Abstract
A single stationary mother rotor, located in the fastest activating region and giving rise to activation fronts that propagate throughout the remainder of the myocardium, has been hypothesized to be responsible for the maintenance of ventricular fibrillation (VF). Others have reported a mother rotor in guinea pigs and rabbits. We wanted to see if a mother rotor exists in a larger heart, that is, pigs. Epicardial mapping studies have demonstrated that VF wavefronts in pigs tend to propagate from the posterior basal LV to the anterior LV and on to the anterior RV, raising the possibility of a mother rotor in the posterior LV. However, no sustained reentry consistent with a mother rotor was found on the posterior LV epicardium, even though an intramural mapping study showed that the fastest activating transmural layer was near the epicardium. Many wavefronts in the posterior LV entered the mapped region from the posterior boundary of the mapping array, adjacent to the posterior descending coronary artery, raising the possibility that a mother rotor is located in the right ventricle or septum. Since a previous study has shown that the RV activates more slowly than the LV during VF, the more likely site for a mother rotor was the septum. However, we then performed a study in which we recorded from the right side of the septum and found that reentry was uncommon there also and that the activation rate was slower than the posterobasal LV. Many of the VF wavefronts in the septum passed from the posterior septum toward the anterior septum. This fact coupled with the fact that many wavefronts passed from the posterior LV free wall toward the anterior LV free wall point to the region where the posterior free wall intersects with the septum, the region where the posterior papillary muscle is located, as the possible site of a mother rotor. Indeed, a recent abstract by others reports that, after propranolol, a stable reentrant circuit is present on the endocardium at the insertion of the posterior papillary muscle into the LV free wall in pigs.
Keywords: arrhythmias, ventricular fibrillation, cardiac mapping
ALL PORTIONS OF THE VENTRICLES MAY NOT BE EQUALLY RESPONSIBLE FOR VF MAINTENANCE
While reentry is common in guinea pig and rabbit hearts during ventricular fibrillation (VF), 1 , 2 electrical and optical cardiac mapping in dogs, pigs, and humans indicate that less than approximately 8% of wavefronts mapped on the epicardium exhibit reentry during VF. 3 , 4 , 5 , 6 , 7 , 8 Many of the remaining wavefronts are annihilated by block or collision without reentering. Transmural electrical mapping from a row of plunge needles in swine revealed that intramural reentry during VF is also uncommon and short‐lived in the anterior left ventricular (LV) free wall. 9 One possible reason for these findings is that, instead of all parts of the ventricles being equally responsible for VF maintenance because of wandering wavelets of activation, reentry is more common and more sustained in some regions of the ventricles than others and the studies described above did not record from these regions in which reentry is more common.
Recent experimental evidence from Jalife's and Pertsov's groups suggest that VF is maintained by reentrant circuits that are localized to a single myocardial region. 7 , 10 , 11 These same investigators determined that the peak in the power spectrum at each optical recording site varied across the surface of isolated slabs of sheep ventricular myocardium and in isolated guinea pig hearts. They also provided evidence that the peak in the power spectrum corresponded to the activation rate in the optical recordings during VF and that areas with a higher peak frequency had faster activation rates and shorter refractory periods than regions with lower peak frequencies. Their findings are consistent with those of Janse's group that determined that the activation rate from epicardial electrical recordings during VF in dog hearts supported by cardiopulmonary bypass correlated highly with the effective refractory period (ERP) before VF was induced. 12 , 13
Zaitsev et al. found that the power spectrum during VF had the same peak frequency over a mean area of 1.1 cm2 of isolated, perfused sheep RV. 7 A region in which the peak of the power spectrum was the same was called a domain. Optical recordings indicated that a single domain of highest peak frequency was present, called the dominant domain, that was surrounded by domains of lower peak frequencies that in turn were bordered by domains of even lower peak frequencies. Many of the wavefronts that arose in the dominant domain propagated into domains with lower peak frequencies. 1 Other wavefronts in the dominant domain blocked at the boundary with other domains in a Wenckebach pattern. These findings suggest that all portions of the myocardium are not equally responsible for VF maintenance. Rather, VF is maintained by a single, stationary, stable reentrant circuit, that is, the mother rotor, in the dominant domain, which has the shortest refractory period, and from which activations propagate into domains with longer refractory periods. According to this concept, the reason reentry is uncommon in the studies mentioned in the first paragraph is that they did not record from the mother rotor in the dominant domain. Not all investigators agree with these ideas. For example, Salama's group did not find evidence for stable frequency domains in the guinea pig heart. 14
If a dominant domain is present, it has important implications for the treatment or prevention of VF. Even if a long‐lived, fixed location rotor is not present in the dominant domain, less stable reentrant circuits there may still give rise to daughter wavefronts that propagate into the slower activating domains to help maintain VF. For example, ablation, pacing, or a small shock delivered to the dominant domain containing the mother rotor might prevent VF from being initiated or prevent it from being sustained.
VF IS NOT A SINGLE ELECTROPHYSIOLOGIC STATE WITH A SINGLE LEVEL OF ORGANIZATION
High‐speed cinematography and electrical mapping have shown that VF passes through stages with different levels of organization as its duration lengthens. 15 , 16 Several lines of evidence suggest that VF in different animals or patients can differ markedly, even after the same duration. Besides differences among species, causes for these differences include cardiac diseases and drugs. For example, some drugs raise the activation rate during VF while other drugs lower it. 17 In some species such as rabbits and armadillos, VF will frequently terminate spontaneously, while in others such as pigs and sheep, VF is almost always sustained. 18 While VF is usually sustained in humans, it occasionally breaks spontaneously. 19 When VF is induced during implantable cardioverter‐defibrillator implantation in patients with a low ejection fraction (EF) and dilated ventricles, the activation rate is typically slower than in other patients 20 and the DFT is not markedly increased even though their hearts are much larger than in other patients. 21 In dogs, the DFT is greatly elevated in the presence of acute ischemia, 22 , 23 but not MI. 24 , 25
In a series of recent articles, Chen's laboratory has reported that there are two distinct types of VF. 26 , 27 , 28 In Type I VF, the activation rate is rapid, reentry is uncommon and short‐lived, and wavefronts follow constantly changing pathways with little repetition, consistent with wandering wavelets. While the conduction velocity restitution curve is flat, the action potential duration (APD) restitution curve is steep, and Chen and coworkers believe this steep APD restitution curve is responsible for conduction block during Type I VF. In Type II VF, the activation rate is slower and many wavefronts are large and follow similar pathways, which Chen et al. believe is consistent with VF maintenance by a mother rotor. Whereas the APD restitution slope is flat, excitability is reduced and the conduction velocity restitution curve is broad, leading Chen et al. to believe that this broad conduction velocity restitution is responsible for conduction block in Type II VF. The type of VF appears to be influenced by the duration of VF, the presence of drugs, and the presence of heart disease. 26 , 27 , 28 All these findings indicate that VF is not a single electrophysiologic state with a single level of organization.
THE IMPORTANCE OF DIFFERENT MECHANISMS FOR CAUSING BLOCK AND REENTRY DURING VF IS NOT KNOWN
Several mechanisms for block, which can lead to reentry, have been proposed. The classical mechanism is nonuniform dispersion of refractoriness. 29 An increase in the nonuniformity of dispersion of refractoriness increases the inducibility of VF 30 and also increases the duration of sustained VF in animals in which VF tends to stop spontaneously. 31 This mechanism is also supported by the finding that a nonuniform dispersion of refractoriness is present during VF. 32 Anatomic structure has also been proposed as a mechanism that can influence the occurrence of block and reentry. For example, studies from Chen's laboratory indicate that block during VF occurs frequently along the paths of coronary vessels 33 and that reentry circuits occur around the insertion of papillary muscles into the ventricular walls. 34 , 35 , 36 Block has been reported more likely to occur when wavefronts travel along the long axis of the myocardial fibers, 37 while under other conditions block has been reported to be more likely when wavefronts travel across the long axis of the fibers. 38
Recently, mechanisms for block and reentry have been suggested that can occur even in tissue whose properties are totally homogeneous. In computer and chemical models of excitable media, block leading to reentry can be induced in a homogeneous medium. 39 An alternans in APD and/or cycle length has been shown to be a precursor to block. 40 , 41 It has been shown by computer simulation that reentrant spirals become unstable and break down into multiple spirals when the slope of the restitution curve for refractoriness or conduction velocity is greater than 1. 42 , 43 , 44 When the slope is greater than 1, oscillations in APDs increase with each oscillatory cycle until block occurs. It has also been shown in a small piece of isolated heart tissue that the slope of the restitution curve is greater than 1 during VF and that a drug that decreases this slope to less than 1 terminated VF. 45
VF may be maintained by more than one of these mechanisms. VF may be maintained by a mother rotor about an anatomic obstacle with drugs that increase VF organization (Type II VF), while VF may be maintained by wandering wavelets with block caused by restitution (Type I VF) with other drugs that decrease VF organization. For example, Wu et al. have reported Type I VF with drifting rotors in normal rabbit hearts, which is converted by the drug D600 to Type II VF with a fixed rotor anchored at the insertion of the anterior papillary muscle within the dominant domain. 34 It is crucial to know which of these hypothesized mechanisms are most important for the maintenance of VF because pharmacologic agents and/or alterations of autonomic tone could be effective in preventing one of these possible mechanisms, but could create conditions more conducive to the maintenance of VF by other possible mechanisms.
GLOBAL EPICARDIAL DISTRIBUTION OF VF ACTIVATION RATE
The studies of the mother rotor by Jalife's and Pertsov's groups were performed in isolated slabs of sheep ventricle 7 , 10 , 46 or in isolated rabbit or guinea pig hearts. 6 , 11 The mass of rabbit and guinea pig hearts is less than 2% of human hearts. The fact that a mother rotor exists in a small heart does not necessarily indicate that it exists in a heart 50 times larger. Therefore, we have performed several studies to determine if a mother rotor exists in swine, in which the heart is about half the size of human hearts. In the first study, recordings were made with a 504 electrode sock pulled over the ventricular epicardium to determine if there are different domains of activation rates during VF in pigs. 47 The mother rotor hypothesis proposes that VF is maintained by a single reentrant rotor in the most rapidly activating portion of the myocardium, giving rise to activation fronts that propagate into slower activating regions, where they frequently block. We tested two predictions of this hypothesis during VF: (1) there should be a single maximum in the distribution of activation rates with the activation rate decreasing with distance away from the maximum and (2) the incidence of block should be greater outside than inside the myocardial region with the fastest activation rate.
Six 25 seconds VF episodes from each of six pigs were recorded from 504 electrodes over the entire ventricular epicardium. The electrodes were divided into four zones of equal area: LV base and apex (LVB and LVA) and RV base and apex (RVB and RVA). A Fast Fourier transform (FFT) was performed on each electrogram and the mean activation rate was estimated from the dominant (peak) frequency (DF) while block was estimated to be present during those time intervals when double peaks (DPs) were present in the power spectrum. 48 The zones had statistically significant distributions of DF incidence (Fig. 1, LVB > LVA > RVA > RVB) and DP incidence (RVA > RVB > LVA > LVB). Thus, LV epicardium has a higher estimated VF activation rate and lower block incidence than RV epicardium, and the apex has a lower estimated activation rate and higher block incidence than the base. These findings raise the possibility of a single rotor maintaining VF in the LV base.
Figure 1.
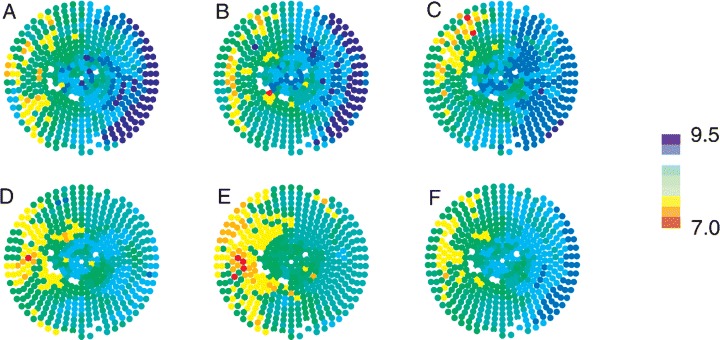
DF distribution during a VF episode. Panels A–E show maps of the mean DF at each electrode for 5–10, 10–15, 15–20, 20–25, and 25–30 seconds, respectively. Panel F shows the mean DF at each electrode over the entire 25 second VF episode. The right of each map denotes the LV and the left denotes the RV. The apex of the heart is central, surrounded by the base. LAD is at top. The color scale on the right denotes mean DF in Hertz. The white sites denote bad electrodes that were not included in the analysis (used with the publisher's permission from Newton et al. 47 ).
GLOBAL TRANSMURAL DISTRIBUTION OF VF ACTIVATION RATE
The main limitation of the previous study was that electrodes were confined to the epicardium, so it was not known if yet faster activating regions were located intramurally. Therefore, we performed a similar study with plunge needle electrodes to determine if there are different transmural domains of activation rate during VF. 49 We quantified the distribution of activation rate, conduction block, and organization transmurally throughout both ventricular free walls during VF in pigs and dogs, in which the Purkinje distribution differs. Plunge needles, containing four electrodes for the RV and six electrodes for the LV spaced 2 mm apart, were inserted into six pig and five dog hearts in vivo. VF was initiated and allowed to continue for 45 seconds in five episodes and for over 3 minutes in a sixth episode. From the FFT power spectrum, DF and DP were calculated for each electrode recording.
DF increased (Fig. 2) while DP decreased from endocardium to epicardium of the entire LV and the RV base in pigs and dogs for the first 70 seconds of VF. These transmural distributions of DF and DP reversed in dogs by 2 minutes, but not in pigs. Thus, estimated activation rates, amount of block, and level of organization are not uniformly distributed across the ventricular walls during VF. The faster activating epicardial regions experience less block and are more organized than the slower activating endocardial regions. These VF descriptors have similar distributions in both pigs and dogs, suggesting both species may have a similar mechanism of VF maintenance. The fact that the fastest epicardial domain is on the epicardium is fortuitous for epicardial and electrical optical mapping, since this imaging modality is currently limited to recording from the heart surface.
Figure 2.
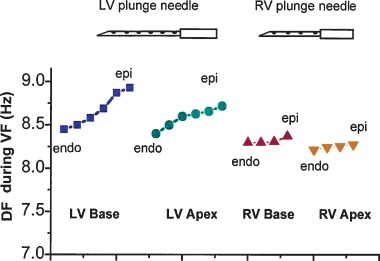
Mean transmural DF distribution in a pig during the first 5 seconds of VF. Each data point represent the mean DF at plunge needle electrodes with the most endocardial (endo) electrode to the left and the most epicardial (epi) electrode to the right for the four regions (LV base, LV apex, RV base, and RV apex). In all four regions, the electrodes near the epicardium had a higher frequency, suggesting a faster activation rate, than the electrodes near the endocardium.
REGIONAL DIFFERENCES IN LV ACTIVATION SEQUENCES DURING VF
The results of the previous two studies indicate that the fastest activation rate in the ventricular free walls during VF is near the epicardium in the basal half of the LV. The electrodes in the two previous studies were too far apart to allow accurate mapping of VF activation sequences. Therefore, we performed a study in which we recorded from 2 of our 528 channel mapping systems simultaneously to record from over 1000 electrodes totally covering the base of the LV with electrodes 2 mm apart, which is sufficiently close to track the epicardial activation sequences. 8 We investigated the quantitative characteristics of the VF wavefronts, separately in the posterior half and the anterior half of the mapped LV region. If the region with the highest activation rate, the dominant domain, contains a mother rotor that spawns daughter wavefronts, which propagate out to maintain VF in the other regions, then the ratio of waves that propagate off a region to those that propagate onto it (propoff/propon) should be greater than 1 for the dominant domain and less than 1 in adjacent domains.
The VF activation rate in the seven pigs studied was significantly faster in the posterior than the anterior LV (10.0 ± 1.3 Hz vs 9.3 ± 1.3 Hz). The anterior LV had a significantly higher fraction of wavefronts that blocked than did the posterior LV and had a propoff/propon ration less than 1. The mean conduction velocity vectors of the VF wavefronts pointed from the posterior to the anterior LV (Fig. 3). While these findings favor a dominant domain in the posterior LV, the facts that the posterior LV had a lower incidence of reentry than the anterior LV and that the posterior LV did not have a propoff/propon ration significantly different from 1 do not. Thus, quantitative regional differences are present over the porcine LV epicardium during VF. However, these differences are not totally consistent with a dominant domain within the base of the LV free wall, even though our previous study showed it was the fastest activating epicardial region.
Figure 3.
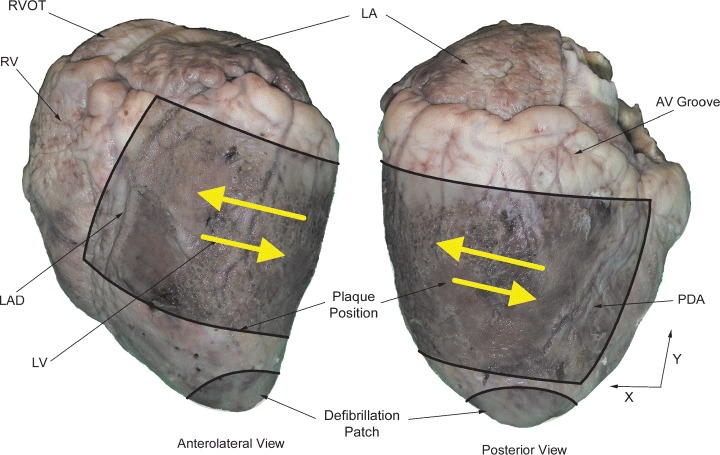
Picture of the mapped region with large arrows indicating the mean direction of propagation of VF wavefronts in six of the seven hearts. The mean direction of propagation for the seventh heart is in the opposite direction as indicated by small arrows.
The propoff/propon ratio of the posterior border of the mapped region adjacent to the posterior descending coronary artery (PDA) was significantly less than 1 while the propoff/propon ratio of the anterior boundary of the mapped region adjacent to the left anterior descending coronary artery (LAD) was significantly greater than 1 (Fig. 4). Thus, there was a significant tendency for activation fronts to enter the mapped region from the posterior interventricular groove and to leave the mapped region toward the anterior interventricular groove. Therefore, if a dominant domain is the source of these wavefronts, it could be located in either the posterior RV or the septum. However, our previous studies demonstrated that the RV activates significantly slower than the LV during VF (Figs. 1 and 2). Thus, we decided to next look for a dominant domain within the septum.
Figure 4.
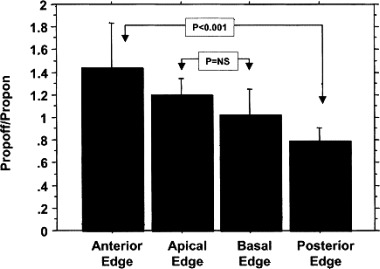
Propoff/propon ratios for the four edges of the mapped region. Wavefronts tended to enter posteriorly and leave anteriorly. Mean ± 95% confidence intervals are shown. P < 0.001 indicates statistical significance; P = ns indicates no statistical significance (used with the publisher's permission). 8
COMPARISON OF VF ACTIVATION PATTERNS IN THE SEPTUM AND LV
Therefore, we next performed a study in which we mapped activation sequences on the right side of the interventricular septum. 50 Recordings during VF were made from two plaques, one on the posterior base of the LV and the other on the base of the right side of the septum (Fig. 5). First, the plaque was sutured to the posterior basal LV and VF was recorded (before pump group). Next, cardiopulmonary bypass was instituted and VF was again recorded (before incision group). Then, an incision was made through the RV free wall to allow access to the right side of the septum. The second mapping plaque was sutured to the septum and VF was again induced while recordings were made simultaneously from both mapping plaques (after incision group). The first two sets of VF recordings from the plaque on the posterior basal LV served as controls to determine if instituting cardiopulmonary bypass and making an incision through the RV altered VF activation in the posterior LV.
Figure 5.
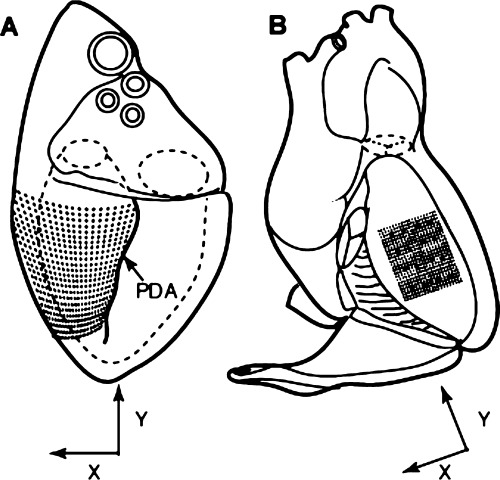
Diagrams of the heart indicating the location of the mapping electrodes on the LV (A) and the right side of the septum (B). The black dots represent the individual plaque recording electrodes while the 16 stars represent the locations of the plunge needle electrodes. The directions of the x and y components of the conduction velocity vectors of the VF wavefronts are also indicated. PDA = posterior descending coronary artery (used with the publisher's permission). 50
The activation rate in the septum, 8.6 ± 3.0 Hz, was significantly slower than in the posterior basal LV, 10.4 ± 3.4 Hz. Also, reentry was rare in the septal recordings, being present in only 1% of wavefront families, which is even lower than the 2% incidence of reentry in the posterior LV. The mean direction of the VF wavefronts was not altered by bypass and RV incision (Fig. 6). The mean x velocity for the LV was in the direction from the PDA around the free wall to the LAD, consistent with our previous study (Fig. 3). 8 The y‐velocity vector for the posterior LV was from the apex toward the base (Fig. 6). On the right side of the septum, the x‐velocity vector pointed in the opposite direction compared to the LV, from the PDA through the septum toward the LAD (Fig. 6). The y‐velocity vector in the septum was not significantly different than 0.
Figure 6.
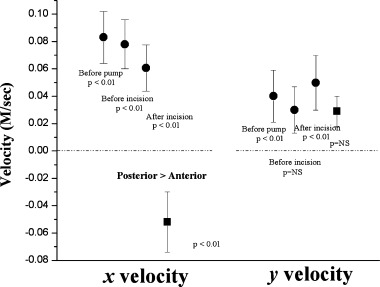
Mean x and y components of the velocity vectors for VF wavefronts in the LV (circles) and septum (squares). The mean ± 95% confidence intervals in m/s are shown. P values represent the probability that the mean velocity is different than 0 (used with the publisher's permission). 50
Toward the end of the study, the mapping array on the right side of the septum was removed, and 16 plunge needles were inserted to form a 4 × 4 matrix distributed throughout the region previously mapped with the plaque (Fig. 5). Each plunge needle contained three electrodes spaced 1 cm apart to record from the subendocardium of the LV side of the septum, the middle of the septum, and the subendocardium of the RV side of the septum. The peak frequency measured from the left side, mid, and right side of the septum was 8.6 ± 2.2, 8.7 ± 1.6, and 8.9 ± 1.9 Hz, respectively (P = ns).
The following findings suggest that a dominant domain is not located in the central portion of the septum: (1) reentry is less frequent on the right side of the septum than on the posterior basal LV epicardium, (2) the DF at the three electrodes on each septal plunge needle are not significantly different, suggesting there was not a faster activating intramural domain beneath the mapped RV side of the septum, and (3) the septal DF is slower than the LV DF. In both the LV and septal mapped regions, the mean direction of the spread of activation was away from the intersection of the posterior LV free wall and the posterior septum. This finding suggests that, if a dominant domain containing a mother rotor exists, it is where the posterior LV free wall joins the septum, which includes the posterior papillary muscle insertion. This region is on the opposite side of the heart from the anterior LV where the dominant domain and mother rotor have been reported in the guinea pigs and rabbits. 1 , 34
An abstract presented at the last Heart Rhythm Society meeting 51 supports the possible existence of a dominant domain at the posterior papillary insertion. In their study, epicardial recordings were made over the papillary muscles in pigs and endocardial noncontact mapping was performed in the LV cavity of pigs and dogs during VF. They found, particularly after giving propranolol, that reentry was present on the endocardium anchored around the posterior papillary muscle while break through was simultaneously present on the epicardium overlying the papillary muscle. They also reported that ablation lesions extending away from the posterior papillary muscle made VF much more difficult to induce. Thus, the studies reviewed in this article suggest that, if a mother rotor is present in swine, it may be present near the posterior papillary muscle.
Acknowledgments
Acknowledgments: The authors wish to thank Catherine M. Sreenan for her help in preparing this manuscript. This study was supported in part by the National Institutes of Health grants HL‐28429 and HL‐66256.
[ Michel Mirowski in Poland, 1940s Used with permission of Ariella M. Rosengard, MD. This photograph may not be reproduced, stored, or transmitted in any form or by any means without the prior permission in writing from Dr. Rosengard. ]
REFERENCES
- 1. Samie FH, Berenfeld O, Anumonwo J, et al Rectification of the background potassium current: A determinant of rotor dynamics in ventricular fibrillation. Circ Res 2001;89: 1216–1223. [DOI] [PubMed] [Google Scholar]
- 2. Jalife J, Gray RA. Drifting vortices of electrical waves underlie ventricular fibrillation in the rabbit heart. Acta Physiol Scand 1996;157: 123–131. [DOI] [PubMed] [Google Scholar]
- 3. Chen P‐S, Wolf PD, Dixon EG, et al Mechanism of ventricular vulnerability to single premature stimuli in open‐chest dogs. Circ Res 1988;62: 1191–1209. [DOI] [PubMed] [Google Scholar]
- 4. Lee JJ, Kamjoo K, Hough D, et al Reentrant wavefronts in Wiggers' stage II ventricular fibrillation. Circ Res 1996;78: 660–675. [DOI] [PubMed] [Google Scholar]
- 5. Rogers JM, Huang J, Smith WM, et al Quantitative characteristics of reentrant pathways change during the first 44 seconds of ventricular fibrillation. Circulation 1997;96: I–122. [Google Scholar]
- 6. Chen J, Mandapati R, Berenfeld O, et al High‐frequency periodic sources underlie ventricular fibrillation in the isolated rabbit heart. Circ Res 2000;86: 86–93. [DOI] [PubMed] [Google Scholar]
- 7. Zaitsev AV, Berenfeld O, Mironov SF, et al Distribution of excitation frequencies on the epicardial and endocardial surfaces of fibrillating ventricular wall of the sheep heart. Circ Res 2000;86: 408–417. [DOI] [PubMed] [Google Scholar]
- 8. Nanthakumar K, Huang J, Rogers JM, et al Regional differences in ventricular fibrillation in the open‐chest porcine left ventricle. Circ Res 2002;91: 733–740. [DOI] [PubMed] [Google Scholar]
- 9. Rogers JM, Huang J, Melnick SB, et al Sustained reentry in the left ventricle of fibrillating pig hearts. Circ Res 2003;92: 539–545. [DOI] [PubMed] [Google Scholar]
- 10. Pertsov AM, Mironov SF, Zaitsev AV, et al Visualization of stable intramural reentry during fibrillatory activity in perfused sheep right ventricle. Circulation 1999;100: I–872. [Google Scholar]
- 11. Samie FH, Mironov S, Mandapati R, et al A gradient of excitation frequencies in the ventricles of the isolated Langendorff‐perfused guinea pig during ventricular fibrillation. Circulation 1999;100: I–874. [Google Scholar]
- 12. Opthof T, Ramdat Misier AR, Coronel R, et al Dispersion of refractoriness in canine ventricular myocardium: Effects of sympathetic stimulation. Circ Res 1991;68: 1204–1215. [DOI] [PubMed] [Google Scholar]
- 13. Opthof T, Dekker LRC, Coronel R, et al Interaction of sympathetic and parasympathetic nervous system on ventricular refractoriness assessed by local fibrillation intervals in the canine heart. Cardiovasc Res 1993;27: 753–759. [DOI] [PubMed] [Google Scholar]
- 14. Choi BR, Nho W, Liu T, et al Life span of ventricular fibrillation frequencies. Circ Res 2002;91: 339–345. [DOI] [PubMed] [Google Scholar]
- 15. Wiggers CJ. Studies of ventricular fibrillation caused by electric shock: Cinematographic and electrocardiographic observations of the natural process in the dog's heart: Its inhibition by potassium and the revival of coordinated beats by calcium. Am Heart J 1930;5: 351–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Huang J, Rogers JM, Killingsworth CR, et al Evolution of activation patterns during long‐duration ventricular fibrillation in dogs. Am J Physiol Heart Circ Physiol 2004;286: H1193–H1200. [DOI] [PubMed] [Google Scholar]
- 17. Babbs CF. Effects of drugs on defibrillation threshold In: Tacker WA., Jr. (ed.) Defibrillation of the Heart: ICDs, AEDs, and Manual. St. Louis , Mosby‐Year Book, Inc, 1994, pp. 223–258. [Google Scholar]
- 18. Manoach M, Netz H, Erez M, et al Ventricular self‐defibrillation in mammals: Age and drug dependence. Age Ageing 1980;9: 112–116. [DOI] [PubMed] [Google Scholar]
- 19. Cafri C, Ilia R, Battler A. Self‐terminating ventricular fibrillation during variant angina–a case report. Angiology 1998;49: 581–584. [DOI] [PubMed] [Google Scholar]
- 20. Fenelon G, Stambler BS, Huvelle E, et al Left ventricular dysfunction is associated with prolonged average ventricular fibrillation cycle length in patients with implantable cardioverter defibrillators. J Interv Card Electrophysiol 2002;7: 249–254. [DOI] [PubMed] [Google Scholar]
- 21. Muratore C, Rabinovich R, Iglesias R, et al Implantable cardioverter defibrillators in patients with Chaga's disease: Are they different from patients with coronary disease? Pacing Clin Electrophysiol 1997;20: 194–197. [DOI] [PubMed] [Google Scholar]
- 22. Ouyang P, Brinker JA, Buckley BH, et al Ischemic ventricular fibrillation: The importance of being spontaneous. Am J Cardiol 1981;48: 455–459. [DOI] [PubMed] [Google Scholar]
- 23. Qin H, Walcott GP, Killingsworth CR, et al Impact of myocardial ischemia and reperfusion on ventricular defibrillation patterns, energy requirements, and detection of recovery. Circulation 2002;105: 2537–2542. [DOI] [PubMed] [Google Scholar]
- 24. Wharton JM, Richard VJ, Murry CE Jr, et al Electrophysiological effects of monophasic and biphasic stimuli in normal and infarcted dogs. Pacing Clin Electrophysiol 1990;13: 1158–1172. [DOI] [PubMed] [Google Scholar]
- 25. Chang MS, Inoue H, Kallok MJ, et al Double and triple sequential shocks reduce ventricular defibrillation threshold in dogs with and without myocardial infarction. J Am Coll Cardiol 1986;8: 1393–1405. [DOI] [PubMed] [Google Scholar]
- 26. Wu TJ, Lin SF, Weiss JN, et al Two types of ventricular fibrillation in isolated rabbit hearts: Importance of excitability and action potential duration restitution. Circulation 2002;106: 1859–1866. [DOI] [PubMed] [Google Scholar]
- 27. Chen PS, Wu TJ, Ting CT, et al A tale of two fibrillations. Circulation 2003;108: 2298–2303. [DOI] [PubMed] [Google Scholar]
- 28. Liu Y‐B, Pak H‐N, Lamp ST, et al Co‐existence of two types of ventricular fibrillation during acute regional ischemia in rabbit ventricles. J Cardiovasc Electrophysiol 2004: 1433–1440. [DOI] [PubMed] [Google Scholar]
- 29. Moe GK, Abildskov JA, Han J. Factors responsible for the initiation and maintenance of ventricular fibrillation In: Surawicz B, Pellegrino ED. (eds.) Sudden Cardiac Death. New York, NY , Grune and Stratton, 1964. [Google Scholar]
- 30. Han J. Ventricular vulnerability to fibrillation In: Dreifus LS, Likoff W. (eds.) Cardiac Arrhythmias. New York , Grune and Stratton, Inc., 1973, pp. 87–95. [Google Scholar]
- 31. Tovar OH, Jones JL. Epinephrine facilitates cardiac fibrillation by shortening action potential refractoriness. Mol Cell Cardiol 1997;29: 1447–1455. [DOI] [PubMed] [Google Scholar]
- 32. Janse MJ. Vulnerability to ventricular fibrillation. Chaos 1998;8: 149–156. [DOI] [PubMed] [Google Scholar]
- 33. Valderrábano M, Chen PS, Lin SF. Spatial distribution of phase singularities in ventricular fibrillation. Circulation 2003;108: 354–359. [DOI] [PubMed] [Google Scholar]
- 34. Wu TJ, Lin S‐F, Baber A, et al Mother rotors and the mechanisms of D600‐induced type 2 ventricular fibrillation. Circulation 2004;110: 2110–2118. [DOI] [PubMed] [Google Scholar]
- 35. Kim YH, Xie F, Yashima M, et al Role of papillary muscle in the generation and maintenance of reentry during ventricular tachycardia and fibrillation in isolated swine right ventricle. Circulation 1999;100: 1450–1459. [DOI] [PubMed] [Google Scholar]
- 36. Valderrábano M, Lee M‐H, Ohara T, et al Dynamics of intramural and transmural reentry during ventricular fibrillation in isolated swine ventricles. Circ Res 2001;88: 839–848. [DOI] [PubMed] [Google Scholar]
- 37. Spach MS, Dolber PC, Heidlage JF. Influence of the passive anisotropic properties on directional differences in propagation following modification of the sodium conductance in human atrial muscle: A model of reentry based on anisotropic discontinuous propagation. Circ Res 1988;62: 811–832. [DOI] [PubMed] [Google Scholar]
- 38. Delgado C, Steinhaus B, Delmar M, et al Directional differences in excitability and margin of safety for propagation in sheep ventricular epicardial muscle. Circ Res 1990;67: 97–110. [DOI] [PubMed] [Google Scholar]
- 39. Winfree AT. When Time Breaks Down: The Three‐Dimensional Dynamics of Electrochemical Waves and Cardiac Arrhythmias. Princeton , NJ , Princeton University Press, 1987, pp. 1–153. [Google Scholar]
- 40. Vinet A, Roberge FA. The dynamics of sustained reentry in a ring model of cardiac tissue. Ann Biomed Eng 1994;22: 568–591. [DOI] [PubMed] [Google Scholar]
- 41. Pastore JM, Girouard SD, Laurita KR, et al Mechanism linking T‐wave alternans to the genesis of cardiac fibrillation. Circulation 1999;99: 1385–1394. [DOI] [PubMed] [Google Scholar]
- 42. Karma A. Spiral breakup in model equations of action potential propagation in cardiac tissue. Phys Rev Lett 1993;71: 1103–1106. [DOI] [PubMed] [Google Scholar]
- 43. Qu Z, Weiss JN, Garfinkel A. Cardiac electrical restitution properties and stability of reentrant spiral waves: A simulation study. Am J Physiol 1999;276: H269–H283. [DOI] [PubMed] [Google Scholar]
- 44. Cao JM, Qu Z, Kim YH, et al Spatiotemporal heterogeneity in the induction of ventricular fibrillation by rapid pacing: Importance of cardiac restitution properties. Circ Res 1999;84: 1318–1331. [DOI] [PubMed] [Google Scholar]
- 45. Gilmour RF, Watanabe M. Dynamics of circus movement re‐entry across canine Purkinje fibre‐muscle junctions. J Physiol 1994;476: 473–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zaitsev AV, Berenfeld O, Mironov SF, et al Spatial distribution of frequencies on the endocardium and epicardium of the fibrillating ventricle of the sheep heart. Circulation 1999;100: I–872. [Google Scholar]
- 47. Newton JC, Johnson PL, Justice RK, et al Estimated global epicardial distribution of activation rate and conduction block during porcine ventricular fibrillation. J Cardiovasc Electrophysiol 2002;13: 1035–1041. [DOI] [PubMed] [Google Scholar]
- 48. Evans FG, Rogers JM, Smith WM, et al Automatic detection of conduction block based on time‐frequency analysis of unipolar electrograms. IEEE Trans Biomed Eng 1999;46: 1090–1097. [DOI] [PubMed] [Google Scholar]
- 49. Newton JC, Ideker RE. Estimated global transmural distribution of activation rate and conduction block during porcine and canine ventricular fibrillation. Circ Res 2004;94: 836–842. [DOI] [PubMed] [Google Scholar]
- 50. Huang J, Walcott GP, Killingsworth CR, et al Quantification of activation patterns during ventricular fibrillation in open‐chest porcine left ventricle and septum. Heart Rhythm 2005;2: 720–728. [DOI] [PubMed] [Google Scholar]
- 51. Pak H‐N, Lim HE, Chou C‐C, et al Reentrant wavefronts anchoring to the papillary muscle during ventricular fibrillation in open chest swine and dogs (abstract). Heart Rhythm 2005;2: S87. [Google Scholar]