Abstract
Visible T‐wave alternans (TWA), a beat‐to‐beat alternation in the morphology and amplitude of the ST segment or T wave, has been observed for over a century to occur in association with life‐threatening arrhythmias in patients with acute coronary syndrome, heart failure, and cardiac channelopathies. This compelling linkage prompted development of quantitative techniques leading to FDA‐cleared commercial methodologies for measuring nonvisible levels of TWA in the frequency and time domains. The first aim of this review is to summarize evidence from more than a hundred studies enrolling a total of >12,000 patients that support the predictivity of TWA for cardiovascular mortality and sudden cardiac death.
The second focus is on the usefulness of TWA in guiding therapy. Until recently, TWA has been used primarily in decision making for cardioverter‐defibrillator implantation. Its potential utility in guiding pharmacologic therapy has been underappreciated. We review clinical literature supporting the usefulness of TWA as an index of antiarrhythmic effects and proarrhythmia for different drug classes. Beta‐adrenergic and sodium channel‐blocking agents are the most widely studied drugs in clinical TWA investigations, with both reducing TWA magnitude; the exception is patients in whom sodium channel blockade discloses the Brugada syndrome and provokes macroscopic TWA. An intriguing possibility is that TWA may help to detect beneficial effects of nonantiarrhythmic agents such as the angiotensin II receptor blocker valsartan, which indirectly protects from arrhythmia through improving myocardial remodeling. We conclude that quantitative analysis of TWA has considerable potential to guide pharmacologic intervention and thereby serve as a therapeutic target.
Ann Noninvasive Electrocardiol 2010;15(3):276–288
Keywords: T‐wave alternans, sudden cardiac death, risk stratification, antiarrhythmic effects, proarrhythmia
Decreasing the incidence of sudden death, a leading cause of mortality in the industrially developed world, necessitates a reliable index of risk that reflects fundamental electrophysiologic properties underlying arrhythmogenesis in diverse forms of cardiac disease. Such a marker should be an integral component of the causal pathway of arrhythmogenesis and thereby serve as a therapeutic target. Moreover, as the propensity for arrhythmias changes across the natural course of cardiovascular disease or in response to an intervention such as pharmacologic therapy, this tool would ideally track the concomitant alterations in susceptibility to life‐threatening arrhythmias.
As discussed below, T‐wave alternans (TWA), a beat‐to‐beat alternation in the morphology and amplitude of the ST segment or T wave, reflects temporal‐spatial heterogeneity of repolarization. 1 The evidence linking TWA with arrhythmias spans more than a century, dating from the pioneering observations of Hering in 1908. Macroscopic levels of TWA have been detected under diverse clinical conditions in association with life‐threatening arrhythmias, including acute myocardial ischemia and infarction, Prinzmetal's angina, heart failure, and channelopathies such as the Brugada and long QT syndromes. Although TWA is a unitary phenomenon, its morphology varies considerably as a function of the underlying pathology. In ambulatory patients with stable coronary heart disease, in whom ischemia is usually subendocardial, alternation is localized primarily in the first half of the T wave (Fig. 1). 2 TWA is particularly prevalent in congenital long QT syndrome patients, in whom the T wave frequently alternates above and below the isoelectric line without concomitant ST‐segment changes 3 and heralds initiation of torsades de pointes. 4 In Brugada syndrome patients, the signature ST‐T‐wave pattern is the locus of alternation. 5 Collectively, these observations indicate that repolarization alternans is fundamentally linked to arrhythmogenesis but that the underlying pathophysiology differs, requiring that therapy be tailored to address the electrophysiologic derangement.
Figure 1.
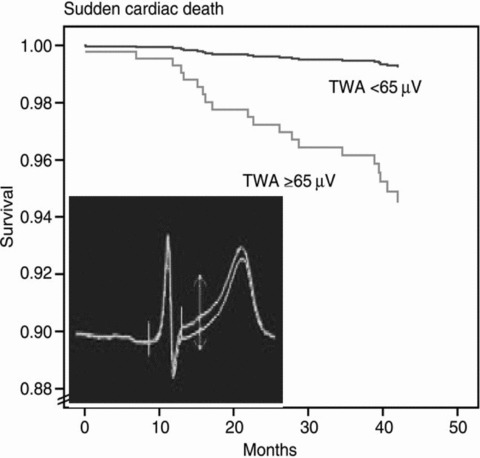
Survival curves from the Finnish Cardiovascular Study (FINCAVAS), which enrolled >1000 consecutive patients referred for routine exercise testing. 36 Inset is a high‐resolution QRS‐aligned template from a FINCAVAS patient illustrating T‐wave alternans (TWA) as the separation between successive T waves. 2 TWA was measured by the Modified Moving Average method. (Reprinted with permission from Oxford University Press)
This review has two major aims. First, we will discuss the evidence based on more than a hundred investigations enrolling a total of more than 12,000 patients that TWA is a robust marker of risk of sudden cardiac death and cardiovascular mortality. The second focus will be on the literature demonstrating the applicability of TWA in evaluating the efficacy of antiarrhythmic drugs as well as agents with proarrhythmic potential.
PHYSIOLOGIC CONSIDERATIONS FOR TWA AS A MARKER OF VULNERABILITY TO VENTRICULAR FIBRILLATION
The fundamental link between TWA and arrhythmia vulnerability is underscored by the finding that TWA magnitude exhibits a parallel time course with the spontaneous occurrence of ventricular fibrillation and tachycardia during myocardial ischemia and reperfusion. 6
Heterogeneity of Repolarization
The detailed electrophysiologic and ionic mechanisms underlying TWA have been recently reviewed. 7 , 8 , 9 , 10 Both experimental 1 , 11 , 12 and clinical evidence 13 indicates that TWA magnitude appears to parallel changes in temporal‐spatial heterogeneity of repolarization, which is a critical factor in arrhythmogenesis arising in different cardiac pathologies associated with sudden death, 9 such as ischemic heart disease, heart failure, and cardiomyopathies including Chagas’ disease. 14 The association between TWA and heterogeneity of repolarization is particularly evident during discordant TWA, wherein myocardial cells in neighboring regions alternate out‐of‐phase, thereby markedly enhancing heterogeneity of repolarization and establishing the preconditions for conduction block, reentry, and life‐threatening arrhythmias.
Intracellular Calcium Cycling
The network of related ionic mechanisms is complex, but several lines of evidence from experimental investigations affirm that instabilities in calcium handling in the sarcoplasmic reticulum give rise to cellular alternation of Ca2+, action potential duration (APD) alternans, and beat‐to‐beat alternation during repolarization. Calcium channel blockers and agents such as ryanodine, which blocks calcium release from the sarcoplasmic reticulum, have been shown to suppress pacing‐ and ischemia‐induced APD alternans in ventricular muscle fibers. 15 , 16 Moreover, APD alternans was augmented by calcium channel agonist Bay K 8644, presumably secondary to changing amounts of calcium entering through the sarcolemmal calcium channel. 16 Adenoviral overexpression of SERCA2a enhanced calcium reuptake into sarcoplasmic reticulum and reduces calcium alternans in isolated cardiomyocytes 16 and ADP alternans in Langendorff‐perfused hearts, 17 providing evidence that SERCA2a is a potential therapeutic target. Clusin 8 observed that ischemia‐induced alternation in calcium transients was temporally correlated with APD alternans and TWA. Intrapericardial delivery of the classic nitric oxide donor nitroglycerin effectively and in parallel suppressed ischemia‐induced ventricular fibrillation and TWA in intact porcines. 18 A follow‐up study 19 determined the mechanism to be an improvement in calcium handling and reduction in T‐wave heterogeneity. In a study of heart failure patients, Narayan and coworkers 20 reported that alternans of action potential amplitude, attributed to abnormalities of calcium cycling, strongly predicted ventricular tachycardia and fibrillation during a 2.6‐year follow‐up.
Heart Rate
Heart rate plays a role in TWA largely because of its impact on intracellular calcium cycling. 10 In patients with ischemic heart disease or heart failure, the capacity of the sarcoplasmic reticulum to reuptake calcium may be impaired, and TWA can be induced at low heart rates. 10 However, heart rate is not the sole determinant of TWA, as autonomic neurotransmitters and changes in myocardial substrate can provoke elevated levels of TWA during fixed rate pacing. 6 , 21 Pacing alone did not replicate the enhancement in TWA achieved by adrenergic stimulation or myocardial ischemia to a comparable heart rate. 21 In patients with a history of cardiac arrest, beta‐adrenergic stimulation with isoproterenol elicited a 2.8‐fold greater increase in TWA during electrophysiologic study compared to pacing to the same heart rate. 9 , 22
Continuum of Electrical Instability
TWA magnitude reflects the continuum of cardiac electrical instability. The higher the level of TWA, the more likely is the onset of ventricular tachyarrhythmia. Both experimentally 23 and clinically, 24 , 25 life‐threatening arrhythmias are preceded by a crescendo in the level of TWA. This continuum of instability underlies estimation of risk by quantitation of TWA 2 , 26 and the opportunity to monitor the efficacy of pharmacologic therapy.
CLINICAL MEASUREMENT OF TWA AND EVIDENCE OF PREDICTIVITY
Over the last two decades, evaluation of TWA has evolved from visual inspection of the ECG to the use of computerized analytical methods for detection of nonvisible TWA in the microvolt range. We summarize the two analytical approaches with FDA clearance in the United States. 27
Spectral Method
Briefly, the Spectral Method employs the Fast Fourier Transform to analyze the ECG across 128 consecutive J‐T segments in the frequency domain (Cambridge Heart, Inc., Tewksbury, MA, USA). The generated power spectrum at 0.5 cycle/beat, that is, occurring on every other beat, is defined as the alternans power. The test is usually conducted during bicycle or treadmill exercise. An alternans level >1.9 μV is considered a positive test, whereas test results below this level are negative. On account of relatively high incidence of indeterminate test results, between 20–40% of all cases, a new classification of “abnormal” due to patient factors has been introduced. 28 This classification is used when the test is associated with excessive ectopy, lack of capacity to reach a target rate of 105–110 beats/min, or nonsustained TWA. Abnormal test results due to these patient factors indicate the same level of risk as positive test results. Quantification of TWA with the Spectral Method provides additional insights 29 but is clearly less widely used than the binary approach.
Predictivity for sudden cardiac death and cardiovascular mortality by TWA analysis with the Spectral Method has been evaluated in >9000 patients with various types of cardiovascular disease, including ischemic heart disease, nonischemic cardiomyopathy, and heart failure. Overall, as reviewed in recent meta‐analyses, 30 , 31 TWA testing with the Spectral Method exhibits valuable predictive capacity. However, concern about its suitability to guide ICD implantation has been raised based on the results of two trials in patients with implantable cardioverter defibrillators (ICDs). TWA stratified total mortality in the MASTER trial 32 but did not predict ICD discharge in MASTER or SCD‐HeFT TWA substudy. 32 , 33 These results were disappointing in light of the fact that spectral TWA testing was advocated mainly to rule out the need for ICD devices, as the test performs particularly well in terms of negative predictive value, which is excellent, in the range of 97%. 30 A recent meta‐analysis addressing this concern indicated that predictive accuracy was strong in the TWA studies enrolling relatively few patients with ICDs, with a composite hazard ratio for prediction of abnormal vs. negative TWA of 13.6 (95% CI 8.5–30.4), although predictive accuracy in studies with high ICD use was low at 1.6 (95% CI 1.2–2.1). 30 These findings suggest that use of ICD discharge as a surrogate end point for lethal arrhythmias may have underrepresented the utility of the test. As will be discussed, there is recurring evidence of the promise of spectral TWA analysis in evaluating antiarrhythmic agents, although this was not its primary application.
To date, only the Alternans Before Cardioverter Defibrillator (ABCD) trial 34 has tested TWA's capacity to guide prophylactic ICD implantation. The study enrolled 566 patients with coronary heart disease, ejection fraction ≤40%, and nonsustained ventricular tachycardia on Holter. All patients underwent both microvolt TWA testing and electrophysiology study, with ICD implantation mandated for patients in whom either test was positive. ICDs were also inserted in 67% of patients with both negative/indeterminate TWA and negative EP study results. Event rates at the prespecified primary time point of 1 year were significantly higher in patients with either a positive TWA (hazard ratio 2.1, P = 0.03) or a positive EP study (hazard ratio 2.4, P = 0.007) than in those with both tests negative/indeterminate. Moreover, the event rate at 1 year in patients with both negative TWA and EP studies was lower than in patients with two positive tests (2% vs. 12%; P = 0.017), suggesting synergy between the tests. The ABCD study was also the first to suggest that TWA predictivity is time dependent, as TWA predicted end point events at 1 year but not at 2 years.
Modified Moving Average Method
The time‐domain Modified Moving Average (MMA) method is a more recently developed 35 and commercialized approach (GE Healthcare, Inc., Milwaukee, WI). This technique was designed to allow TWA analysis during routine exercise and ambulatory monitoring by circumventing the stationarity requirements of the Spectral Method, which mandates stabilization of heart rate for several minutes by a specialized exercise protocol or pacing. The requirement for specialized electrodes is also eliminated through advanced noise‐reduction software. The MMA algorithm continuously streams odd and even beats into separate bins and creates median complexes for each bin. These complexes are then superimposed, and the peak difference between the odd and even median complexes at any point within the JT segment is reported as the TWA value, which is updated every 10 to 15 seconds. The moving average allows control of the influence of new incoming beats on the median complexes with an adjustable update factor. A more rapid update factor provides greater sensitivity in detecting transient but clinically important surges in TWA. The recommended update factor is 1/8.
An essential component of MMA testing is the display of high‐resolution QRS‐aligned templates of the superimposed complexes, which permit visual examination to verify TWA's presence and magnitude (Fig. 1). Noise measurements are in part derived from mismatch of the median complexes outside the JT segment.
Predictivity by the two methods is comparable, consistent with the fact that they are measuring the same electrophysiologic property, although the TWA values reported with the MMA algorithm are consistently larger by a factor of 4 to 10. This difference is mainly attributable to the fact that the Spectral Method reports the average TWA level across the entire JT segment for 128 beats, whereas the MMA method reports the peak TWA level at any point within the JT segment.
Experience with the MMA TWA test, which has been used both during exercise testing and ambulatory ECG monitoring, extends to >3000 patients with preserved as well as with depressed ejection fraction (Table 1). In the initial investigation of 1000 consecutive patients referred for routine exercise testing in the Finnish Cardiovascular Study (FINCAVAS), Nieminen and coworkers 36 reported that MMA‐based TWA had a high level of predictivity for cardiovascular mortality (relative risk 6.0, 95% confidence interval [CI] 2.8–12.8) and sudden cardiac death (relative risk 7.4, 95% CI 2.8–19.4, Fig. 1). These findings were subsequently confirmed in an extended database of 2000 patients. 2 In a prospective trial of ambulatory ECG‐based TWA testing in 295 patients with left ventricular dysfunction, Sakaki, Ikeda, and colleagues 37 reported relative risks of 17.1 (95% CI 6.3–46.6, P < 0.0001) for cardiovascular mortality and 22.6 (95% CI 2.6–193.7, P < 0.005) for witnessed sudden death (Fig. 2).
Table 1.
Hazard Ratios Generated by MMA in Clinical Studies*
| Test Setting | Patient Population (Disease, Enrollment) | Left Ventricular Ejection Fraction | Hazard Ratios for End Point at Follow‐up Period |
|---|---|---|---|
| Routine Exercise Stress Testing | |||
| • FINCAVAS (Minkkinen 2009) 2 | 2119 consecutive patients referred for routine exercise testing | Mostly preserved | 6.4 for CV death, 4.6 for SCD at 47 months |
| Exercise Recovery | |||
| • REFINE/Exner 2007 75 | 322 post‐MI at 10–14 weeks after event | Moderately depressed (38–48%) | 2.94 for CV death or resuscitated cardiac arrest at 47 months |
| • REFINE/FINCAVAS/Slawnych 2009 26 | 322 post‐MI patients and 681 CAD patients | Moderately depressed (38–48%) and mostly preserved groups | 2.5 for CV death at 48 months |
| • FINCAVAS/Leino 2009 76 | Consecutive patients referred for routine exercise testing in FINCAVAS database | Mostly preserved | 3.5 for CV death at 48 months |
| Ambulatory ECG | |||
| • ATRAMI/Verrier 2003 70 | Acute post‐MI; Case control analysis (15 cases: 29 controls) | Moderately preserved (42 ± 3%) | 4.2 to 7.9 for cardiac arrest or arrhythmic death at 21 months |
| • EPHESUS/Stein 2008 72 | Acute post‐MI, LVEF ≤ 40%, and heart failure; Case control analysis (46 cases: 92 controls) | Depressed (34 ± 5%) | 5.2 to 5.5 for sudden death at 16.4 months |
| • Sakaki 2009 37 | 295 ischemic or nonischemic LV dysfunction | Depressed (34 ± 6%) | 17.1 for CV death, 22.6 for witnessed SCD at 1 year |
| • Maeda 2009 73 | 63 consecutive patients including 21 controls, 21 post‐MI without VT, and 21 post‐MI with VT | Depressed (36–43%) for post‐MI group | 6.1 for sustained VT/VF at 6 years |
| • Cardiovascular Health Study/Stein 2010 74 | Case‐control (49 cases: 98 controls) analysis of patients ≥65 years old | Not tested, assumed preserved | 4.8 for SCD at 14 years |
Figure 2.
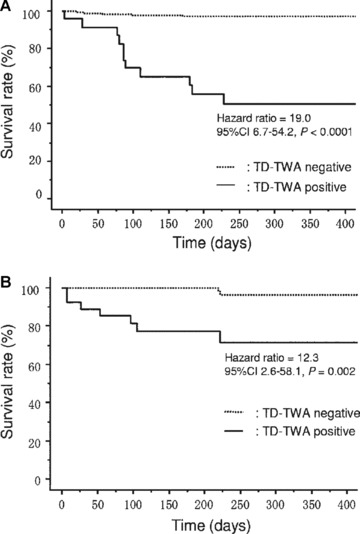
Event‐free curves for cardiac mortality using maximal voltage of time‐domain T‐wave alternans (TD‐TWA) from 24‐hour Holter ECGs in ischemic (A) and nonischemic (B) study subgroups. 37 TWA was analyzed by the Modified Moving Average method. (Reprinted with permission from Elsevier, Inc.)
CALCIUM CHANNEL BLOCKADE
Despite extensive experimental evidence suggesting the capacity of nondihydropyridine calcium channel blockers verapamil and diltiazem to suppress TWA in several experimental studies, no clinical studies regarding effects of this class of compounds on TWA have been published. In addition, definitive evidence that these agents reduce sudden cardiac death in humans is lacking. A potential explanation for this disparity is that inotropic interventions affecting interplay between uptake and release of calcium may influence cardiac outcomes by different mechanisms depending on the mechanical state of the heart. In the case of decompensated heart failure, depression of contractility by calcium channel blockade can lead to systemic hypotension, thereby reducing coronary perfusion and predisposing to myocardial ischemia and arrhythmias. In these patients, the negative inotropic effect of calcium channel blockers would be expected to overcome possible cardioprotective actions on electrophysiologic function. It is likely but untested that calcium sensitizers such as levosimendan, which is widely prescribed in Europe for acute heart failure and effectively improves pump function, might actually decrease repolarization alternans following attenuation of mechanical alternans. These surmises are based on results presented by the Multicenter Diltiazem Post‐Infarction Trial Research Group, 38 who observed a diltiazem‐related reduction in cardiac death (hazard ratio 0.77, 95% CI 0.61–0.98) for patients with preserved ventricular function but a significant diltiazem‐related increase in cardiac death (hazard ratio 1.41, 95% CI 1.01–1.96) for those with pulmonary congestion. The increase in cardiac death rate was similar among patients with left ventricular ejection fraction <40%.
BETA‐ADRENERGIC BLOCKADE
Beta‐adrenergic blocking agents are well known to decrease incidence of sudden cardiac death, and they also reduce TWA with both acute and chronic use. These agents influence calcium handling through the cyclic nucleotide cascade and, in contrast to calcium channel blockers, improve cardiac mechanical function in patients with heart failure. Klingenheben and colleagues 39 found that infusions of metoprolol, a pure beta‐blocker, or d,l‐sotalol, a beta‐blocker with Class III antiarrhythmic effects, diminished TWA magnitude in patients with documented or suspected malignant ventricular tachycardia during electrophysiologic testing. The reduction in TWA was comparable with metoprolol (by 35%, from 7.9 to 4.9 μV by spectral analysis, n = 25) and d,l‐sotalol (by 38%, from 8.6 to 4.4 μV, n = 29) (Fig. 3).
Figure 3.
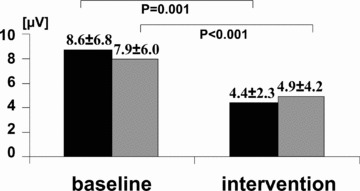
Changes in T‐wave alternans (TWA) amplitude following drug administration in patients with d,l‐sotalol (dark bars) and metoprolol (gray bars). 39 TWA was measured by the Spectral Method. (Reprinted with permission from Elsevier, Inc.)
Also during electrophysiologic testing, Rashba and colleagues 40 infused the beta‐blocker esmolol (n = 20) in patients with ischemic cardiomyopathy and inducible sustained ventricular tachycardia. Esmolol significantly diminished the absolute values of TWA and diminished the number of TWA tests classified as positive by 50%. Komiya and coworkers 41 reported a greater decrement in TWA magnitude following propranolol infusion among patients with a history of ventricular tachycardia (n = 15) than among those with a history of supraventricular tachycardia (n = 20). Despite this effect, TWA remained more sizeable in the ventricular tachycardia group than in the supraventricular tachycardia group during pacing at 110 beats/min, reflecting the greater level of cardiac electrical instability in the former group.
Murata and colleagues 42 reported a decrease in positive TWA test results along with improvement in several measures of sympathetic nerve activity and in left ventricular ejection fraction following a 3‐month course of oral beta‐adrenergic blockade. (123)I‐metaiodobenzylguanidine (MIBG) imaging and echocardiography were performed at baseline and after beta‐blocker therapy in 26 patients with nonischemic heart disease and positive TWA test results during rest or exercise. After treatment with metoprolol (mean dose 26 mg), carvedilol (11 mg), bisoprolol (5 mg), or atenolol (5 mg), TWA became negative in eight patients but remained positive although decreased in magnitude in the remaining 18 patients.
A critical consideration in TWA test protocols is whether or not to withdraw beta‐blocking agents prior to spectral TWA determination to allow patients to raise heart rates to target levels. Several investigators have advised, based on experience, against washing out antiarrhythmic medication prior to TWA testing. 39 , 43 A broader consideration is that this practice may lead to false positive test results, since beta‐blockade in long‐term use is protective against cardiovascular death.
SODIUM CHANNEL BLOCKADE
Kavesh and coworkers 44 reported that procainamide infusion decreased TWA magnitude by 43–65% during electrophysiologic study in 24 patients with inducible sustained ventricular tachycardia, but no outcomes were reported.
The prototypical late INa blocking agent ranolazine decreased ventricular vulnerability and TWA in a large animal model 45 , 46 and suppressed ventricular tachyarrhythmias in the MERLIN TIMI 36 clinical study. 47 Recently, Murdock and coworkers 48 reported parallel suppression of ventricular tachycardia and TWA with ranolazine in a patient with cardiomyopathy (Fig. 4).
Figure 4.
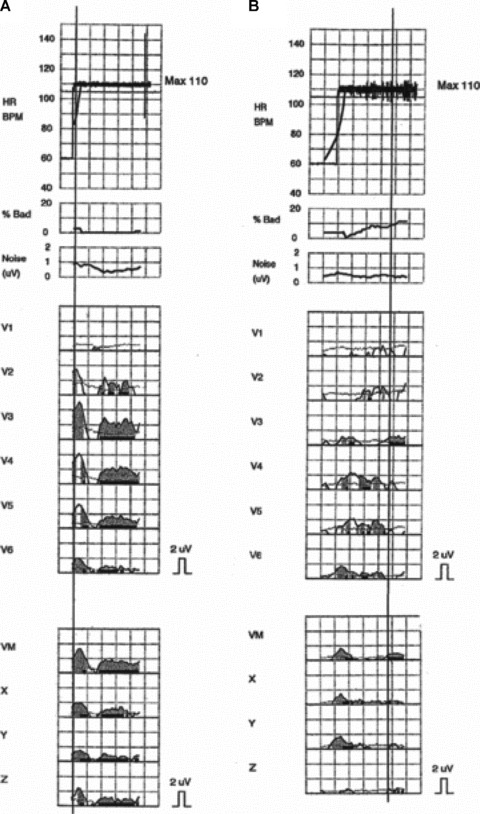
Significant microvolt T‐wave alternans, determined by the Spectral Method, was present at 110 beats/min just before (A) but not after (B) ranolazine was restarted. 48
Pharmacologic Testing to Disclose Brugada Syndrome
The Brugada syndrome is an autosomal‐dominant ion channel disorder that predisposes individuals with structurally normal hearts to ventricular arrhythmias and sudden cardiac death. Guidelines indicate ICD implantation for primary or secondary prevention of cardiac arrest. 49 The spontaneous appearance of the Brugada ECG is diagnostic, namely distinct ST‐segment elevation in the right precordial leads (V1 to V3) and incomplete or complete right bundle branch block. Visible TWA may also appear spontaneously. 50 , 51 As TWA is masked by exercise or atrial pacing in patients with this syndrome, 51 these platforms may be inappropriate for TWA‐based risk assessment. Rather, provocative testing with pilsicainide, a Class Ic drug, can be used to disclose TWA along with the diagnostic Brugada ECG. Specifically, Morita and colleagues 52 administered pilsicainide orally or intravenously to 65 patients with Brugada syndrome during electrophysiologic study to evaluate the occurrence of TWA and ventricular arrhythmia. Prior to drug delivery, TWA was not visually detected. Pilsicainide provoked macroscopic TWA in 6 of 10 patients with drug‐induced sustained polymorphic ventricular tachycardia or ventricular fibrillation but in only one of 55 patients without arrhythmia. Tada and colleagues 53 also investigated the association between ventricular fibrillation and TWA induced by intravenous pilsicainide. None of the patients exhibited TWA at baseline, but the agent provoked visible TWA in 17 (22.1%) of 77 Brugada patients. Patients with TWA also experienced a significantly higher incidence of spontaneous VF (52.9% vs 8.3%) and syncope (58.8% vs 26.7%) than their counterparts without TWA.
MULTICHANNEL BLOCKADE
Surprisingly, only limited experimental and clinical information is available regarding the effects of agents with multichannel actions, particularly amiodarone, on TWA, despite their clinical importance. Groh and colleagues 54 reported a decreased prevalence of TWA in ICD patients with ischemic or nonischemic cardiomyopathy and a history of ventricular tachycardia who were treated with amiodarone. Specifically, TWA test results were positive in only 1 (11%) of 9 patients treated with amiodarone as compared with 14 (64%) of 22 patients without the drug. Importantly, TWA status served as a predictor of appropriate ICD therapy over the follow‐up of 0.9 ± 0.2 years.
Sakabe and colleagues 55 investigated whether use of antiarrhythmic therapy disrupts TWA's predictive capacity. They prospectively evaluated 49 patients with ischemic or nonischemic‐dilated cardiomyopathy and history of sustained ventricular tachycardia or ventricular fibrillation. Amiodarone, prescribed for 28 (57%) patients, was the most common antiarrhythmic medication; additional medications included beta‐blockade in 17 (35%) and angiotensin converting enzyme (ACE) inhibition in all 49 patients. TWA was measured only once, after 2–4 weeks with antiarrhythmics, and was positive in 61% of cases. During the 13 ± 11‐month follow‐up, the investigators found that TWA test results (negative/positive), but not left ventricular ejection fraction, significantly predicted the recurrence of tachycardia (Fig. 5). These investigators concluded that TWA is capable of predicting recurrence of ventricular arrhythmias in patients receiving empirically guided pharmacologic therapy for sustained ventricular tachycardia or fibrillation.
Figure 5.
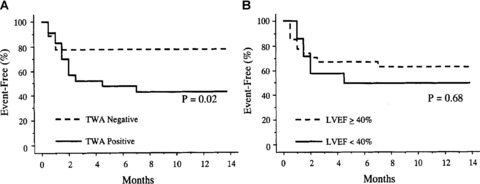
Kaplan‐Meier event‐free curves for ventricular tachyarrhythmias according to T‐wave alternans (TWA) (Panel A) and left ventricular ejection fraction (LVEF) (Panel B). 55 TWA‐positive patients had more frequent event recurrences than TWA‐negative patients (P = 0.02), whereas LVEF ≤ 40% did not distinguish between patients with and without events (P = 0.68).
Proarrhythmia
Case reports of macroscopic levels of TWA in association with proarrhythmia following amiodarone administration have appeared (Fig. 6). 56 , 57 , 58 Houltz and coworkers 59 reported visible TWA in association with torsades de pointes in patients receiving almokalant, another Class III agent, prescribed to revert atrial fibrillation or flutter. Torsades de pointes occurred in 6 of 100 patients, 3 of whom had exhibited visible TWA prior to drug infusion. Only 16 of the remaining 94 patients (17%) exhibited TWA without torsades de pointes.
Figure 6.
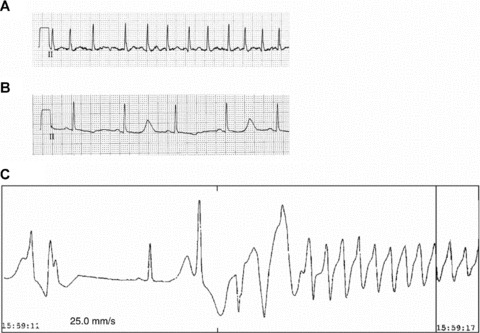
Case report of macroscopic T‐wave alternans (TWA) heralding torsades de pointes induced by intravenous amiodarone in a 62‐year‐old male patient with new‐onset atrial fibrillation and a rapid ventricular response. (A) Baseline ECG; (B) Prominent QT prolongation and macroscopic TWA appeared after restoration of sinus rhythm following 48 hours of drug exposure; and (C) Onset of torsades de pointes tachycardia that generated into ventricular fibrillation. The patient was resuscitated and QT interval normalized after amiodarone was discontinued. Genetic testing revealed a mutation at the KCNE2 gene, indicating congenital long QT syndrome VI, although the patient had no prior history of QT prolongation or ventricular tachyarrhythmias. 58 (Reprinted with permission from Oxford University Press.)
DRUGS WITH “UPSTREAM” ANTIARRHYTHMIC EFFECTS
A number of agents that effectively reduce total mortality and sudden cardiac death are not, strictly speaking, “antiarrhythmic agents” but act on “upstream” events and processes to improve the electrical stability of the myocardial substrate in patients with atherosclerosis, hypertension, ischemic heart disease, or heart failure. 60 These agents may reduce ischemia and/or fibrosis and include statins, dihydropyridine calcium channel blockers, and drugs affecting renin‐angiotensin‐aldosterone system. Their proarrhythmic potential is limited as they do not act directly on cardiac ion channels, a focus that can slow conduction, predispose to reentrant tachycardias, or prolong the long QT interval, thereby conducing to torsades de pointes.
Recently, Kubo and colleagues 61 investigated whether TWA magnitude reflects the antifibrillatory potential of the angiotensin II receptor antagonist valsartan. Sixteen of the 50 patients enrolled initially tested positive for TWA. Treatment with valsartan (80 mg/day, orally) for 3 days markedly decreased TWA from 6.1 ± 3.8 to 2.5 ± 1.9 μV (by spectral analysis), without accompanying changes in blood pressure, resting heart rate, or echocardiographic parameters.
PROARRHYTHMIA AND TWA WITH NONCARDIAC MEDICATIONS
Clinical reports concur that agents that markedly prolong the QT interval may also induce macroscopic levels of TWA that herald torsades de pointes. 56 , 57 , 58 , 59 , 62 , 63 QT interval prolongation sets the stage for increased spatial heterogeneity of repolarization across the ventricular wall, 64 leading to increased intracellular calcium levels and predisposing to afterdepolarizations.
CONCLUSIONS
The evidence cited in this review supports the pivotal role of this phenomenon in arrhythmogenesis and indicates the broad utility of TWA in sudden death risk evaluation and its potential for assessment of antiarrhythmic and proarrhythmic effects of diverse agents across differing pathologies. Although the focus of this review has been to discuss the value of TWA in guiding pharmacologic therapy, this marker may also prove to be useful in guiding nonpharmacologic approaches. The scope may include interventions such as rehabilitation following myocardial infarction, during significant substrate and neural remodeling 65 , 66 with changes in cardiac electrical instability potentially quantifiable by TWA. In heart failure patients, sleep apnea has been shown to result in high levels of TWA. 67 An intriguing question that deserves exploration is whether apnea treatment by CPAP and recent approaches such as resynchronization therapy may reduce susceptibility to cardiac arrhythmias reflected by reduction in TWA levels. 68 The recent availability of TWA testing based on ambulatory ECG recordings 24 , 37 , 69 , 70 , 71 , 72 , 73 , 74 is an important pragmatic advance, as this platform is routinely used in drug evaluation trials as well as in medical practice.
Invited review for Revista Argentina de Cardiología and Annals of Noninvasive Electrocardiology.
Conflict of Interest: Dr. Verrier is co‐inventor of the Modified Moving Average method for T‐wave alternans analysis, with patent assigned to Beth Israel Deaconess Medical Center and licensed by GE Healthcare. Dr. Nieminen declares no conflicts of interest.
REFERENCES
- 1. Nearing BD, Verrier RL. Tracking cardiac electrical instability by computing interlead heterogeneity of T‐wave morphology. J Appl Physiol 2003;95:2265–2272. [DOI] [PubMed] [Google Scholar]
- 2. Minkkinen M, Kähönen M, Viik J, et al Enhanced predictive power of quantitative TWA during routine exercise testing in the Finnish Cardiovascular Study. J Cardiovasc Electrophysiol 2009;20:408–415. [DOI] [PubMed] [Google Scholar]
- 3. Schwartz PJ, Malliani A. Electrical alternation of the T‐wave: Clinical and experimental evidence of its relationship with the sympathetic nervous system and with the long Q‐T syndrome. Am Heart J 1975;89:45–50. [DOI] [PubMed] [Google Scholar]
- 4. Noda T, Shimizu W, Satomi K, et al Classification and mechanism of Torsades de Pointes initiation in patients with congenital long QT syndrome. Eur Heart J 2004;25:2149–2154. [DOI] [PubMed] [Google Scholar]
- 5. Fish JM, Antzelevitch C. Cellular mechanism and arrhythmogenic potential of T‐wave alternans in the Brugada syndrome. J Cardiovasc Electrophysiol 2008;19:301–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nearing BD, Oesterle SN, Verrier RL. Quantification of ischaemia induced vulnerability by precordial T wave alternans analysis in dog and human. Cardiovasc Res 1994;28:1440–1449. [DOI] [PubMed] [Google Scholar]
- 7. Shimizu W, Antzelevitch C. Cellular and ionic basis for T‐wave alternans under long‐QT conditions. Circulation 1999;99:1499–1507. [DOI] [PubMed] [Google Scholar]
- 8. Clusin WT. Mechanisms of calcium transient and action potential alternans in cardiac cells and tissues. Am J Physiol Heart Circ Physiol 2008;294:H1–H10. [DOI] [PubMed] [Google Scholar]
- 9. Verrier RL, Kumar K, Nearing BD. Basis for sudden cardiac death prediction by T‐wave alternans from an integrative physiology perspective. Heart Rhythm 2009;6:416–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cutler MJ, Rosenbaum DS. Explaining the clinical manifestations of T wave alternans in patients at risk for sudden cardiac death. Heart Rhythm 2009;6:S22–S28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Konta T, Ikeda K, Yamaki M, et al Significance of discordant ST alternans in ventricular fibrillation. Circulation 1990;82:2185–2189. [DOI] [PubMed] [Google Scholar]
- 12. Pastore JM, Girouard SD, Laurita KR, et al Mechanism linking T‐wave alternans to the genesis of cardiac fibrillation. Circulation 1999;99:1385–1394. [DOI] [PubMed] [Google Scholar]
- 13. Selvaraj RJ, Picton P, Nanthakumar K, et al Endocardial and epicardial repolarization alternans in human cardiomyopathy: Evidence for spatiotemporal heterogeneity and correlation with body surface T‐wave alternans. J Am Coll Cardiol 2007;49:338–346. [DOI] [PubMed] [Google Scholar]
- 14. Garcia E de V, Samesima N, Filho HG, et al Comparison of quantitative T‐wave alternans profiles of healthy subjects and ICD patients. Ann Noninvasive Electrocardiol 2009;14:108–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Saitoh H, Bailey JC, Surawicz B. Action potential duration alternans in dog Purkinje and ventricular muscle fibers. Further evidence in support of two different mechanisms. Circulation 1989;80:1421–1431. [DOI] [PubMed] [Google Scholar]
- 16. Xie LH, Sato D, Garfinkel A, et al Intracellular Ca alternans: Coordinated regulation by sarcoplasmic reticulum release, uptake, and leak. Biophys J 2008;95:3100–3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cutler MJ, Wan X, Laurita KR, et al Targeted SERCA2a gene expression identifies molecular mechanism and therapeutic target for arrhythmogenic cardiac alternans. Circ Arrhythm Electrophysiol 2009;2:686–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kumar K, Waxman S, Nearing BD, et al Potent antifibrillatory effects of intrapericardial nitroglycerin in the ischemic porcine heart. J Am Coll Cardiol 2003;41:1831–1837. [DOI] [PubMed] [Google Scholar]
- 19. Zhao SX, Nearing BD, Verrier RL. Suppression of calcium‐induced repolarization heterogeneity as a mechanism of nitroglycerin's antiarrhythmic action. J Cardiovasc Pharmacol 2006;48:22–29. [DOI] [PubMed] [Google Scholar]
- 20. Narayan SM, Bayer JD, Lalani G, et al Action potential dynamics explain arrhythmic vulnerability in human heart failure: A clinical and modeling study implicating abnormal calcium handling. J Am Coll Cardiol 2008;52:1782–1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kovach JA, Nearing BD, Verrier RL. Angerlike behavioral state potentiates myocardial ischemia‐induced T‐wave alternans in canines. J Am Coll Cardiol 2001;37:1719–1725. [DOI] [PubMed] [Google Scholar]
- 22. Kaufman ES, Mackall JA, Julka B, et al Influence of heart rate and sympathetic stimulation on arrhythmogenic T wave alternans. Am J Physiol Heart Circ Physiol 2000;279:H1248–H1255. [DOI] [PubMed] [Google Scholar]
- 23. Nearing BD, Verrier RL. Progressive increases in complexity of T‐wave oscillations herald ischemia‐induced ventricular fibrillation. Circ Res 2002;91:727–732. [DOI] [PubMed] [Google Scholar]
- 24. Shusterman V, Goldberg A, London B. Upsurge in T‐wave alternans and nonalternating repolarization instability precedes spontaneous initiation of ventricular tachyarrhythmias in humans. Circulation 2006;113:2880–2887. [DOI] [PubMed] [Google Scholar]
- 25. Nearing BD, Mittleman MA, Burger AJ, et al Crescendo T‐wave alternans prior to VT in hospitalized decompensated heart failure patients in the PRECEDENT trial (abstract). Circulation 2008;118:S833. [Google Scholar]
- 26. Slawnych MP, Nieminen T, Kähönen M, et al REFINE (Risk Estimation Following Infarction Noninvasive Evaluation), FINCAVAS (Finnish Cardiovascular Study) Investigators. Post‐exercise assessment of cardiac repolarization alternans in patients with coronary artery disease using the modified moving average method. J Am Coll Cardiol 2009;53:1130–1137. [DOI] [PubMed] [Google Scholar]
- 27. Garcia E de V. T‐wave alternans: Reviewing the clinical performance, understanding limitations, characterizing methodologies. Ann Noninvasive Electrocardiol 2008;13:401–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kaufman ES, Bloomfield DM, Steinman RC, et al “Indeterminate” microvolt T‐wave alternans tests predict high risk of death or sustained ventricular arrhythmias in patients with left ventricular dysfunction. J Am Coll Cardiol 2006;48:1399–1404. [DOI] [PubMed] [Google Scholar]
- 29. Klingenheben T, Ptaszynski P, Hohnloser SH. Quantitative assessment of microvolt T‐wave alternans in patients with congestive heart failure. J Cardiovasc Electrophysiol 2005;16:620–624. [DOI] [PubMed] [Google Scholar]
- 30. Gehi AK, Stein RH, Metz LD, et al Microvolt T‐wave alternans for the risk stratification of ventricular tachyarrhythmic events: A meta‐analysis. J Am Coll Cardiol 2005;46:75–82. [DOI] [PubMed] [Google Scholar]
- 31. Hohnloser SH, Ikeda T, Cohen RJ. Evidence regarding clinical use of microvolt T‐wave alternans. Heart Rhythm 2009;6:S36–S44. [DOI] [PubMed] [Google Scholar]
- 32. Chow T, Kereiakes DJ, Onufer J, et al MASTER Trial Investigators. Does microvolt T‐wave alternans testing predict ventricular tachyarrhythmias in patients with ischemic cardiomyopathy and prophylactic defibrillators? The MASTER (Microvolt T Wave Alternans Testing for Risk Stratification of Post‐Myocardial Infarction Patients) trial. J Am Coll Cardiol 2008;52:1607–1615. [DOI] [PubMed] [Google Scholar]
- 33. Gold MR, Ip JH, Costantini O, et al Role of microvolt T‐wave alternans in assessment of arrhythmia vulnerability among patients with heart failure and systolic dysfunction: Primary results from the T‐wave alternans sudden cardiac death in heart failure trial substudy. Circulation 2008;118:2022–2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Costantini O, Hohnloser SH, Kirk MM, et al ABCD Trial Investigators. The ABCD (Alternans Before Cardioverter Defibrillator) Trial: Strategies using T‐wave alternans to improve efficiency of sudden cardiac death prevention. J Am Coll Cardiol 2009;53:471–479. [DOI] [PubMed] [Google Scholar]
- 35. Nearing BD, Verrier RL. Modified moving average analysis of T‐wave alternans to predict ventricular fibrillation with high accuracy. J Appl Physiol 2002;92:541–549. [DOI] [PubMed] [Google Scholar]
- 36. Nieminen T, Lehtimäki T, Viik J, et al T‐wave alternans predicts mortality in a population undergoing a clinically indicated exercise test. Eur Heart J 2007;28:2332–2337. [DOI] [PubMed] [Google Scholar]
- 37. Sakaki K, Ikeda T, Miwa Y, et al Time‐domain T‐wave alternans measured from Holter electrocardiograms predicts cardiac mortality in patients with left ventricular dysfunction: A prospective study. Heart Rhythm 2009;6:332–337. [DOI] [PubMed] [Google Scholar]
- 38. Multicenter Diltiazem Postinfarction Trial Research Group . The effect of diltiazem on mortality and reinfarction after myocardial infarction. N Engl J Med 1988;319:385–392. [DOI] [PubMed] [Google Scholar]
- 39. Klingenheben T, Gronefeld G, Li YG, et al Effect of metoprolol and d,l‐sotalol on microvolt‐level T‐wave alternans. Results of a prospective, double‐blind, randomized study. J Am Coll Cardiol 2001;38:2013–2019. [DOI] [PubMed] [Google Scholar]
- 40. Rashba EJ, Cooklin M, MacMurdy K, et al Effects of selective autonomic blockade on T‐wave alternans in humans. Circulation 2002;105:837–842. [DOI] [PubMed] [Google Scholar]
- 41. Komiya N, Seto S, Nakao K, et al The influence of beta‐adrenergic agonists and antagonists on T‐wave alternans in patients with and without ventricular tachyarrhythmia. Pacing Clin Electrophysiol 2005;28:680–684. [DOI] [PubMed] [Google Scholar]
- 42. Murata M, Harada M, Shimizu A, et al Effect of long‐term beta‐blocker therapy on microvolt‐level T‐wave alternans in association with the improvement of the cardiac sympathetic nervous system and systolic function in patients with non‐ischemic heart disease. Circ J 2003;67:821–825. [DOI] [PubMed] [Google Scholar]
- 43. Zacks ES, Morin DP, Ageno S, et al Effect of oral beta‐blocker therapy on microvolt T‐wave alternans and electrophysiology testing in patients with ischemic cardiomyopathy. Am Heart J 2007;153:392–397. [DOI] [PubMed] [Google Scholar]
- 44. Kavesh NG, Shorofsky SR, Sarang SE, et al The effect of procainamide on T wave alternans. J Cardiovasc Electrophysiol 1999;10:649–654. [DOI] [PubMed] [Google Scholar]
- 45. Kumar K, Nearing BD, Bartoli CR, et al Effect of ranolazine on ventricular vulnerability and defibrillation threshold in the intact porcine heart. J Cardiovasc Electrophysiol 2008;19:1073–1079. [DOI] [PubMed] [Google Scholar]
- 46. Nieminen T, Nanbu DY, Datti IP, et al Potent antifibrillatory effect of ranolazine during severe coronary stenosis in the intact porcine heart [abstract]. Heart Rhythm 2010;7:S111–S112. [DOI] [PubMed] [Google Scholar]
- 47. Scirica BM, Morrow DA, Hod H, et al Effect of ranolazine, an antianginal agent with novel electrophysiological properties, on the incidence of arrhythmias in patients with non ST‐segment elevation acute coronary syndrome: Results from the Metabolic Efficiency With Ranolazine for Less Ischemia in Non ST‐Elevation Acute Coronary Syndrome Thrombolysis in Myocardial Infarction 36 (MERLIN‐TIMI 36) randomized controlled trial. Circulation 2007;116:1647–1652. [DOI] [PubMed] [Google Scholar]
- 48. Murdock DK, Kaliebe J, Overton N. Ranolazine‐induced suppression of ventricular tachycardia in a patient with nonischemic cardiomyopathy: A case report. Pacing Clin Electrophysiol 2008;31:765–768. [DOI] [PubMed] [Google Scholar]
- 49. Antzelevitch C, Fish JM. Therapy for the Brugada syndrome. Handb Exp Pharmacol 2006;171:305–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ikeda T, Takami M, Sugi K, et al Noninvasive risk stratification of subjects with a Brugada‐type electrocardiogram and no history of cardiac arrest. Ann Noninvasive Electrocardiol 2005;10:396–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Nishizaki M, Fujii H, Sakurada H, et al Spontaneous T wave alternans in a patient with Brugada syndrome–responses to intravenous administration of class I antiarrhythmic drug, glucose tolerance test, and atrial pacing. J Cardiovasc Electrophysiol 2005;16:217–220. [DOI] [PubMed] [Google Scholar]
- 52. Morita H, Morita ST, Nagase S, et al Ventricular arrhythmia induced by sodium channel blocker in patients with Brugada syndrome. J Am Coll Cardiol 2003;42:1624–1631. [DOI] [PubMed] [Google Scholar]
- 53. Tada T, Kusano KF, Nagase S, et al Clinical significance of macroscopic T‐wave alternans after sodium channel blocker administration in patients with Brugada syndrome. J Cardiovasc Electrophysiol 2008;19:56–61. [DOI] [PubMed] [Google Scholar]
- 54. Groh WJ, Shinn TS, Engelstein EE, et al Amiodarone reduces the prevalence of T wave alternans in a population with ventricular tachyarrhythmias. J Cardiovasc Electrophysiol 1999;10:1335–1339. [DOI] [PubMed] [Google Scholar]
- 55. Sakabe K, Ikeda T, Sakata T, et al Predicting the recurrence of ventricular tachyarrhythmias from T‐wave alternans assessed on antiarrhythmic pharmacotherapy: A prospective study in patients with dilated cardiomyopathy. Ann Noninvasive Electrocardiol 2001;6:203–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hohnloser SH. Macroscopic T wave alternans as a harbinger of sudden death. J Cardiovasc Electrophysiol 1999;10:625. [DOI] [PubMed] [Google Scholar]
- 57. Tomcsanyi J, Somloi M, Horvath L. Amiodarone‐induced giant T wave alternans hastens proarrhythmic response. J Cardiovasc Electrophysiol 2002;13:629. [DOI] [PubMed] [Google Scholar]
- 58. Wegener FT, Ehrlich JR, Hohnloser SH. Amiodarone‐associated macroscopic T‐wave alternans and torsades de pointes unmasking the inherited long QT syndrome. Europace 2008;10:112–113. [DOI] [PubMed] [Google Scholar]
- 59. Houltz B, Darpo B, Edvardsson N, et al Electrocardiographic and clinical predictors of torsades de pointes induced by almokalant infusion in patients with chronic atrial fibrillation or flutter: a prospective study. Pacing Clin Electrophysiol 1998;21:1044–1057. [DOI] [PubMed] [Google Scholar]
- 60. Das MK, Zipes DP. Antiarrhythmic and nonantiarrhythmic drugs for sudden cardiac death prevention. J Cardiovasc Pharmacol 2010;55:438–449. [PubMed] [Google Scholar]
- 61. Kubo S, Yoshida A, Kitamura H, et al Acute effects of angiotensin II receptor blocker on ventricular repolarization alternans in chronic heart failure. Kobe J Med Sci 2008;53:365–374. [PubMed] [Google Scholar]
- 62. Yamazaki K, Terada H, Satoh H, et al Arrhythmogenic effects of arsenic trioxide in patients with acute promyelocytic leukemia and an electrophysiological study in isolated guinea pig papillary muscles. Circ J 2006;70:1407–1414. [DOI] [PubMed] [Google Scholar]
- 63. Kroll CR, Gettes LS. T wave alternans and Torsades de Pointes after the use of intravenous pentamidine. J Cardiovasc Electrophysiol 2002;13:936–938. [DOI] [PubMed] [Google Scholar]
- 64. Lakireddy V, Baweja P, Syed A, et al Contrasting effects of ischemia on the kinetics of membrane voltage and intracellular calcium transient underlie electrical alternans. Am J Physiol Heart Circ Physiol 2005;288:H400–H1407. [DOI] [PubMed] [Google Scholar]
- 65. Verrier RL, Kwaku KF. Frayed nerves in myocardial infarction: The importance of rewiring. Circ Res 2004;95:5–6. [DOI] [PubMed] [Google Scholar]
- 66. Zhou S, Chen LS, Miyauchi Y, et al Mechanisms of cardiac nerve sprouting after myocardial infarction in dogs. Circ Res 2004;95:76–83. [DOI] [PubMed] [Google Scholar]
- 67. Takasugi N, Nishigaki K, Kubota T, et al Sleep apnoea induces cardiac electrical instability assessed by T‐wave alternans in patients with congestive heart failure. Eur J Heart Fail 2009;11:1063–1070. [DOI] [PubMed] [Google Scholar]
- 68. Verrier RL, Josephson ME. Impact of sleep on arrhythmogenesis. Circ Arrhythm Electrophysiol 2009;2:450–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Verrier RL, Nearing BD, Kwaku KF. Noninvasive sudden death risk stratification by ambulatory ECG‐based T‐wave alternans analysis: Evidence and methodological guidelines. Ann Noninvasive Electrocardiol 2005;10:110–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Verrier RL, Nearing BD, La Rovere MT, et al Ambulatory electrocardiogram‐based tracking of T wave alternans in postmyocardial infarction patients to assess risk of cardiac arrest or arrhythmic death. J Cardiovasc Electrophysiol 2003;14:705–711. [DOI] [PubMed] [Google Scholar]
- 71. Lampert R, Shusterman V, Burg M, et al Anger‐induced T‐wave alternans predicts future ventricular arrhythmias in patients with implantable cardioverter‐defibrillators. J Am Coll Cardiol 2009;53:774–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Stein PK, Sanghavi D, Domitrovich PP, et al Ambulatory ECG‐based T‐wave alternans predicts sudden cardiac death in high‐risk post‐MI patients with left ventricular dysfunction in the EPHESUS study. J Cardiovasc Electrophysiol 2008;19:1037–1042. [DOI] [PubMed] [Google Scholar]
- 73. Maeda S, Nishizaki M, Yamawake N, et al Ambulatory ECG‐based T‐wave alternans and heart rate turbulence predict high risk of arrhythmic events in patients with old myocardial infarction. Circ J 2009;73:2223–2228. [DOI] [PubMed] [Google Scholar]
- 74. Stein PK, Sanghavi D, Sotoodehnia N, et al Association of Holter‐based measures including T‐wave alternans with risk of sudden cardiac death in the community‐dwelling elderly. J Electrocardiol 2010;43:251–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Exner DV, Kavanagh KM, Slawnych MP, et al REFINE Investigators. Noninvasive risk assessment early after a myocardial infarction the REFINE study. J Am Coll Cardiol 2007;50:2275–2284. [DOI] [PubMed] [Google Scholar]
- 76. Leino J, Minkkinen M, Nieminen T, et al Combined assessment of heart rate recovery and T‐wave alternans during routine exercise testing improves prediction of total and cardiovascular mortality: The Finnish Cardiovascular Study. Heart Rhythm 2009;6:1765–1771. [DOI] [PubMed] [Google Scholar]


