Abstract
Objective
The electrocardiography (ECG) was the simplest and common adjunctive diagnostic tool for cardiac amyloidosis (CA). We sought to clarify the findings of ECG in patients with CA in order to early identification of CA according to the findings of ECG.
Methods
A total of 276 patients with diagnosis of systemic amyloidosis admitted to Peking Union Medical College Hospital from January 2000 to December 2011, were enrolled. Two groups were classified according to the cardiac involvement or not, namely CA (n = 189) and control (n = 87) groups. The low voltage on limb leads defined by the amplitude of the QRS complex in each limb leads ≤0.5 mV. The pseudo‐infarct pattern defined by the presence of pathologic Q waves on at least two contiguous leads on ECG without obstructive coronary artery disease.
Results
The mean age was 55 ± 12 (15–88) years, 168 patients (61%) were male. Atrial arrhythmia (15.9% vs 3.4%, P = 0.003), low voltage on limb leads (54.5% vs 20.7%, P < 0.001), atrioventricular block (14.8% vs 1.1%, P = 0.001) and pseudo‐infarct pattern (40.2% vs 4.6%, P < 0.001) were more prevalent in CA than control groups. The combination of low voltage on limb leads and pseudo‐infarct pattern was more common (28.0% vs 2.3%, P < 0.001) in CA than control groups. The sensitivity, specificity, positive and negative predictive values of the presence of low voltage on limb leads and pseudo‐infarct pattern for the diagnosis of CA were 28%, 98%, 96%, and 39%, respectively.
Conclusion
In CA patients, low voltage on limb leads and pseudo‐infarct pattern were the most common ECG findings. Atrial arrhythmia and atrioventricular block were the most common arrhythmias in CA patients. The combination of low voltage on limb leads and pseudo‐infarct pattern had high specificity and positive predictive value for the diagnosis of CA.
Keywords: electrocardiography, cardiac amyloidosis, low voltage on limb leads, pseudo‐infarct pattern
Amyloidosis frequently involves the heart. Deposition of amyloid fibrils in myocardial tissue results in reduced ventricular compliance with impairment of relaxation and eventually contraction. Amyloid fibrils may also deposit in myocardial vessels and cause local ischemia. Amyloid deposition is associated with fibrosis of conduction tissue, resulting in conduction abnormalities and arrhythmias. Cardiac amyloidosis (CA) is associated with a variable but generally poor prognosis,1, 2, 3, 4 and early recognition may is the simplest and common adjunctive diagnostic tool for CA. However, the findings of ECG in patients with CA remain unclear. In this study, we aimed to determine the findings of ECG in patients with CA and thus as a clue for the early identification and diagnosis of CA.
METHODS
Study Population
This was a single center, retrospective study of 276 patients with systemic amyloidosis (at least one tissue or organ biopsy with positive Congo red staining and demonstration of apple‐green birefringence under polarized light) who admitted to Peking Union Medical College Hospital from January 2000 to December 2011, were enrolled. A total of 189 patients diagnosed of CA (or systemic amyloidosis with cardiac involvement) who met one of the following criteria, were classified into CA group.
Endomyocardial biopsy (EMB) confirmed the diagnosis of CA.
The presence of clinical and laboratory evidence of amyloidosis and echocardiographic features of amyloidosis, include a mean left ventricular wall thickness (septum and posterior wall) greater than 12 mm in the absence of hypertension or other potential causes of left ventricular hypertrophy; right ventricular free wall thickening in the presence of left ventricular thickening and in the absence of pulmonary or systemic hypertension.1
The remaining 87 patients without cardiac involvement were classified into control group.
Data Collection
We performed a detailed examination of the medical records of the 276 patients after obtaining the approval from Institutional Review Board of Peking Union Medical College Hospital. A review of the medical records was performed by a team of physicians blinded to the grouping.
The ECG was analyzed for the following characteristics: rhythm, conduction abnormalities (such as right or left bundle branch block), a low voltage pattern (defined by the amplitude of the QRS complex in each limb leads ≤ 0.5 mV5, a pseudo‐infarct pattern (pathologic Q waves on at least two contiguous leads on ECG without obstructive coronary artery disease by coronary angiography or computed tomography angiography). In addition, we calculated the voltages of QRS complex in all 12 leads, and the QRS duration, QT as well as QTc interval. The QTc interval was QT interval corrected for the heart rate according to Bazett's formula (QTc = QT/√RR). The unit of ECG leads voltage is millivolt.
The ECHO was analyzed for the following characteristics: left atrial (LA) size, interventricular septal (IVS) thickness, left ventricular posterior wall (LVPW) thickness, left ventricular end of diastolic diameter (LVEDD), left ventricular end of systolic diameter (LVESD), pericardial effusion, left ventricular ejection fraction (LVEF), and E/A ratio. Transthoracic ECHO was performed using commercially available GE Vivid seven Ultrasound machines (Horten, Norway). IVS and LVPW thicknesses were measured in standard fashion according to American Society of Echocardiography guidelines.6 Also, LA size and LVEDD as well as LVESD were measured in standard fashion. LVEF was assessed using the biplane Simpson's equation from apical two‐chamber views. Mitral inflow peak velocities of E and A waves, were measured in patients in normal sinus rhythm, E/A‐wave ratio was calculated in standard fashion.
Statistical Analysis
Data were reported as mean ± standard deviation (SD) for continuous variables or percentage for categorical variables. Differences in baseline characteristics between the two groups were evaluated with paired t‐tests or Mann‐Whitney U test for all continuous variable, all categorical variables were compared using chi‐square test or Fisher's exact test. All P values were two‐sided and the results were considered statistically significant at the level of P < 0.05. SPSS statistical software of version 13.0 for windows was used for all statistical analyses.
RESULTS
Patients
A total of 276 patients with systemic amyloidosis were enrolled. The mean age was 55 ± 12 (15–88) years, 168 patients (61%) were male. Twenty‐eight patients (10%) died during the stay of hospitalization, including heart failure in 16 patients (5.8%), sudden death in six patients (2.2%), septic shock in four patients (1.4%) and pulmonary infection in two patients (0.7%). The concomitant diseases were diabetes mellitus in 19 patients (6.9%), ischemic stroke in eight patients (2.9%). The classification of amyloidosis were primary (immunoglobulin light chains) CA in 170 patients (61.6%), CA associated with multiple myeloma in 52 patients (18.8%), secondary CA in 16 patients (5.8%), hereditary CA in 6 patients (2.2%), and unclassified CA in 32 patients (11.6%).
Electrocardiographic Findings
Tables 1, 2, 3 summarized the ECG findings of the 276 patients. Atrial arrhythmia (15.9% vs 3.4%, P = 0.003), low voltage on limb leads (54.5% vs 20.7%, P < 0.001), atrioventricular block (14.8% vs 1.1%, P = 0.001) and pseudo‐infarct pattern (40.2% vs 4.6%, P < 0.001) were more prevalent in CA than control groups. The combination of low voltage on limb leads and pseudo‐infarct pattern was more common (28.0% vs 2.3%, P < 0.001) in CA than control groups. The sensitivity, specificity, positive and negative predictive values of the presence of low voltage on limb leads and pseudo‐infarct pattern for the diagnosis of CA were 28%, 98%, 96%, and 39%, respectively. The voltages of leads I (0.40 ± 0.27 vs 0.58 ± 0.34 mV, P < 0.001), II (0.48 ± 0.30 vs 0.69 ± 0.28 mV, P < 0.001), aVR (0.37 ± 0.24 vs 0.59 ± 0.25 mV, P < 0.001), V1 (0.81 ± 0.53 vs 0.95 ± 0.49 mV, P = 0.009), V4 (1.42 ± 0.76 vs 1.68 ± 0.72 mV, P = 0.003), V5 (1.14 ± 0.75 vs 1.44 ± 0.70 mV, P < 0.001) and V6 (0.80 ± 0.54 vs 1.13 ± 0.61 mV, P < 0.001) were significantly lower in CA than control groups. QRS duration (95 ± 22 vs 90 ± 38 ms, P = 0.002), QT (382 ± 49 vs 368 ± 44 ms, P = 0.019) and QTc (451 ± 40 vs 428 ± 32 ms, P < 0.001) intervals were significantly longer in CA than control groups. Figure 1 showed the typical ECG findings of low voltage on limb leads and pathologic Q waves in one 46‐year old male patient with primary CA.
Table 1.
Electrocardiographic Findings
| Variables | All (n = 276) | CA (n = 189) | Control (n = 87) | P value |
|---|---|---|---|---|
| Rhythm | ||||
| Atrial arrhythmiaa | 33 (12.0%) | 30 (15.9%) | 3 (3.4%) | 0.003 |
| Low voltage on limb leads | 121 (43.8%) | 103 (54.5%) | 18 (20.7%) | <0.001 |
| Conduction abnormalities | 42 (15.2%) | 39 (20.6%) | 3 (3.4%) | <0.001 |
| Atrioventricular block | 29 (10.5%) | 28 (14.8%) | 1 (1.1%) | 0.001 |
| Third degree | 4 (1.4%) | 3 (1.6%) | 1 (1.1%) | 1.000 |
| First degree | 25 (9.1%) | 25 (13.2%) | 0 | <0.001 |
| Left bundle branch block | 4 (1.4%) | 4 (2.1%) | 0 | 0.312 |
| Right bundle branch block | 14 (5.1%) | 13 (6.9%) | 1 (1.1%) | 0.072 |
| Pseudo‐infarct pattern | 80 (29.0%) | 76 (40.2%) | 4 (4.6%) | <0.001 |
| Combination of low voltage on limbleads and pseudo‐infarct pattern | 55 (19.9%) | 53 (28.0%) | 2 (2.3%) | <0.001 |
Including atrial fibrillation, atrial flutter and atrial tachycardia.
Table 2.
The Voltages of QRS Complex of the 12 Leads
| Leads | All (n = 276) | CA (n = 189) | Control (n = 87) | P value |
|---|---|---|---|---|
| I (mV) | 0.46 ± 0.30 | 0.40 ± 0.27 | 0.58 ± 0.34 | <0.001 |
| II (mV) | 0.55 ± 0.31 | 0.48 ± 0.30 | 0.69 ± 0.28 | <0.001 |
| III (mV) | 0.46 ± 0.31 | 0.48 ± 0.34 | 0.44 ± 0.23 | 0.918 |
| aVR (mV) | 0.44 ± 0.26 | 0.37 ± 0.24 | 0.59 ± 0.25 | <0.001 |
| aVF (mV) | 0.47 ± 0.36 | 0.45 ± 0.39 | 0.50 ± 0.27 | 0.016 |
| aVL (mV) | 0.39 ± 0.25 | 0.38 ± 0.26 | 0.40 ± 0.22 | 0.120 |
| V1 (mV) | 0.85 ± 0.52 | 0.81 ± 0.53 | 0.95 ± 0.49 | 0.009 |
| V2 (mV) | 1.67 ± 1.96 | 1.68 ± 2.32 | 1.65 ± 0.73 | 0.150 |
| V3 (mV) | 1.60 ± 0.84 | 1.58 ± 0.89 | 1.65 ± 0.73 | 0.290 |
| V4 (mV) | 1.50 ± 0.76 | 1.42 ± 0.76 | 1.68 ± 0.72 | 0.003 |
| V5 (mV) | 1.23 ± 0.75 | 1.14 ± 0.75 | 1.44 ± 0.70 | <0.001 |
| V6 (mV) | 0.91 ± 0.58 | 0.80 ± 0.54 | 1.13 ± 0.61 | <0.001 |
Table 3.
QRS Duration, QT, and QTc Intervals
| Variables | All (n = 276) | CA (n = 189) | Control (n = 87) | P value |
|---|---|---|---|---|
| QRS duration (ms) | 93 ± 28 | 95 ± 22 | 90 ± 38 | 0.002 |
| QT interval (ms) | 377 ± 48 | 382 ± 49 | 368 ± 44 | 0.019 |
| QTc interval (ms) | 444 ± 39 | 451 ± 40 | 428 ± 32 | <0.001 |
Figure 1.
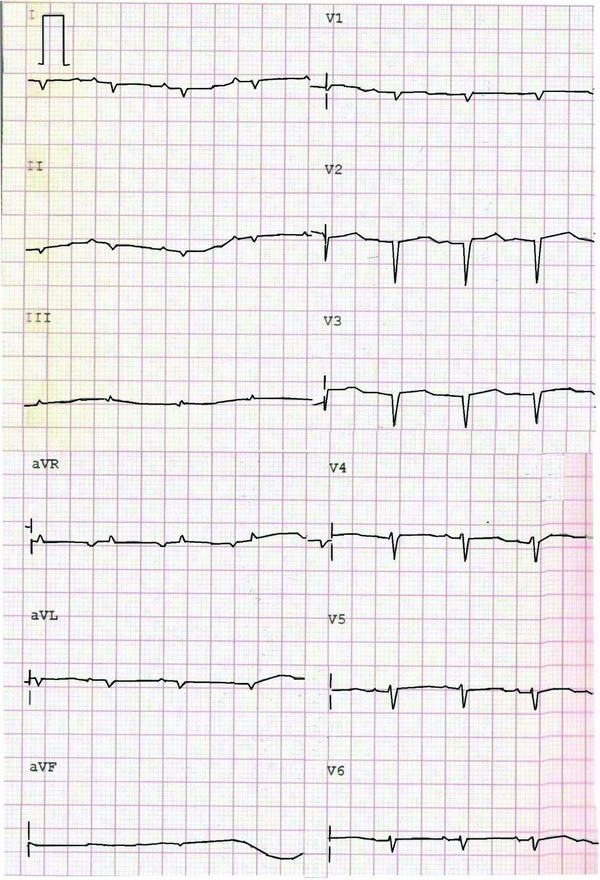
The ECG of one 46‐year old male patient with primary cardiac amyloidosis revealed the low voltage on limb leads and pseudo‐infarct pattern (pathologic Q waves on leads V1 to V3).
Echocardiographic Findings
Table 4 summarized the ECHO findings of the 276 patients. The LVEDD was significantly smaller in CA than control groups (44 ± 6 vs 47 ± 6 mm, P < 0.001). The LVEF was significantly lower in CA than control groups (56 ± 14% vs 65 ± 7%, P < 0.001). The LA dimension was significantly larger in CA than control groups (41 ± 7 vs 36 ± 6 mm, P < 0.001). The thicknesses of IVS (14 ± 2 vs 9 ± 2 mm, P < 0.001) and LVPW (13 ± 1 vs 8 ± 1 mm, P < 0.001) were significantly thicker in CA than control groups. The incidence of LVEF ≤ 50% (32.3% vs 4.6%, P < 0.001) and pericardial effusion (65.1% vs 24.1%, P < 0.001) were significantly more common in CA than control groups.
Table 4.
Echocardiographic Features
| Variables | All (n = 276) | CA (n = 189) | Control (n = 87) | P value |
|---|---|---|---|---|
| LVEDD (mm) | 45 ± 7 | 44 ± 6 | 47 ± 6 | <0.001 |
| LVESD (mm) | 31 ± 6 | 31 ± 7 | 31 ± 6 | 0.764 |
| LVEF (%) | 59 ± 13 | 56 ± 14 | 65 ± 7 | <0.001 |
| LA (mm) | 40 ± 7 | 41 ± 7 | 36 ± 6 | <0.001 |
| E/A ratio | 1.4 ± 0.9 | 1.6 ± 0.9 | 0.9 ± 0.4 | <0.001 |
| IVS (mm) | 12 ± 3 | 14 ± 2 | 9 ± 2 | <0.001 |
| LVPW (mm) | 12 ± 3 | 13 ± 1 | 8 ± 1 | <0.001 |
| LVEF ≤ 50% | 65(23.6%) | 61(32.3%) | 4(4.6%) | <0.001 |
| Pericardial effusion | 144(53.0%) | 123(65.1%) | 21(24.1%) | <0.001 |
CA = cardiac amyloidosis; LVEDD = left ventricular end of systolic dimension; LVESD = left ventricular end of systolic dimension; LVEF = left ventricular ejection fraction; LA = left atrial dimension; IVS = interventricular septum; LVPW = left ventricular posterior wall.
DISCUSSION
This was a large CA cohort. The prognosis of CA was poor once the occurrence of symptoms, therefore, the early diagnosis was important for the early treatment.7 ECG was an important adjunctive diagnostic tool and clue for the identification of possible CA. This study revealed several classical and important findings of ECG in CA patients. The low voltage on limb leads was the most common finding, followed by pseudo‐infarct pattern and atrioventricular block. The combination of low voltage on limb leads and pseudo‐infarct pattern was more common in CA than control groups, it had high specificity and positive predictive value for the diagnosis of CA. Atrial arrhythmia was very common in CA patients. The results of the present study were similar with previous reports.8, 9, 10, 11 Murtagh et al.8 from the Mayo Clinic reported that 127 patients with biopsy‐proven primary CA and found that the low voltage was present in 46% of patients and a pseudo‐infarct pattern was present in 47% of patients. The pseudo‐infarct patterns were anterior (36%), inferior (12%), and lateral (14%). Both low voltage and pseudo‐infarct pattern were present in 25% of patients. Atrial fibrillation and flutter was the most common arrhythmia. Rahman et al.9 reported that the low voltage was in 56% and pseudo‐infarct pattern was in 60% of patients. Austin et al.10 reported that 45 patients with biopsy‐proven CA, the low voltage was seen in 27% of patients. Another important finding was the increased thicknesses of IVS and LVPW, its reverse relationship with the voltages of limb leads, the similar finding with previous reports. The presence of above mentioned findings of ECG or the reverse relationship between the thickness of IVS/LVPW and voltages of limb leads strongly supported the possibility of CA after excluding other potential diseases. Rahman et al.9 reported that the low voltage on limb leads associated with IVS thick‐ness > 1.98 cm, the diagnosis of CA could be made with a sensitivity of 72% and a specificity of 91%, the positive and negative predictive values were 79% and 88%, respectively. Cheng et al.11 studied the ECG and ECHO features of 11 patients with biopsy‐proven CA, concluded the ratio of R(I) /LVPW < 0.4 for the diagnosis of CA had the sensitivity of 91% and specificity of 100%, the positive and negative predictive values of 100% and 91%, respectively; the ratios of R(V5/6) /LVPW < 0.7 had the sensitivity of 91% and specificity of 89%, the positive and negative predictive values of 91% and 89%, respectively.
Limitations
This study had several limitations. First, the diagnosis of CA wasn't all based on EMB, the majority of patients in CA group diagnosed from typical ECHO features of CA and pathological evidence of extra‐cardiac tissue or organ. Second, the ECG features of different type of CA would be variable. Rapezzi et al.12 reported that hereditary CA patients less often displayed low voltage than primary CA patients (25% vs 60%; P < 0.0001). This study didn't classify all the CA patients and determine the different ECG findings of different type of CA. Third, the ECG findings of CA was the clue for identification of possible CA, couldn't substitute the EMB of the golden standard for the diagnosis of CA. Although the above‐mentioned limitations, combination of low voltage on limb leads and pseudo‐infarct pattern had high specificity and positive predictive value for the identification and early diagnosis of CA.
CONCLUSION
In the large cohort of CA patients, low voltage on limb leads and pseudo‐infarct pattern were the most common ECG findings. Atrial arrhythmia and atrioventricular block were the most common arrhythmias in CA patients. The combination of low voltage on limb leads and pseudo‐infarct pattern had high specificity and positive predictive value for the diagnosis of CA.
Funding: This study was supported by the Research Fund from the Janssen Research Council China.
REFERENCES
- 1. Gertz MA, Comenzo R, Falk RH, et al. Definition of organ involvement and treatment response in immunoglobulin light chain amyloidosis (AL): A consensus opinion from the 10th International Symposium on Amyloid and Amyloidosis, Tours, France, 18–22 April 2004. Am J Hematol 2005;79:319–328. [DOI] [PubMed] [Google Scholar]
- 2. Selvanayagam JB, Hawkins PN, Paul B, et al. Evaluation and management of the cardiac amyloidosis. J Am Coll Cardiol 2007;50:2101–2110. [DOI] [PubMed] [Google Scholar]
- 3. Hess EP, White RD. Out‐of‐hospital cardiac arrest in patients with cardiac amyloidosis: Presenting rhythms, management and outcomes in four patients. Resuscitation 2004;60:105–111. [DOI] [PubMed] [Google Scholar]
- 4. Shah KB, Inoue Y, Mehra MR. Amyloidosis and the heart: A comprehensive review. Arch Intern Med 2006;166:1805–1813. [DOI] [PubMed] [Google Scholar]
- 5. Falk RH. Diagnosis and management of the cardiac amyloidoses. Circulation 2005;112:2047–2060. [DOI] [PubMed] [Google Scholar]
- 6. Gardin JM, Adams DB, Douglas PS, et al. American Society of Echocardiography. Recommendations for a standardized report for adult transthoracic echocardiography: A report from the American Society of Echocardiography's Nomenclature and Standards Committee and Task Force for a Standardized Echocardiography Report. J Am Soc Echocardiogr 2002;15:275–290. [DOI] [PubMed] [Google Scholar]
- 7. Kholová I, Kautzner J. Current treatment in cardiac amyloidosis. Curr Treat Options Cardiovasc Med 2006;8:468–473. [DOI] [PubMed] [Google Scholar]
- 8. Murtagh B, Hammill SC, Gertz MA, et al. Electrocardiographic findings in primary systemic amyloidosis and biopsy‐proven cardiac involvement. Am J Cardiol 2005;95:535–537. [DOI] [PubMed] [Google Scholar]
- 9. Rahman JE, Helou EF, Gelzer‐Bell R, et al. Noninvasive diagnosis of biopsy‐proven cardiac amyloidosis. J Am Coll Cardiol 2004;43:410–415. [DOI] [PubMed] [Google Scholar]
- 10. Austin BA, Duffy B, Tan C, et al. Comparison of functional status, electrocardiographic, and echocardiographic parameters to mortality in endomyocardial‐biopsy proven cardiac amyloidosis. Am J Cardiol 2009;103:1429–1433. [DOI] [PubMed] [Google Scholar]
- 11. Cheng Z, Kang L, Tian Z, et al. Utility of combined indexes of electrocardiography and echocardiography in the diagnosis of biopsy proven primary cardiac amyloidosis. Ann Noninvasive Electrocardiol 2011;16:25–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rapezzi C, Merlini G, Quarta CC, et al. Systemic cardiac amyloidoses: Disease profiles and clinical courses of the 3 main types. Circulation 2009;120:1203–1212. [DOI] [PubMed] [Google Scholar]


