Abstract
First‐degree atrioventricular (AV) block is a delay within the AV conduction system and is defined as a prolongation of the PR interval beyond the upper limit of what is considered normal (generally 0.20 s). Up until recently, first‐degree AV block was considered an entirely benign condition. In fact, some complain that it is a misnomer since there is only delay and no actual block in the AV conduction system (usually within the AV node). However, it has long been acknowledged that extreme forms of first‐degree AV block (typically a PR interval exceeding 0.30 s) can cause symptoms due to inadequate timing of atrial and ventricular contractions, similar to the so‐called pacemaker syndrome. Consequently, the current guidelines state that permanent pacemaker implantation is reasonable for first‐degree AV block with symptoms similar to those of pacemaker syndrome or with hemodynamic compromise, but also stresses that there is little evidence to suggest that pacemakers improve survival in patients with isolated first‐degree AV block. Recent reports suggest that it may be time to revisit the impact of first‐degree AV block. Also, several findings in post hoc analyses of randomized device trials give important insights in possible treatment options. The present review aims to provide an update on the current knowledge concerning the impact of first‐degree AV block and also to address the issue of pacing in patients with this condition.
Keywords: clinical, electrophysiology—conduction disturbances, epidemiology/clinical trials, implantable devices—pacemaker‐bradyarrhythmias
First‐degree atrioventricular (AV) block is a delay within the AV conduction system and is defined as a prolongation of the PR interval beyond the upper limit of what is considered normal (generally 0.20 s).1, 2, 3 The PR interval tends to lengthen with increasing age and is on average longer (approximately five to 10 ms) in men compared to women.2, 3 The electrophysiological properties of first‐degree AV block may vary and can be due to delay within several different structures of the heart (i.e., intra atrial delay, AV nodal delay, delay in His‐bundle or in the branches).4, 5 Up until recently, first‐degree AV block was considered an entirely benign condition.6, 7, 8, 9, 10 In fact, some complain that it is a misnomer since there is only delay and no actual block in the AV conduction system (usually within the AV node). However, it has long been acknowledged that extreme forms of first‐degree AV block (typically a PR interval exceeding 0.30 s) can cause symptoms due to inadequate timing of atrial and ventricular contractions, similar to the so‐called pacemaker syndrome.11 Consequently, the current guidelines state that permanent pacemaker implantation is reasonable for first‐degree AV block with symptoms similar to those of pacemaker syndrome or with hemodynamic compromise (Class IIa indication), but also stresses that there is little evidence to suggest that pacemakers improve survival in patients with isolated first‐degree AV block.1
Recent reports suggest that it may be time to revisit the impact of first‐degree AV block. Also, several findings in posthoc analyses of randomized device trials give important insights in possible treatment options.
FIRST‐DEGREE AV BLOCK IN HEALTHY POPULATIONS
The incidence of first‐degree AV block was initially reported in apparently healthy, relatively young, male populations such as aviators. 6, 7, 10 In good accordance, the incidence was reported to be somewhere between five and 10 in 1,000 in most of these studies 6, 7, 10, 12 and no association with age, height, weight, heart rate, or blood pressure was found in these very homogenous populations. 7 Packard et al. investigated 1,000 aviators (all men, mean age 23.7 years, range 20 to 30) at baseline and at follow‐up after 10 to 12 years. 6 Over time the PR interval seemed to lengthen somewhat, 6 but none of the individuals with prolonged PR interval at baseline or “marked prolongation” of the PR interval during follow‐up, showed any clinical evidence of heart disease at the end of the study. 6 However, in addition to the fact the population as a whole was healthy and young, the number of patients with first‐degree AV block in the study was low (n = 11).6
A somewhat older, but still healthy and all male, population was studied by Blackburn et al. in the International Study (12,770 men between the ages of 40 and 59). 13 Baseline ECG parameters were recorded and their predictive properties for coronary heart disease were explored during a 5‐year follow‐up. Although the event‐rate was low, a small, but significant excess of subsequent coronary heart disease was seen in individuals with first‐degree AV block. 13 This was the first data indicating that first‐degree AV block may actually not be an entirely benign finding but the authors concluded that the finding “varies somewhat from insurance and military data, showing that it [first‐degree AV block] is of little consequence in mortality prediction at younger age.”
Another healthy population of 1,832 men, with identical age span as in the International Study was investigated and followed for 7 years by Erikssen et al. 9 A higher prevalence of first‐degree AV block than previously reported was seen (about 5%), but progression to higher degrees of AV nodal block was very rare. 9 Moreover, almost half of the patients with prolonged PR interval at baseline normalized their value during follow‐up. 9 Surprisingly, and in stark contrast to the findings reported by Blackburn et al., the incidence of coronary heart disease during follow‐up was, if anything (a nonsignificant trend), lower among patients with first‐degree AV block at baseline, yet again suggesting that first‐degree AV block was an essentially benign condition.9
In 1986, Mymin et al. reported findings in 3,983 healthy male aviators (the Manitoba cohort), initially evaluated right after the second world‐war and subsequently followed over a 30‐year period. 10 Again it was shown that over time the PR interval prolongs, with a higher incidence of first‐degree AV block in the cohort at the later stages of follow‐up. 10 In contrast to the finding by Erikssen et al. 9 it was shown that the intraindividual variation in PR interval was small, with the vast majority of the individuals (84%) exhibiting no more than 0.04 s change over time. 10 During the 30‐year follow‐up, the highest rates of morbidity or mortality from heart disease were seen in individuals with first‐degree AV block. However, these trends did not reach statistical significance. 10 This together with the fact that very few of the patients with first‐degree AV block at entry progressed to second‐ or third‐degree AV block (as reported by Erikssen), led the authors to conclude that first‐degree AV block was a benign condition.10
In summary, the early studies, primarily analyzing young, healthy and very homogeneous populations indicate that first‐degree AV block is a relatively rare condition with no apparent clinical meaning. 6, 7, 8, 9 However, the few studies including older subjects and/or with extended follow‐up indicate that the lack of association between first‐degree AV block and variables of outcome, may primarily be due to lack of statistical power. 10, 13
STUDIES IN COMMUNITY‐BASED POPULATIONS
In a study published 1971, Perlman and colleagues explored the impact of first‐degree AV block in a community‐based health study. 14 From the Tecumseh Community Health Study, 4,678 individuals were studied with respect to the prevalence of first‐degree AV block. 14 None of the individuals younger than 20 years had a PR interval exceeding 0.22 s. The prevalence was then rather stable (about 10–15 per 1000) until the sixth decade in life after which the prevalence rose linearly (to about 145 per 1000 among individuals older than 80 years). 14 Like Erikssen et al. later reported. 9 the authors noted that on many occasions PR‐prolongation in younger individuals resolved during follow‐up. However, primarily in the elderly, the prevalence of concomitant heart disease was higher among patients with first‐degree AV block.14 Moreover, in patients with pronounced PR‐prolongation (exceeding 0.24 s) a higher mortality rate was observed.14 Since this was primarily seen in elderly individuals, the authors concluded, “In otherwise healthy persons less than 60 years of age, a prolonged PR interval appears to be a benign and often transient finding.”14
The first report to address possible ethnic differences in PR interval studied healthy employees and their relatives of a major oil company in the Middle East. 15 The study population (n = 597) included individuals from the Middle East (65%), Asia (26%), and of European ancestry (9.5%) and did not show any significant differences in PR interval between the ethnic groups.15
In another study, by Upshaw, 2,123 patients attending an urban hospital between the ages of 20 and 99 years were investigated and the findings were analyzed separately based on race (African American and Caucasian). 16 Concomitant diseases or reason for hospital visit was not reported. Overall, the differences were small and both races showed a similar pattern with an increasing prevalence of first‐degree AV block after the age of 50. 16 African‐Americans tended to have a somewhat higher incidence of first‐degree AV block, most clearly seen among the oldest individuals (90 to 99 years). 16 Another population‐based epidemiological study later confirmed these findings. In the Atherosclerosis Risk in Communities (ARIC) study African Americans were found to have a higher proportion of individuals with abnormal PR duration (defined as longer than the 95th percentile in the study population) than individuals with European ancestry.17
Two studies from the Framingham cohort shed further light on the potential impact of first‐degree AV block on morbidity and mortality. 18, 19 In the study by Schnabel et al., a risk score for atrial fibrillation development was created. Alongside classical risk factors, such as increasing age, hypertension, heart failure etc., PR prolongation turned out to be predictive of atrial fibrillation development. 18 The magnitude of difference between having a “short” PR interval (less than 0.16 s) and a “prolonged” (greater than or equal to 0.20 s) was in the same order as adding 10 years of age or having hypertension and being obese. 18 In the second study, Cheng et al. investigated 7,575 individuals (mean age 47 years, 54% women) included in the Framingham cohort, without a history of atrial fibrillation, anti‐arrhythmic treatment or pacemaker treatment. 19 At baseline, 124 individuals (1.6%) were found to have a PR interval exceeding 0.20 s. 19 Individuals with first‐degree AV block were found to have a higher incidence of atrial fibrillation (approximately doubled risk), pacemaker implantation (approximately tripled risk) and a moderately increased risk of all cause‐mortality. These numbers held true also after adjusting for conventional risk factors, excluding patients using nodal‐blocking agents and excluding patients with wide QRS‐complexes. 19 Additionally, there was no evidence of sex interactions. 19 Interestingly, 27% of the patients with first‐degree AV block at baseline developed higher‐grade conduction abnormalities (second‐ or third‐degree AV block and/or complete bundle‐branch block), 13% developed further PR‐prolongation (additional prolongation exceeding 0.04 s). 19 These findings led the authors to question the previous notion of first‐degree AV block being an entirely benign condition.19
Using the National Health and Nutrition Examination Survey, Magnani et al. investigated 7486 individuals in sinus rhythm (mean age 60 years, 52% women, 50% ethnic minorities). 20 Their objective was to explore the association between “P wave indices” (i.e., P wave duration, P wave amplitude, and PR interval) and clinical outcome. As before, the PR interval was reported to be longer in men and to increase with increasing age. 20 In the univariate analysis PR interval was found to be associated with mortality, but P wave duration was the only P wave parameter to be independently associated with mortality.20
In a subsequent study, in an older population included in Health ABC (mean age 74 years), Magnani et al. reported that PR‐prolongation (exceeding 200 ms) was associated with an 46% increase in incident heart failure over a 10‐year period. 21 However, the increased risk of heart failure was not confined to those individuals with first degree AV block (i.e., PR interval exceeding 200 ms), instead every SD increase in PR interval was associated with an 13% increased risk of incident heart failure. In contrast with previous studies, no association between race and PR‐interval was found.21
The community‐based studies have shown that first‐degree AV block is more common than first assumed and that it becomes increasingly prevalent with increasing age regardless of race or gender. 14, 16, 21 The thorough reports from the Framingham population shows without any doubt that first‐degree AV block is associated with worse outcome. 18, 19 The key question that remains open is whether first‐degree AV block is merely a sign of increased risk or if it is part of the problem in itself.
FIRST‐DEGREE AV BLOCK IN PATIENTS WITH CORONARY HEART DISEASE
Crisel et al. investigated 938 patients with stable coronary artery disease using data from the Heart and Soul Study. 22 As expected, the prevalence of first‐degree AV block was substantially higher than observed in the healthy populations, with a prevalence around 9%. 22 Patients with first‐degree AV block were shown to be older (mean of 73 vs. 65 years), more likely to be male (91 vs. 81%), had a higher prevalence of heart failure history (26 vs. 16%) and were less likely to smoke (11 vs. 21%), than patients with normal AV conduction. 22 In addition, they had a lower ejection fraction (mean 59 vs. 62%), were more likely to have inducible ischemia and more often had a QRS duration exceeding 100 ms. 22 Well in keeping with the Framingham data, during follow‐up (mean 6.2 years) patients with first‐degree AV block were more than twice as likely to be hospitalized for heart failure and/or die from cardiovascular causes and had a more than 50% increased risk of dying from any cause. 22 The only covariates to be significantly associated with the observed risk estimates were lower ejection fraction or a history of heart failure, which led the authors to speculate that first‐degree AV block may indeed be a cause of heart failure in patients with stable coronary artery disease.22
In the Finnish cardiovascular study, 1979 patients (mean age in the late fifties) referred for clinically motivated exercise testing were studied. 23 Roughly a third of the patients had coronary heart disease and a fifth had a previous myocardial infarction. First‐degree AV block (PR interval exceeding 0.20 s) pre‐ and postexercise were both shown to be predictive of mortality during the 47 months follow‐up, but the post exercise measure was the only independently predictive factor. 23 The authors suggested that the postexercise assessment of first‐degree AV block may offer improved prediction because of functional abnormalities that become manifest only during this physiologic challenge to the heart.23
The two studies available focusing on first‐degree AV block in patients with known 22 or suspected 23 coronary heart disease reinforces the findings from the larger community‐based studies that first‐degree AV block is associated with worse outcome, but they do not answer the question whether or not the PR prolongation is the cause of the problem. The findings regarding the prevalence and possible impact of first‐degree AV block are summarized in Figure 1.
Figure 1.
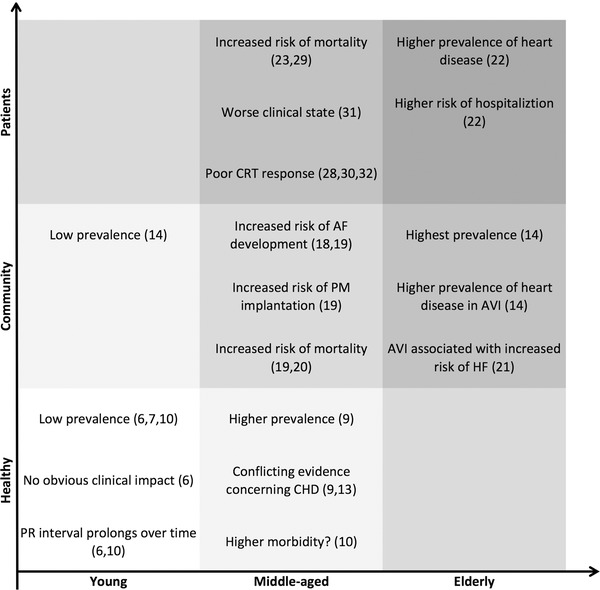
A summary of the prevalence and impact of first‐degree AV block in various populations. Findings in studies including healthy individuals, community‐based studies and studies including patients with concomitant diseases. Notably, the prevalence of first‐degree AV block seems to increase with age and more advanced clinical state. Likewise, the affect of first‐degree AV block on outcome and clinical state is primarily seen in the older and/or more comorbid populations. AF = atrial fibrillation; AVI = first‐degree AV block; CHD = coronary heart disease; CRT = cardiac resynchronization therapy; HF = heart failure; PM = pacemaker.
INSIGHTS FROM DEVICE STUDIES INCLUDING PATIENTS WITH CONGESTIVE HEART FAILURE
The modern era of cardiac resynchronization trials have not only generated a lot of data on optimal treatment of patients with congestive heart failure and dyssynchrony, 24, 25, 26, 27 but also some additional information on the possible prognostic meaning of first‐degree AV block. 28, 29, 30
The first piece of information in this patient category came from a much smaller study, though. Tedrow et al. studied 75 patients from a database of patients receiving cardiac resynchronization therapy (CRT) (mean age 66 years, mean left ventricular ejection fraction LVEF] about 20%) and explored possible predictors of poor outcome (the combined end point of death, cardiac transplantation or left ventricular assist device implantation) during a 1‐year follow‐up. 31 Although, a prolonged PR interval was shown to be associated with a worse clinical state, it was not independently predictive of poor clinical outcome.31
The Multicenter InSync Randomized Clinical Evaluation (MIRACLE)24 and Multicenter InSync ICD Randomized Clinical Evaluation (MIRACLE‐ICD) 25 trials included very similar patient populations (LVEF less than 35%, QRS duration exceeding 130 ms, New York Heart Association NYHA] class III or IV) who were randomized to optimal medical therapy or CRT (MIRACLE) or CRT‐D, respectively (MIRACLE‐ICD). In a subanalysis of these two trials Pires et al. identifies predictors of CRT response (defined as improvement in NYHA‐class). 30 In spite of the similar patient populations, the identified predictors differed substantially between the two trials, with virtually no overlap. 30 First‐degree AV block was identified as an independent marker for poor CRT response in the MIRACLE trial. 30 The fact that this was not seen in MIRACLE‐ICD (P = 0.64), although the study populations were very similar, raises the question whether the finding is genuine or merely a chance finding.
On the other hand, although the approach was slightly different, a similar finding was seen in a subanalysis of the Cardiac Resynchronization – Heart Failure (CARE‐HF) trial.26, 28 In a similar patient population (e.g., left ventricular ejection fraction less than 35%, QRS duration exceeding 120 ms, NYHA‐class III or IV) a prolonged PR interval was identified as being a predictor, not of nonresponse as in the MIRACLE trial, but of unfavorable outcome (death, unplanned hospitalization for management of a major cardiac event). 28 This held true also after adjusting for CRT treatment or not, i.e., PR prolongation was associated with unfavorable outcome regardless of treatment arm.28
Moreover, the findings in CARE‐HF and the finding in MIRACLE are both supported by findings in Danish registry study from Kronborg et al. 32 In this study 659 patients who received either a clinically indicated CRT‐P or CRT‐D were studied (mean age 66 years, mean LVEF 25%). 32 After adjustment for relevant covariables, a prolonged PR interval (exceeding 0.20 s) was associated with nonresponse (left ventricular ejection fraction improvement less than 5%) as well as with a poor clinical outcome (all‐cause mortality and cardiac mortality).32
Hence, it seems as if the earlier findings of first‐degree AV block being a predictor of worse clinical outcome hold true also in heart failure patients with clinical indication for CRT. 28, 30, 31, 32 However, there was an additional finding in CARE‐HF that warrants further notice. Not only was a PR prolongation found to be a predictor of poor clinical outcome, a shortening of the PR interval from baseline to 3‐month follow‐up was a strong predictor of favorable outcome, indicating that applying CRT to patients with PR prolongation may improve their prognosis. 28 This brings us closer to the remaining question, is first‐degree AV block more than just a sign of advanced disease?
FIRST‐DEGREE AV BLOCK—SIGN OF DISEASE OR THE PROBLEM ITSELF?
At least three substudies of large, randomized clinical trials on patients with congestive heart failure treated with ICD or CRT address this question, with somewhat diverging results. 29, 33, 34 Another study the Danish Multicenter Randomized Trial on Single Lead Atrial Pacing versus Dual Chamber Pacing in Sick Sinus Syndrome (DANPACE) randomized patients with sick sinus syndrome to either AAIR or DDDR pacing.35
The dual chamber and VVI implantable defibrillator (DAVID) trial randomized patients (mean age in the mid‐sixties, more than 80% men, mean LVEF circa 27%) with standard indications for implantable defibrillator but without pacing indication, to either DDDR pacing or to standard backup ventricular pacing. 36 The authors showed that DDDR pacing did not offer any advantages over backup ventricular pacing, in fact it seemed to be associated with an increase in the combined end point of heart failure hospitalization or death. 36 In a subsequent subanalysis it was further explored if patients without formal pacing indications, but with sinus bradycardia (heart rate less than 60 beats per minute) and/or first‐degree AV block (PR interval exceeding 0.20 s), did benefit from pacing. 33 Consistent with the main findings in DAVID, Kutalek et al. showed that the 169 patients with “soft indications” for pacing (91 of whom had first‐degree AV block, median PR interval 0.22 s) did not differ from the study group as a whole, i.e., DDDR pacing was still detrimental.33
In the Managed Ventricular pacing versus VVI 40 Pacing (MVP) trial, patients (mean age 62 years, 80% male) with an indication for ICD according to current guidelines but without significant bradycardia were randomized to either atrial pacing with ventricular backup at 60 beats per minute or only ventricular backup pacing at a lower rate (40 beats per minute). 34 The vast majority of the randomized patients (80%) received the ICD as primary prevention, 84% had dilated cardiomyopathy (of whom three quarters had ischemic heart disease) and the mean ejection fraction in the study population was 35%. 34 Overall, the primary end point of all‐cause mortality or worsening heart failure favored ventricular backup pacing. 34 But of particular interest for the current review, was the finding in a posthoc analysis of the subgroup of patients with prolonged PR interval (exceeding 0.23 s, 10% of the entire population). In that subgroup, patients randomized to atrial pacing was found to do significantly worse than patients with ventricular backup pacing, with a substantially higher rate of death or heart failure events. In contrast, the event rate of patients with PR prolongation with ventricular pacing was similar to that of patients without PR prolongation and when looking only at patients with normal AV conduction (PR interval less than 0.23 s) no differences were seen between patients randomized to either pacing strategy. 34 That is, the overall difference observed in the MVP trial was primarily due to a high event rate in patients with first‐degree AV block randomized to atrial pacing. 34 However, a word of caution is warranted, the prespecified cutoff for PR prolongation was 0.22 s and using this value patients with PR prolongation did not differ from the study population as a whole.34
In DANPACE, where 1415 patients (mean age circa 73 years, two‐thirds female, 10% of the population had subnormal LVEF) with sick sinus syndrome were randomized to either AAIR or DDDR pacing, it was reported that the incidence of AF was higher among patients treated with AAIR pacing. 35 Interestingly, a subsequent posthoc analysis revealed that the AAIR associated risk‐increase was predominately seen in patients with baseline PR longer than the mean (exceeding 0.18 s), well in keeping with the findings in the MVP trial. 37 Moreover, in patients randomized to DDDR pacing no association between percentage ventricular pacing or the programmed AV interval and risk of AF could be seen. 37 There was a nonsignificant trend toward a difference between patients with prolonged PR interval randomized to AAIR versus DDDR, with a better outcome in the DDDR group, indicating that DDDR pacing may attenuate the negative effects of first‐degree AV block or at least, not be as harmful as AAIR pacing. 37 The findings in MVP and DANPACE suggest that atrial pacing may amplify the negative effects of having a first‐degree AV block. This in turn, may indicate that first‐degree AV block is not merely a “sign” of advanced disease, it may in fact be a problem in itself.
The most recent piece of information of this sort comes from the recently published posthoc, subanalysis of the Comparison of Medical Therapy, Pacing, and Defibrillation in Heart Failure (COMPANION). The COMPANION trial included patients with congestive heart failure (ischemic or nonischemic) and QRS duration exceeding 120 ms who were randomized to either CRT (‐D or ‐P) or optimal medical therapy (mean age 67 years, two‐thirds men, mean LVEF around 20%). 27 In a subanalysis by Olshansky et al., patients with prolonged PR interval (exceeding or equal to 0.20 s, 52% of the population) were compared with patients with normal PR interval. 29 In striking resemblance with the Heart and Soul Study. 22 patients with prolonged PR interval were older, more likely to be male and had somewhat wider QRS complexes. 29 In addition, they were more likely to have heart failure of ischemic etiology and renal disease was more prevalent. 29 In the patient subset treated with optimal medical therapy, patients with first‐degree AV block had a significantly higher event‐rate (all‐cause mortality or heart failure hospitalization) than patients with normal AV conduction, again emphasizing the fact that first‐degree AV block is to be considered as a negative prognostic factor. 29 In contrast, in the subset of patients treated with CRT, there were no significant differences between patients with first‐degree AV block and patients with normal AV conduction. 29 Unlike the findings in the DAVID and MVP trials, these findings indicate that the negative effect inferred by the AV‐block may possibly be attenuated, is this case using biventricular pacing.
According to these reports, treating first‐degree AV block with pacing could either amplify (AAI pacing in the MVP and DANPACE trial),34, 37 not affect (DDDR pacing in DAVID) 33 or attenuate (CRT pacing in COMPANION) 29 the negative effects inferred by the PR prolongation. Although seemingly conflicting, at least the difference between DDDR and CRT pacing may be explained by the fact that right ventricular pacing has been shown to worsen congestive heart failure and increase the risk of atrial fibrillation. 38, 39, 40, 41 It may be, that the magnitude of the negative effects inferred by the PR prolongation is not enough to balance the harm induced by right ventricular pacing only, but may be enough to balance the less detrimental effects of biventricular pacing. At this stage this is pure speculation and the concept needs to be proven in prospective, randomized trials. In any case, the findings in DAVID, MVP and COMPANION corroborate the notion that first‐degree AV block is not merely a sign of disease. Clearly, it is a factor in itself that could be influenced, either amplified or attenuated, depending on treatment strategy. The findings regarding first‐degree AV block and pacing are summarized in Figure 2.
Figure 2.
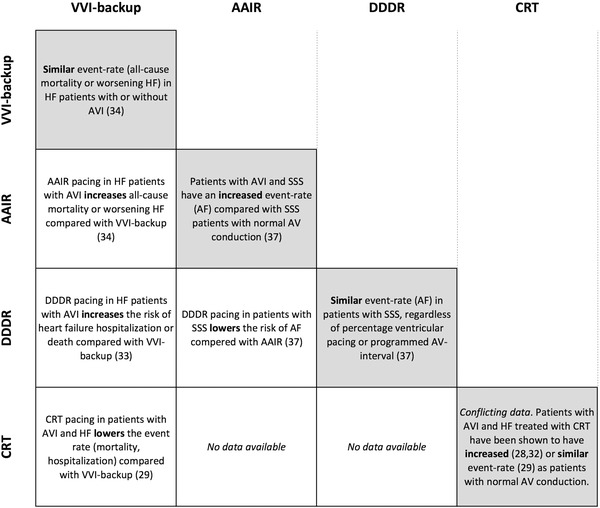
A summary of pacing and outcome in patients with first‐degree AV block. The gray boxes comprise data regarding patients with first‐degree AV block compared with patients with normal AV conduction. The white boxes comprise data regarding different pacing methods in patients with first‐degree AV block. AF = atrial fibrillation; AVI = first‐degree AV block; HF = heart failure; SSS = sick sinus syndrome.
CONCLUSIONS AND FUTURE PERSPECTIVES
In the literature, there is now overwhelming evidence that first‐degree AV block is not an entirely benign condition. Instead, the more interesting question seems to be whether a prolonged PR interval primarily identifies high‐risk patients or if the prolonged PR interval is a part of the problem per se (e.g., via delayed ventricular activation). The data from the studies discussed above, support the hypothesis that first‐degree AV block may, at least in part, be a part of the problem in itself (e.g., atrial pacing augments the negative effects, while CRT attenuates it). 29, 34, 37 If first‐degree AV block were simply a sign of advanced disease, one would not expect the effect to be modulated by different pacing modalities.
The proof of this concept in its purest form would be if first‐degree AV block were induced in otherwise healthy subjects and the outcome of these subjects was monitored and compared with controls. For obvious reasons this cannot be done, but one potentially important piece of information with respect to this question was given by a small study by Wang et al. 42 In that study the authors explored the long‐term impact of PR prolongation following ablation of AV nodal reentrant tachycardia. Seventeen patients (mean age 43 years, nine female; 3.9% of the entire cohort) developed first‐degree AV block following the ablation. During a follow‐up of three to 6 years none of these patients’ AV conduction deteriorated further, they all remained symptom free and their left ventricular ejection fraction, measured using echocardiography, remained unchanged. 42 Although the study actually provides some support for the notion that first‐degree AV block in itself may not be the problem, there is an obvious risk to once again having to rely on studies that are underpowered to detect clinically relevant differences.
Should PR interval be considered a useful predictor and more broadly used, it is probably necessary to look into more detail regarding the rate and age dependence of PR prolongation. It is likely that some kind of correction formula needs to be developed.43
Another open question is how to optimally pace in first‐degree AV block. As briefly mentioned above there is compelling evidence that right ventricular pacing may be detrimental at least in patients with heart failure. 38, 39, 40, 41 Due to the PR prolongation, these patients will require continual forced pacing when paced. 44 At least in patients with concomitant heart failure it may seem reasonable to consider CRT in those case pacing is initiated 1. 29 In a small pilot study, Iliev et al. compared AAI and DDD pacing in 19 patients with SSS and first‐degree AV block. Using echocardiography, the study showed that the optimal pacing method (as measured using the aortic flow time velocity integral) depended on intrinsic AV interval and desired pacing frequency. 45 This indicates that the optimal pacing method may well vary between individuals.
Recently, a heritable component to atrioventricular conduction was described, with a substantial portion of variability in PR interval duration attributable to heritable factors 46, 47 and genetic studies have begun to unravel genetic variants which cause prolonged PR duration. 47, 48, 49, 50, 51, 52 Interestingly, several of these genetic variants have been associated with increased risk of atrial fibrillation, heart block, and pacemaker implantation.
First‐degree AV block is without any doubt a sign of worse clinical prognosis. This may or may not be partly instituted by the PR prolongation in itself, but the truth is most probably somewhere in between. The predominantly retrospective, posthoc findings in the device trials that have given us most of the information regarding the potential to “correct” the delay in AV conduction needs to be confirmed in future, prospective studies.
FH was supported by travel grants from Sweden‐America Foundation, Swedish Heart‐Lung Foundation, Swedish Heart Association and the Fulbright Commission.
Conflicts of interest: There are no conflicts of interest to report.
REFERENCES
- 1. Epstein AE, DiMarco JP, Ellenbogen KA, et al. ACC/AHA/HRS 2008 Guidelines for Device‐Based Therapy of Cardiac Rhythm Abnormalities: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices): Developed in collaboration with the American Association for Thoracic Surgery and Society of Thoracic Surgeons. Circulation 2008;117:e350–e408. [DOI] [PubMed] [Google Scholar]
- 2. Mason JW, Ramseth DJ, Chanter DO, et al. Electrocardiographic reference ranges derived from 79,743 ambulatory subjects. J Electrocardiol 2007;40:228–234. [DOI] [PubMed] [Google Scholar]
- 3. Wu J, Kors JA, Rijnbeek PR, et al. Normal limits of the electrocardiogram in Chinese subjects. Int J Cardiol 2003;87:37–51. [DOI] [PubMed] [Google Scholar]
- 4. Massumi RA, Ali N. Determination of the site of impaired conduction in atrio‐ventricular block. J Electrocardiol 1970;3:193–209. [DOI] [PubMed] [Google Scholar]
- 5. Narula OS, Cohen LS, Samet P, et al. Localization of A‐V conduction defects in man by recording of the His bundle electrogram. Am J Cardiol 1970;25:228–237. [DOI] [PubMed] [Google Scholar]
- 6. Packard JM, Graettinger JS, Graybiel A. Analysis of the electrocardiograms obtained from 1000 young healthy aviators: Ten year follow‐up. Circulation 1954;10:384–400. [DOI] [PubMed] [Google Scholar]
- 7. Johnson RL, Averill KH, Lamb LE. Electrocardiographic findings in 67,375 asymptomatic subjects. VII. Atrioventricular block. Am J Cardiol 1960;6:153–177. [DOI] [PubMed] [Google Scholar]
- 8. Bexton RS, Camm AJ. First degree atrioventricular block. Euro Heart J 1984;5 Suppl A:107–109. [DOI] [PubMed] [Google Scholar]
- 9. Erikssen J, Otterstad JE. Natural course of a prolonged PR interval and the relation between PR and incidence of coronary heart disease. A 7‐year follow‐up study of 1832 apparently healthy men aged 40–59 years. Clin Cardiol 1984;7:6–13. [DOI] [PubMed] [Google Scholar]
- 10. Mymin D, Mathewson FA, Tate RB, Manfreda J. The natural history of primary first‐degree atrioventricular heart block. N Engl J Med 1986;315:1183–1187. [DOI] [PubMed] [Google Scholar]
- 11. Schuller H, Brandt J. The pacemaker syndrome: Old and new causes. Clin Cardiol 1991;14:336–340. [DOI] [PubMed] [Google Scholar]
- 12. Kobza R, Cuculi F, Abacherli R, et al. Twelve‐lead electrocardiography in the young: Physiologic and pathologic abnormalities. Heart Rhythm 2012;9:2018–2022. [DOI] [PubMed] [Google Scholar]
- 13. Blackburn H, Taylor HL, Keys A. Coronary heart disease in seven countries. XVI. The electrocardiogram in prediction of five‐year coronary heart disease incidence among men aged forty through fifty‐nine. Circulation 1970;41:I154–I161. [DOI] [PubMed] [Google Scholar]
- 14. Perlman LV, Ostrander LD, Jr. , Keller JB, et al. An epidemiologic study of first degree atrioventricular block in Tecumseh, Michigan. Chest 1971;59:40–46. [DOI] [PubMed] [Google Scholar]
- 15. Mansi IA, Nash IS. Ethnic differences in electrocardiographic intervals and axes. J Electrocardiol 2001;34:303–307. [DOI] [PubMed] [Google Scholar]
- 16. Upshaw CB, Jr. Comparison of the prevalence of first‐degree atrioventricular block in African‐American and in Caucasian patients: An electrocardiographic study III. J Nat Med Assoc 2004;96:756–760. [PMC free article] [PubMed] [Google Scholar]
- 17. Soliman EZ, Prineas RJ, Case LD, Zhang ZM, Goff DC, Jr. Ethnic distribution of ECG predictors of atrial fibrillation and its impact on understanding the ethnic distribution of ischemic stroke in the Atherosclerosis Risk in Communities (ARIC) study. Stroke: J Cerebral Circulation 2009;40:1204–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schnabel RB, Sullivan LM, Levy D, et al. Development of a risk score for atrial fibrillation (Framingham Heart Study): A community‐based cohort study. Lancet 2009;373:739–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cheng S, Keyes MJ, Larson MG, et al. Long‐term outcomes in individuals with prolonged PR interval or first‐degree atrioventricular block. Jama 2009;301:2571–2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Magnani JW, Gorodeski EZ, Johnson VM, et al. P wave duration is associated with cardiovascular and all‐cause mortality outcomes: The National Health and Nutrition Examination Survey. Heart Rhythm 2011;8:93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Magnani JW, Wang N, Nelson KP, et al. Electrocardiographic PR interval and adverse outcomes in older adults: The health, aging, and body composition study. Circulation Arrhythmia Electrophysiol 2013;6:84–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Crisel RK, Farzaneh‐Far R, Na B, Whooley MA. First‐degree atrioventricular block is associated with heart failure and death in persons with stable coronary artery disease: Data from the Heart and Soul Study. Euro Heart J 2011;32:1875–1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nieminen T, Verrier RL, Leino J, et al. Atrioventricular conduction and cardiovascular mortality: Assessment of recovery PR interval is superior to pre‐exercise measurement. Heart Rhythm 2010;7:796–801. [DOI] [PubMed] [Google Scholar]
- 24. Abraham WT, Fisher WG, Smith AL, et al. Cardiac resynchronization in chronic heart failure. N Engl J Med 2002;346:1845–1853. [DOI] [PubMed] [Google Scholar]
- 25. Young JB, Abraham WT, Smith AL, et al. Combined cardiac resynchronization and implantable cardioversion defibrillation in advanced chronic heart failure: The MIRACLE ICD Trial. Jama 2003;289:2685–2694. [DOI] [PubMed] [Google Scholar]
- 26. Cleland JG, Daubert JC, Erdmann E, et al. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med 2005;352:1539–1549. [DOI] [PubMed] [Google Scholar]
- 27. Bristow MR, Saxon LA, Boehmer J, et al. Cardiac‐resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med 2004;350:2140–2150. [DOI] [PubMed] [Google Scholar]
- 28. Gervais R, Leclercq C, Shankar A, et al. Surface electrocardiogram to predict outcome in candidates for cardiac resynchronization therapy: A sub‐analysis of the CARE‐HF trial. Euro J Heart Failure. 2009;11:699–705. [DOI] [PubMed] [Google Scholar]
- 29. Olshansky B, Day JD, Sullivan RM, et al. Does cardiac resynchronization therapy provide unrecognized benefit in patients with prolonged PR intervals? The impact of restoring atrioventricular synchrony: An analysis from the COMPANION Trial. Heart Rhythm 2012;9:34–39. [DOI] [PubMed] [Google Scholar]
- 30. Pires LA, Abraham WT, Young JB, et al. Clinical predictors and timing of New York Heart Association class improvement with cardiac resynchronization therapy in patients with advanced chronic heart failure: Results from the Multicenter InSync Randomized Clinical Evaluation (MIRACLE) and Multicenter InSync ICD Randomized Clinical Evaluation (MIRACLE‐ICD) trials. Am Heart J 2006;151:837–43. [DOI] [PubMed] [Google Scholar]
- 31. Tedrow UB, Kramer DB, Stevenson LW, et al. Relation of right ventricular peak systolic pressure to major adverse events in patients undergoing cardiac resynchronization therapy. Am J Cardiol 2006;97:1737–1740. [DOI] [PubMed] [Google Scholar]
- 32. Kronborg MB, Nielsen JC, Mortensen PT. Electrocardiographic patterns and long‐term clinical outcome in cardiac resynchronization therapy. Europace 2010;12:216–222. [DOI] [PubMed] [Google Scholar]
- 33. Kutalek SP, Sharma AD, McWilliams MJ, et al. Effect of pacing for soft indications on mortality and heart failure in the dual chamber and VVI implantable defibrillator (DAVID) trial. Pacing Clin Electrophysiol 2008;31:828–837. [DOI] [PubMed] [Google Scholar]
- 34. Sweeney MO, Ellenbogen KA, Tang ASL, et al. Atrial pacing or ventricular backup‐only pacing in implantable cardioverter‐defibrillator patients. Heart Rhythm 2010;7:1552–1560. [DOI] [PubMed] [Google Scholar]
- 35. Nielsen JC, Thomsen PE, Hojberg S, et al. A comparison of single‐lead atrial pacing with dual‐chamber pacing in sick sinus syndrome. Euro Heart J 2011;32:686–696. [DOI] [PubMed] [Google Scholar]
- 36. Wilkoff BL, Cook JR, Epstein AE, et al. Dual‐chamber pacing or ventricular backup pacing in patients with an implantable defibrillator: The Dual Chamber and VVI Implantable Defibrillator (DAVID) Trial. Jama 2002;288:3115–3123. [DOI] [PubMed] [Google Scholar]
- 37. Nielsen JC, Thomsen PE, Hojberg S, et al. Atrial fibrillation in patients with sick sinus syndrome: The association with PQ‐interval and percentage of ventricular pacing. Europace 2012;14:682–689. [DOI] [PubMed] [Google Scholar]
- 38. Sweeney MO, Hellkamp AS, Lee KL, Lamas GA. Association of prolonged QRS duration with death in a clinical trial of pacemaker therapy for sinus node dysfunction. Circulation 2005;111:2418–2423. [DOI] [PubMed] [Google Scholar]
- 39. Olshansky B, Day JD, Lerew DR, et al. Eliminating right ventricular pacing may not be best for patients requiring implantable cardioverter‐defibrillators. Heart Rhythm 2007;4:886–891. [DOI] [PubMed] [Google Scholar]
- 40. Sharma AD, Rizo‐Patron C, Hallstrom AP, et al. Percent right ventricular pacing predicts outcomes in the DAVID trial. Heart Rhythm 2005;2:830–834. [DOI] [PubMed] [Google Scholar]
- 41. Steinberg JS, Fischer A, Wang P, et al. The clinical implications of cumulative right ventricular pacing in the multicenter automatic defibrillator trial II. J Cardiovasc Electrophysiol 2005;16:359–365. [DOI] [PubMed] [Google Scholar]
- 42. Wang L, Li J, Yao R, Song S, Guo Z. Long‐term follow‐up of patients with P‐R prolongation after catheter ablation of slow pathway for atrioventricular node re‐entrant tachycardia. Arch Med Res 2004;35:442–445. [DOI] [PubMed] [Google Scholar]
- 43. Soliman EZ, Rautaharju PM. Heart rate adjustment of PR interval in middle‐aged and older adults. J Electrocardiol 2012;45:66–69. [DOI] [PubMed] [Google Scholar]
- 44. Barold SS, Herweg B. Conventional and biventricular pacing in patients with first‐degree atrioventricular block. Europace 2012;14:1414–1419. [DOI] [PubMed] [Google Scholar]
- 45. Iliev, II , Yamachika S, Muta K, et al. Preserving normal ventricular activation versus atrioventricular delay optimization during pacing: The role of intrinsic atrioventricular conduction and pacing rate. Pacing Clin Electrophysiol 2000;23:74–83. [DOI] [PubMed] [Google Scholar]
- 46. Newton‐Cheh C, Guo CY, Wang TJ, O'Donnell C J, Levy D, Larson MG. Genome‐wide association study of electrocardiographic and heart rate variability traits: The Framingham Heart Study. BMC Med Genet 2007;8 (Suppl 1):S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Smith JG, Lowe JK, Kovvali S, et al. Genome‐wide association study of electrocardiographic conduction measures in an isolated founder population: Kosrae. Heart Rhythm 2009;6:634–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Pfeufer A, van Noord C, Marciante KD, et al. Genome‐wide association study of PR interval. Nat. Genet 2010;42:153–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Holm H, Gudbjartsson DF, Arnar DO, et al. Several common variants modulate heart rate, PR interval and QRS duration. Nat Genet 2010;42:117–122. [DOI] [PubMed] [Google Scholar]
- 50. Chambers JC, Zhao J, Terracciano CM, et al. Genetic variation in SCN10A influences cardiac conduction. Nat Genet 2010;42:149–152. [DOI] [PubMed] [Google Scholar]
- 51. Smith JG, Magnani JW, Palmer C, et al. Genome‐wide association studies of the PR interval in African Americans. PLoS Genetics 2011;7:e1001304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Butler AM, Yin X, Evans DS, et al. Novel Loci associated with PR interval in a genome‐wide association study of ten African American cohorts. Cir Cardiovasc Genet 2012. 5:639–646. [DOI] [PMC free article] [PubMed] [Google Scholar]


