaged. Only studies, where mean ± SD values were presented, were included. In cases when more than one technique was employed for P‐wave measurements, only manual measurements were included. Healthy athletes were also referred to as controls. In cases where both averaged beats and nonaveraged beats were calculated, the randomly chosen beat was used.
Statistical Analysis
Results were expressed as mean and standard deviation (SD). Correlation analyses were performed using the linear, logarithmic, and quadratic regressions. The number of patients was used as a weighted variable. A probability value of P <0.05 was considered significant; two‐tailed P values were used for all statistics. Normal distribution was evaluated with the Shapiro–Wilk test. Analyses were performed using SPSS 15 for Windows software (SPSS, Chicago, IL, USA) and JMP version 7.0 (SAS Institute, Cary, NC, USA). The cutoff for normal values was determined according to 95% confidence interval.
RESULTS
After the exclusion of references traced in more than one search strategy and exclusion of five irrelevant papers, 251 references were included in the initial screening; 103 references did not report any control group and were therefore excluded from the analysis. Eight articles were excluded due to unorthodox techniques of Pd calculations (i.e., evaluation of Pd from Magnetocardiography, when dispersion was calculated as SD of P‐wave durations or calculation of Pd from 16 precordial leads). Eleven were excluded since they were not written in English. Three articles were excluded due to insufficient data on the health status of the participants; 38 were excluded due to the inclusion of a control group of unhealthy individuals. Two articles were excluded due to the presentation of results in medians and not means. Overall 80 articles were included in the meta‐analysis (Fig. 1). 3 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 Fourteen of these articles utilized only a computer‐assisted technique for P‐wave evaluation.
Figure 1.
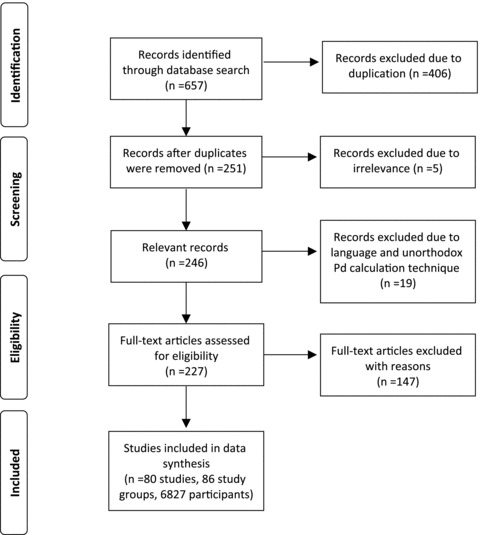
Methods for inclusion of articles in the study.
The meta‐analysis results of mean P wave parameters were of normal distribution. Pd results were available in 80 studies, which included 86 eligible study groups. One paper reported on Pmax, Pmin, and mean P wave duration but not on Pd. The highest reported Pd values were 58.56 ± 16.24 ms (the patients were healthy athletes, N = 810 participants). 84 The highest value for normal non‐athletic patients was 55 ± 15 ms (N = 37). 14 The lowest Pd values were 7 ± 2.7 ms (N = 70), 58 and 7 ± 2.9 ms (N = 62). 30 The weighted mean was 33.46 ± 9.65 ms; weighted median was 32.2 ms. The biggest cohort, which included 1,353 patients, reported on mean Pd values of 38.36 ± 10.38 ms. 39 Figure 2 demonstrates the association between logarithmic presentation of the number of included participants (logN), and Pd values. The scattered lines represent the funnel‐like shaped range of the 99% confidence interval of expected Pd values, according to the biggest cohort. 39 Twenty‐nine groups only were found to be in the expected zone (33.7% of the study groups). Thirteen studies reported mean Pd values of >40 ms (15.1% of all the study groups). Pmax was evaluated in 75 studies. The highest reported Pmax values were 125 ± 15 ms; 31 the lower were 64 ± 9.4 ms. 28 Weighted mean was 99.3 ± 11.5 ms; the weighted median was 99.4 ms. Pmin was reported in 62 studies. The highest reported Pmin values were 104 ± 9.2 ms. 10 The lowest reported Pmin values were 31.1 ± 5.4 ms. 82 Weighted mean Pmin was 65.3 ± 13.0 ms; weighted median was 66 ms.
Figure 2.
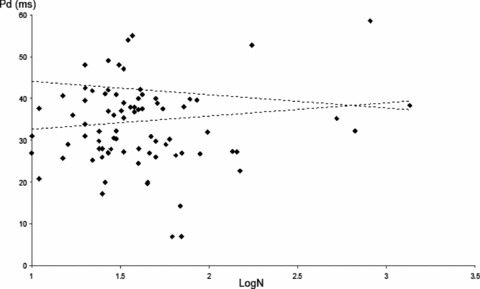
Pd values compared with logarithmic scale of number of participants (N). The funnel‐like scattered lines represent the expected 99% confidence interval of Pd values according to the biggest scaled study. Only 29 groups were found in the expected zone (33.7% of the study groups).
No association was found between gender and P‐wave parameters. In addition, no significant association was found between Pmax and Pmin, and BMI. Nevertheless, a significant negative association was found between Pd and BMI according to all models (Fig. 3, R square 0.47–0.48, P < 0.001). A separate analysis of studies which included more than 30 participants, and which have specified BMI values (18 study groups, including 1646 participants), was associated with an even stronger association (R square ∼0.58, P < 0.001). According to the calculated 95% confidence interval, the upper cutoff was 54.8 ms for Pd, 124.8 ms for Pmax, and 97.3 for Pmin. The upper normal values for Pd in patients with BMI values lower than 25 kg/m2 was 58.56 ms, and for patients with BMI values higher than 25 kg/m2 was 42.5 ms.
Figure 3.
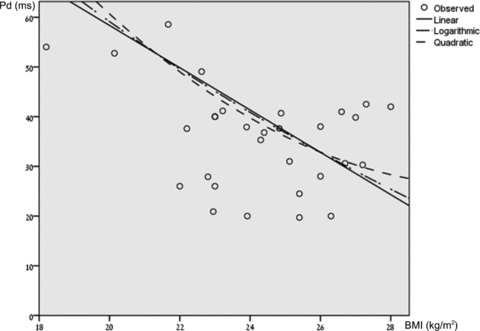
Bivariate fit of mean Pd by BMI according to linear, logarithmic, and quadratic models. A significant negative association was found between Pd and BMI consistent with all models (R square 0.47–0.48, P < 0.001).
No correlation, by any regression analysis, was found between age and Pd, Pmax and Pmin values (R square ∼0.1). A separate analysis of studies which included more than 30 individuals (49 study groups) did not alter the results.
DISCUSSION
In the present study, P‐wave parameters were evaluated in healthy individuals. Although 40 ms was proposed as a value distinguishing patients with a high risk for developing AF from low risk patients, 3 , 4 , 5 we have found that these high values were commonly reported in healthy individuals as well. Therefore, it seems that in Pd (as was previously observed in QT dispersion measurement) overlapping of the results between normal and abnormal conditions occurred. 86 We suggest that small sample size may by associated with inappropriately high or low Pd values, compared with expected values, according to big cohorts.
We did not find that a higher male percentage was associated with higher Pd values and higher Pmax. This association has been investigated in only a few studies. Kose et al. did not observe gender differences in children. 55 Sari et al. reported similar findings in a small group of healthy males and females. 65 In contrast, Yildiz et al. included a very large cohort and reported that male gender was associated with higher Pd and Pmax values. 84 Sari et al. and Kose et al. results are consistent with the findings in our meta‐analysis. It should be emphasized that although in general AF is more common in males, 2 none of the included investigated groups reported that their male control patients ultimately developed AF.
The negative associations found between increased BMI and Pd is intriguing, since clinically an opposite association was reported by most groups. In a recently published review by Rosiak et al., it was reported that an increase of a single BMI unit was associated with an increase of about 8% in the risk for developing AF. 2 Also, higher Pd was reported in obese patients compared with patients with normal weight in studies specifically aimed at evaluating such an association. 68 , 87 A negative association between BMI and Pd, consistent with the findings of the meta‐analysis, was also reported by Magnani et al. after applying a multivariable adjusted model (P = 0.04), 88 further supporting our results. Nevertheless, mean BMI values were in the current study within the normal‐to‐overweight range and none of the included studies had patients with mean BMI values higher than 28 kg/m2. Similarly, Magnani et al. included patients with a BMI lower than 30 kg/m2. 88 Therefore, it is reasonable to assume that our conclusions should be applied only to patients with a BMI lower than 28 kg/m2. We speculated that the association between BMI and Pd was inverted in BMI values above this range. Therefore, we hypothesized that different meta‐analysis or a large prospective cohort design including only obese patients with higher BMIs might yield a different association. Furthermore, it is possible that the studies which included thinner patients had unrecognizable conditions predisposing them to increased risk for supraventricular arrhythmias, hence higher Pd values. Interestingly, the weighted upper normal values of Pd in patients with mild overweight (i.e., 42.5 ms) were similar to those suggested as the upper normal values which distinguish high‐risk patients for AF development (i.e. approximately 40 ms). In some studies which suggested a Pd cutoff, the BMI values were not reported. 15 , 19 , 33 A different study reported on both Pd and BMI. Nevertheless, despite BMI values lower than 25 kg/m2, their Pd suggested cutoff for identifying high risk patients was not increased. 89
We did not find any significant association between increased age and Pd values. Although the risk of AF increased dramatically in older patients, 90 in the majority of the studies, mean age was 25–45 years, in which range AF is less frequent. Yildiz et al. also found no association between age and Pd. 84 Magnani et al., in contrast, reported a positive association between age and all P‐wave parameters. 88
The major limitation of the present study was the inclusion of heterogeneous cohorts in diverse research groups utilizing different P‐wave measurement methodology. For instance, accuracy and reproducibility may significantly increase when PC‐based on‐screen measurements are applied. 15 Studies may differ in applying manual versus automated measurements, ECG on paper compared with digitalized ECG, paper speed and printed resolutions, ECG sampling rate, the number of investigators who evaluated P‐wave parameters, the number of included leads, etc. A comparison of different studies, applying measurement techniques, due to the lack of measurement standardization, is challenging. Also, P‐wave parameters and Pd may vary due to external influences such as seasonal effects. 9
Internal influences such as anxiety may also dramatically alter the results, 77 and since anxiety levels were not routinely measured, statistical standardization could not be performed. Another internal factor that may have altered the results was whether the healthy patients received adequate sleep. It was found that following sleep deprivation in young adults, Pmin significantly decreases, Pmax increases and Pd increases. 65 Pd may also be influenced by autonomic function, 4 thus no standardization could be performed in the current study design. Diurnal variation may also pose as an intervening factor since most studies did not elaborate on the time of the day in which ECG measurements were conducted. 8 It should also be emphasized that there was a vast difference in the number of included patients among studies. The mean number of patients was 79.4 ± 184.2 (median 34.0, 75% quartile 52.0, 25% quartile 24.8 patients). Therefore, by definition, studies which included larger cohorts had greater weighted influence on the results.
CONCLUSIONS
Pd, Pmax, and Pmin span a wide range of values in healthy individuals. Gender and age have no significant effects on P‐wave parameters. Higher BMI within the nonobese range is associated with lower Pd values. Seemingly abnormal values of Pd were commonly reported in healthy adults. The high variability of P‐wave parameters in healthy individuals, and overlapping of the results with those reported for patients with increased risk for AF, might suggest that this technique has limited sensitivity and specificity. Since Pd varies with BMI, it should be taken into account when evaluating whether a study group is within the normal range. Moreover, the current studies emphasized the need for Pd measurement standardization. It is quite reasonable to assume that such standardization will increase the reproducibility, sensitivity, and specificity of this test.
Study Limitations
In the current study design, the raw data of all included studies were unavailable for analysis. Therefore, we conducted the meta‐analysis by evaluating weighted means according to the accepted method. We cannot predict if an analysis of the raw data would have yielded different results, and therefore, the interpretation of our results should be done within this context.
Acknowledgments
Acknowledgments: We wish to thank Mrs. Inna Sergienko from the Medical Library of the Chaim Sheba Medical Center, Tel‐Hashomer, Israel, for her assistance in locating some of the scientific references. We also wish to thank Dr. Gabriel Chodik from Tel‐Aviv University for his assistance with the statistical analysis, and Mrs. Phyllis Curchack Kornspan for her editorial assistance. This study is dedicated to the memory of Haim Gueron.
Conflict of interest: none declared
REFERENCES
- 1. Schneider MP, Hua TA, Bohm M, et al Prevention of atrial fibrillation by Renin‐Angiotensin system inhibition a meta‐analysis. J Am Coll Cardiol 2010;55:2299–2307. [DOI] [PubMed] [Google Scholar]
- 2. Rosiak M, Dziuba M, Chudzik M, et al Risk factors for atrial fibrillation: Not always severe heart disease, not always so ‘lonely.’ Cardiol J 2010;17:437–442. [PubMed] [Google Scholar]
- 3. Nussinovitch N, Livneh A, Katz K, et al P wave dispersion in familial Mediterranean fever. Rheumatol Int 2011;31:1591–1594. [DOI] [PubMed] [Google Scholar]
- 4. Michelucci A, Bagliani G, Colella A, et al P wave assessment: State of the art update. Card Electrophysiol Rev 2002;6:215–220. [DOI] [PubMed] [Google Scholar]
- 5. Dilaveris PE, Gialafos JE. P‐wave dispersion: A novel predictor of paroxysmal atrial fibrillation. Ann Noninvasive Electrocardiol 2001;6:159–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Moher D, Liberati A, Tetzlaff J, et al Preferred reporting items for systematic reviews and meta‐analyses: The PRISMA statement. BMJ 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Guntekin U, Gunes Y, Tuncer M, et al The effect of altitude on P‐wave and QT duration and dispersion. Pacing Clin Electrophysiol 2008;31:889–892. [DOI] [PubMed] [Google Scholar]
- 8. Yildirim N, Topaloglu S, Bozboga S, et al Diurnal variation of the P‐wave dispersion in chronic ischemic heart diseases. Coron Artery Dis 2006;17:707–710. [DOI] [PubMed] [Google Scholar]
- 9. Kose S, Aytemir K, Can I, et al Seasonal variation of P‐wave dispersion in healthy subjects. J Electrocardiol 2002;35:307–311. [DOI] [PubMed] [Google Scholar]
- 10. Acampa M, Lazzerini PE, Guideri F, et al Homocysteine and P wave dispersion in patients with heart transplantation. Clin Transplant 2011;25:119–125. [DOI] [PubMed] [Google Scholar]
- 11. Acar G, Akcay A, Sayarlioglu M, et al Assessment of atrial conduction time in patients with familial Mediterranean fever. Pacing Clin Electrophysiol 2009;32:308–313. [DOI] [PubMed] [Google Scholar]
- 12. Acar G, Akcay A, Sokmen A, et al Assessment of atrial electromechanical delay, diastolic functions, and left atrial mechanical functions in patients with type 1 diabetes mellitus. J Am Soc Echocardiogr 2009;22:732–738. [DOI] [PubMed] [Google Scholar]
- 13. Acar G, Sayarlioglu M, Akcay A, et al Assessment of atrial electromechanical coupling characteristics in patients with ankylosing spondylitis. Echocardiography 2009;26:549–557. [DOI] [PubMed] [Google Scholar]
- 14. Altunkeser BB, Ozdemir K, Gok H, et al The effect of Valsalva maneuver on P wave in 12‐lead surface electrocardiography in patients with paroxysmal atrial fibrillation. Angiology 2002;53:443–449. [DOI] [PubMed] [Google Scholar]
- 15. Andrikopoulos GK, Dilaveris PE, Richter DJ, et al Increased variance of P wave duration on the electrocardiogram distinguishes patients with idiopathic paroxysmal atrial fibrillation. Pacing Clin Electrophysiol 2000;23:1127–1132. [DOI] [PubMed] [Google Scholar]
- 16. Aras D, Maden O, Ozdemir O, et al Simple electrocardiographic markers for the prediction of paroxysmal atrial fibrillation in hyperthyroidism. Int J Cardiol 2005;99:59–64. [DOI] [PubMed] [Google Scholar]
- 17. Arat N, Kacar S, Golbasi Z, et al P wave dispersion is prolonged in patients with Wilson's disease. World J Gastroenterol 2008;14:1252–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Atmaca M, Korkmaz H, Korkmaz S. P wave dispersion in patients with hypochondriasis. Neurosci Lett 2010;485:148–150. [DOI] [PubMed] [Google Scholar]
- 19. Aytemir K, Ozer N, Atalar E, et al P wave dispersion on 12‐lead electrocardiography in patients with paroxysmal atrial fibrillation. Pacing Clin Electrophysiol 2000;23:1109–1112. [DOI] [PubMed] [Google Scholar]
- 20. Barutcu I, Esen AM, Ozdemir R, et al Effect of treadmill exercise testing on P wave duration and dispersion in patients with isolated myocardial bridging. Int J Cardiovasc Imaging 2009;25:465–470. [DOI] [PubMed] [Google Scholar]
- 21. Bayar R, Emul M, Turan S, et al Electrocardiographical P wave changes after electroconvulsive therapy in patients with schizophrenia: A preliminary study. J ECT 2009;25:26–30. [DOI] [PubMed] [Google Scholar]
- 22. Bigi MA, Aslani A, Shahrzad S. Clinical predictors of atrial fibrillation in Brugada syndrome. Europace 2007;9:947–950. [DOI] [PubMed] [Google Scholar]
- 23. Buyukoglan H, Kaya MG, Ardic I, et al Assessment of atrial conduction time in patients with sarcoidosis. J Investig Med 2011;59:15–21. [DOI] [PubMed] [Google Scholar]
- 24. Cagirci G, Cay S, Karakurt O, et al P‐wave dispersion increases in prehypertension. Blood Press 2009;18:51–54. [DOI] [PubMed] [Google Scholar]
- 25. Can I, Onat AM, Aytemir K, et al Assessment of atrial conduction in patients with scleroderma by tissue Doppler echocardiography and P wave dispersion. Cardiology 2007;108:317–321. [DOI] [PubMed] [Google Scholar]
- 26. Caron MF, Song J, Ammar R, et al An evaluation of the change in electrocardiographic P‐wave variables after acute caffeine ingestion in normal volunteers. J Clin Pharm Ther 2001;26:145–148. [DOI] [PubMed] [Google Scholar]
- 27. Cetinarslan B, Akkoyun M, Canturk Z, et al Duration of the P wave and P wave dispersion in subclinical hyperthyroidism. Endocr Pract 2003;9:200–203. [DOI] [PubMed] [Google Scholar]
- 28. Dagli N, Karaca I, Yavuzkir M, et al Are maximum P wave duration and P wave dispersion a marker of target organ damage in the hypertensive population? Clin Res Cardiol 2008;97:98–104. [DOI] [PubMed] [Google Scholar]
- 29. Deniz A, Yavuz B, Aytemir K, et al Intra‐left atrial mechanical delay detected by tissue Doppler echocardiography can be a useful marker for paroxysmal atrial fibrillation. Echocardiography 2009;26:779–784. [DOI] [PubMed] [Google Scholar]
- 30. Deveci OS, Aytemir K, Okutucu S, et al Evaluation of the relationship between atrial septal aneurysm and cardiac arrhythmias via P‐wave dispersion and signal‐averaged P‐wave duration. Ann Noninvasive Electrocardiol 2010;15:157–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dilaveris P, Batchvarov V, Gialafos J, et al Comparison of different methods for manual P wave duration measurement in 12‐lead electrocardiograms. Pacing Clin Electrophysiol 1999;22:1532–1538. [DOI] [PubMed] [Google Scholar]
- 32. Dilaveris P, Raftopoulos L, Giannopoulos G, et al Prevalence of interatrial block in healthy school‐aged children: Definition by P‐wave duration or morphological analysis. Ann Noninvasive Electrocardiol 2010;15:17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dilaveris PE, Gialafos EJ, Sideris SK, et al Simple electrocardiographic markers for the prediction of paroxysmal idiopathic atrial fibrillation. Am Heart J 1998;135:733–738. [DOI] [PubMed] [Google Scholar]
- 34. Dogdu O, Yarlioglues M, Kaya MG, et al Assessment of atrial conduction time in patients with systemic lupus erythematosus. J Investig Med 2010;59:281–286. [DOI] [PubMed] [Google Scholar]
- 35. Duru M, Seyfeli E, Kuvandik G, et al Effect of weight loss on P wave dispersion in obese subjects. Obesity (Silver Spring) 2006;14:1378–1382. [DOI] [PubMed] [Google Scholar]
- 36. Emul M, Dalkiran M, Coskun O, et al P wave and QT changes among inpatients with schizophrenia after parenteral ziprasidone administration. Pharmacol Res 2009;60:369–372. [DOI] [PubMed] [Google Scholar]
- 37. Erbay AR, Turhan H, Yasar AS, et al Effects of long‐term beta‐blocker therapy on P‐wave duration and dispersion in patients with rheumatic mitral stenosis. Int J Cardiol 2005;102:33–37. [DOI] [PubMed] [Google Scholar]
- 38. Gen R, Akbay E, Camsari A, et al P wave dispersion in endogenous and exogenous subclinical hyperthyroidism. J Endocrinol Invest 2010;33:88–91. [DOI] [PubMed] [Google Scholar]
- 39. Gialafos EJ, Dilaveris PE, Synetos AG, et al P wave analysis indices in young healthy men: Data from the digital electrocardiographic study in Hellenic Air Force Servicemen (DEHAS). Pacing Clin Electrophysiol 2003;26:367–372. [DOI] [PubMed] [Google Scholar]
- 40. Guler H, Seyfeli E, Sahin G, et al P wave dispersion in patients with rheumatoid arthritis: Its relation with clinical and echocardiographic parameters. Rheumatol Int 2007;27:813–818. [DOI] [PubMed] [Google Scholar]
- 41. Gunes Y, Tuncer M, Guntekin U, et al The effects of trimetazidine on p‐wave duration and dispersion in heart failure patients. Pacing Clin Electrophysiol 2009;32:239–244. [DOI] [PubMed] [Google Scholar]
- 42. Guntekin U, Gunes Y, Tuncer M, et al Long‐term follow‐up of P‐wave duration and dispersion in patients with mitral stenosis. Pacing Clin Electrophysiol 2008; 31:1620–1624. [DOI] [PubMed] [Google Scholar]
- 43. Guntekin U, Gunes Y, Simsek H, et al P wave duration and dispersion in patients with hyperthyroidism and the short‐term effects of antithyroid treatment. Indian Pacing Electrophysiol J 2009;9:251–259. [PMC free article] [PubMed] [Google Scholar]
- 44. Gur M, Yilmaz R, Demirbag R, et al Relation between P‐wave dispersion and left ventricular geometric patterns in newly diagnosed essential hypertension. J Electrocardiol 2008;41:54e1–6. [DOI] [PubMed] [Google Scholar]
- 45. Guray U, Guray Y, Mecit B, et al Maximum p wave duration and p wave dispersion in adult patients with secundum atrial septal defect: The impact of surgical repair. Ann Noninvasive Electrocardiol 2004;9:136–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Guray U, Guray Y, Yylmaz MB, et al Evaluation of P wave duration and P wave dispersion in adult patients with secundum atrial septal defect during normal sinus rhythm. Int J Cardiol 2003;91:75–79. [DOI] [PubMed] [Google Scholar]
- 47. Hallioglu O, Aytemir K, Celiker A. The significance of P wave duration and P wave dispersion for risk assessment of atrial tachyarrhythmias in patients with corrected tetralogy of Fallot. Ann Noninvasive Electrocardiol 2004;9:339–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ho TF, Chia EL, Yip WC, et al Analysis of P wave and P dispersion in children with secundum atrial septal defect. Ann Noninvasive Electrocardiol 2001;6:305–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Imamoglu EY, Oztunc F, Eroglu AG, et al Dispersion of the P wave as a test for cardiac autonomic function in diabetic children. Cardiol Young 2008;18:581–585. [DOI] [PubMed] [Google Scholar]
- 50. Karaahmet T, Tigen K, Gurel E, et al Impact of systemic sclerosis on electromechanical characteristics of the heart. Heart Vessels 2010;25:223–228. [DOI] [PubMed] [Google Scholar]
- 51. Karakaya O, Saglam M, Barutcu I, et al Comparison of the predictors for atrial rhythm disturbances between trained athletes and control subjects. Tohoku J Exp Med 2005;207:165–170. [DOI] [PubMed] [Google Scholar]
- 52. Katircibasi MT, Deniz F, Pamukcu B, et al Effects of short‐term propylthiouracil treatment on p wave duration and p wave dispersion in patients with overt hypertyroidism. Exp Clin Endocrinol Diabetes 2007;115:376–379. [DOI] [PubMed] [Google Scholar]
- 53. Kocer A, Karakaya O, Kargin R, et al P wave duration and dispersion in multiple sclerosis. Clin Auton Res 2005;15:382–386. [DOI] [PubMed] [Google Scholar]
- 54. Koken R, Demir T, Sen TA, et al The relationship between P‐wave dispersion and diastolic functions in diabetic children. Cardiol Young 2010;20:133–137. [DOI] [PubMed] [Google Scholar]
- 55. Kose S, Kilic A, Iyisoy A, et al P wave duration and P dispersion in healthy children. Turk J Pediatr 2003;45:133–135. [PubMed] [Google Scholar]
- 56. Kurt E, Emul M, Ozbulut O, et al Is valproate promising in cardiac fatal arrhythmias? Comparison of P‐ and Q‐wave dispersion in bipolar affective patients on valproate or lithium‐valproate maintenance therapy with healthy controls. J Psychopharmacol 2009;23:328–333. [DOI] [PubMed] [Google Scholar]
- 57. Metin G, Yildiz M, Bayraktar B, et al Assessment of the p wave dispersion and duration in elite women basketball players. Indian Pacing Electrophysiol J 2010;10:10–20. [PMC free article] [PubMed] [Google Scholar]
- 58. Okutucu S, Evranos B, Aytemir K, et al Relationship between atrial septal aneurysms and atrial electromechanical delay. Int J Cardiovasc Imaging 2011;27:505–513. [DOI] [PubMed] [Google Scholar]
- 59. Ozer N, Yavuz B, Can I, et al Doppler tissue evaluation of intra‐atrial and interatrial electromechanical delay and comparison with P‐wave dispersion in patients with mitral stenosis. J Am Soc Echocardiogr 2005;18:945–948. [DOI] [PubMed] [Google Scholar]
- 60. Ozmen N, Cebeci BS, Kardesoglu E, et al P wave dispersion is increased in pulmonary stenosis. Indian Pacing Electrophysiol J 2006;6:25–30. [PMC free article] [PubMed] [Google Scholar]
- 61. Ozmen N, Cebeci BS, Yiginer O, et al P‐wave dispersion is increased in pregnancy due to shortening of minimum duration of P: Does this have clinical significance? J Int Med Res 2006;34:468–474. [DOI] [PubMed] [Google Scholar]
- 62. Ozyilmaz I, Eroglu AG, Guzeltas A, et al Duration and dispersion of the P wave after the Senning operation. Cardiol Young 2009;19:615–619. [DOI] [PubMed] [Google Scholar]
- 63. Pala S, Tigen K, Karaahmet T, et al Assessment of atrial electromechanical delay by tissue Doppler echocardiography in patients with nonischemic dilated cardiomyopathy. J Electrocardiol 2010;43:344–350. [DOI] [PubMed] [Google Scholar]
- 64. Russo V, Ammendola E, De Crescenzo I, et al Severe obesity and P‐wave dispersion: The effect of surgically induced weight loss. Obes Surg 2008;18:90–96. [DOI] [PubMed] [Google Scholar]
- 65. Sari I, Davutoglu V, Ozbala B, et al Acute sleep deprivation is associated with increased electrocardiographic P‐wave dispersion in healthy young men and women. Pacing Clin Electrophysiol 2008;31:438–442. [DOI] [PubMed] [Google Scholar]
- 66. Sari I, Zengin S, Ozer O, et al Chronic carbon monoxide exposure increases electrocardiographic P‐wave and QT dispersion. Inhal Toxicol 2008;20:879–884. [DOI] [PubMed] [Google Scholar]
- 67. Senen K, Turhan H, Riza Erbay A, et al P‐wave duration and P‐wave dispersion in patients with dilated cardiomyopathy. Eur J Heart Fail 2004;6:567–569. [DOI] [PubMed] [Google Scholar]
- 68. Seyfeli E, Duru M, Kuvandik G, et al Effect of obesity on P‐wave dispersion and QT dispersion in women. Int J Obes (Lond) 2006;30:957–961. [DOI] [PubMed] [Google Scholar]
- 69. Sozen AB, Cefle K, Kudat H, et al Atrial and ventricular arryhthmogenic potential in Turner Syndrome. Pacing Clin Electrophysiol 2008;31:1140–1145. [DOI] [PubMed] [Google Scholar]
- 70. Tamer A, Gunduz H, Ozyildirim S. The cardiac effects of a mobile phone positioned closest to the heart. Anadolu Kardiyol Derg 2009;9:380–384. [PubMed] [Google Scholar]
- 71. Tukek T, Akkaya V, Demirel S, et al Effect of Valsalva maneuver on surface electrocardiographic P‐wave dispersion in paroxysmal atrial fibrillation. Am J Cardiol 2000;85:896–899, A10. [DOI] [PubMed] [Google Scholar]
- 72. Tukek T, Yildiz P, Akkaya V, et al Factors associated with the development of atrial fibrillation in COPD patients: The role of P‐wave dispersion. Ann Noninvasive Electrocardiol 2002;7:222–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Turhan H, Yetkin E, Senen K, et al Effects of percutaneous mitral balloon valvuloplasty on P‐wave dispersion in patients with mitral stenosis. Am J Cardiol 2002;89:607–609. [DOI] [PubMed] [Google Scholar]
- 74. Turhan H, Yetkin E, Atak R, et al Increased p‐wave duration and p‐wave dispersion in patients with aortic stenosis. Ann Noninvasive Electrocardiol 2003;8:18–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Turkmen M, Barutcu I, Esen AM, et al Effect of slow coronary flow on P‐wave duration and dispersion. Angiology 2007;58:408–412. [DOI] [PubMed] [Google Scholar]
- 76. Uyarel H, Ozdol C, Karabulut A, et al Acute alcohol intake and P‐wave dispersion in healthy men. Anadolu Kardiyol Derg 2005;5:289–293. [PubMed] [Google Scholar]
- 77. Uyarel H, Kasikcioglu H, Dayi SU, et al Anxiety and P wave dispersion in a healthy young population. Cardiology 2005;104:162–168. [DOI] [PubMed] [Google Scholar]
- 78. Wong T, Davlouros PA, Li W, et al Mechano‐electrical interaction late after Fontan operation: Relation between P‐wave duration and dispersion, right atrial size, and atrial arrhythmias. Circulation 2004;109:2319–2325. [DOI] [PubMed] [Google Scholar]
- 79. Yasar E, Yilmaz B, Yasar AS, et al Effect of autonomic dysfunction on p‐wave dispersion in patients with chronic spinal cord injury. Am J Phys Med Rehabil 2010;89:824–830. [DOI] [PubMed] [Google Scholar]
- 80. Yavuz T, Nisli K, Oner N, et al The effects of surgical repair on P‐wave dispersion in children with secundum atrial septal defect. Adv Ther 2008;25:795–800. [DOI] [PubMed] [Google Scholar]
- 81. Yavuzkir M, Ozturk A, Dagli N, et al Effect of ongoing inflammation in rheumatoid arthritis on P‐wave dispersion. J Int Med Res 2007;35:796–802. [DOI] [PubMed] [Google Scholar]
- 82. Yavuzkir M, Atmaca M, Dagli N, et al P‐wave dispersion in panic disorder. Psychosom Med 2007;69:344–347. [DOI] [PubMed] [Google Scholar]
- 83. Yazici M, Ozdemir K, Altunkeser BB, et al The effect of diabetes mellitus on the P‐wave dispersion. Circ J 2007;71:880–883. [DOI] [PubMed] [Google Scholar]
- 84. Yildiz M, Pazarli P, Semiz O, et al Assessment of P‐wave dispersion on 12‐lead electrocardiography in students who exercise regularly. Pacing Clin Electrophysiol 2008;31:580–583. [DOI] [PubMed] [Google Scholar]
- 85. Yucel O, Yildiz M, Altinkaynak S, et al P‐wave dispersion and P‐wave duration in children with stable asthma bronchiale. Anadolu Kardiyol Derg 2009;9:118–122. [PubMed] [Google Scholar]
- 86. Malik M, Batchvarov VN. Measurement, interpretation and clinical potential of QT dispersion. J Am Coll Cardiol 2000;36:1749–1766. [DOI] [PubMed] [Google Scholar]
- 87. Kosar F, Aksoy Y, Ari F, et al P‐wave duration and dispersion in obese subjects. Ann Noninvasive Electrocardiol 2008;13:3–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Magnani JW, Johnson VM, Sullivan LM, et al P‐wave indices: Derivation of reference values from the Framingham Heart Study. Ann Noninvasive Electrocardiol 2010;15:344–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Ciaroni S, Cuenoud L, Bloch A. Clinical study to investigate the predictive parameters for the onset of atrial fibrillation in patients with essential hypertension. Am Heart J 2000;139:814–819. [DOI] [PubMed] [Google Scholar]
- 90. Michelena HI, Powell BD, Brady PA, et al Gender in atrial fibrillation: Ten years later. Gend Med 2010;7:206–217. [DOI] [PubMed] [Google Scholar]


