Abstract
Background: QRS fragmentation (fQRS) has been shown to be a marker of scar in patients with left ventricular dysfunction. Whether fQRS is associated with progressive left ventricular remodeling and increased mortality in patients receiving cardiac resynchronization therapy (CRT) is unclear.
Methods: We reviewed the preimplant and follow‐up echocardiograms in 233 patients undergoing the new implantation of a CRT device between December 2001 and November 2006. Patients were included if they had a pre‐CRT ECG with appropriate filter settings (filter 0.16–100 or 0.16–150 Hz, 25 mm/s, 10 mm/mV), a left ventricular ejection fraction (LVEF) ≤40%, and New York Heart Association class II–IV symptoms on standard medical therapy. The 12‐lead electrocardiogram (ECG) was interpreted by two blinded reviewers for the presence of fQRS. Remodeling end points, including changes in LVEF and left ventricular end‐diastolic (LVEDV) and systolic (LVESV) volumes, were compared between patients with and without contiguous fQRS, and an assessment of all‐cause mortality was made.
Results: Two hundred thirty‐two patients met inclusion criteria, of which 50 demonstrated fQRS in contiguous leads. There was no difference in improvement in LVEF (%) (7.9 ± 12.9 vs 6.8 ± 11.0, P = 0.60) or reduction in LVEDV (mL) (−30.1 ± 57.2 vs −15.7 ± 47.6) or LVESV (mL) (−33.7 ± 58.1 vs −22.7 ± 50.6, P = 0.40) between patients with and without contiguous fQRS. At a mean follow‐up of 4.4 ± 1.9 years, there were a total of 89 deaths, 22 (44.0%) in patients with contiguous fQRS and 67 (36.8%) without (log rank P = 0.31).
Conclusions: QRS fragmentation is not a predictor of progressive ventricular remodeling or mortality in heart failure patients undergoing CRT.
Ann Noninvasive Electrocardiol 2011;16(2):165–171
Keywords: cardiac resynchronization therapy, electrocardiogram, ECG, QRS fragmentation, left ventricular scar
QRS fragmentation (fQRS), defined as various notching patterns in the QRS complex on a standard 12‐lead ECG, has been reported to be a marker of adverse cardiac events in patients with multiple cardiovascular disease states. 1 In patients with suspected or documented coronary artery disease, fQRS in contiguous leads is associated with myocardial scar, arrhythmic events, and mortality. 2 , 3 , 4 In addition, the location of fQRS on the 12‐lead ECG has been reported to correspond to the affected coronary artery territory. 2 In patients with nonischemic cardiomyopathy, fQRS has been associated with myocardial scar, as defined by gadolinium‐delayed enhancement on cardiac MRI and increased ventricular arrhythmias. 5 , 6 While fQRS was initially defined only in patients with narrow QRS complexes, recently, the definition has been extended to patients with wide QRS complexes (≥120 ms), including those with bundle branch block, paced ventricular rhythms, and premature ventricular contractions. 2 In patients undergoing CRT, increased myocardial scar burden assessed by nuclear imaging and cardiac magnetic resonanance imaging (MRI) has been shown to correlate with progressive remodeling and poor outcomes. 7 , 8 We tested the hypothesis that in patients with left ventricular dysfunction undergoing CRT, the presence of fQRS in contiguous leads on the preimplant ECG is associated with less improvement in left ventricular ejection fraction (LVEF) and reductions in end‐diastolic and systolic volumes and higher all‐cause mortality compared to patients without fQRS.
METHODS
The study was a retrospective review of a prospectively collected cohort of consecutive patients undergoing the new implantation of a CRT device at the Cleveland Clinic, Cleveland, Ohio, between December 27, 2001 and November 3, 2006. Patients were included if they had high‐quality pre‐ and post‐CRT echocardiograms with the post‐CRT echocardiogram occurring at least 2 months after implant, baseline New York Heart Association class II to IV heart failure symptoms, baseline LVEF ≤ 40%, and a 12‐lead ECG prior to CRT with appropriate filter settings to detect fQRS (filter 0.16–100 or 0.16–150 Hz, 25 mm/s, 10 mm/mV). Patients who lacked US social security numbers were excluded from the analysis of mortality. The study was approved by the Institutional Review Board of the Cleveland Clinic for retrospective medical records review and performed according to institutional guidelines.
fQRS DEFINITIONS
The 12‐lead ECG was analyzed by two blinded cardiologists for the presence of fQRS in contiguous leads defined as follows: anterior (V1‐V5), lateral (I, avL, V6), and inferior (II, III, avF). In rare instances of disagreement, the ECG was re‐reviewed by both reviewers and a consensus decision reached. fQRS was defined as follows in accord with definitions previously reported. 2 , 3 In patients with narrow QRS complexes, fQRS was defined as the presence of an additional R wave (R′), notching in the nadir of the R wave or the S wave, or the presence of more than one R′ wave. In patients with wide QRS complexes, fragmented left bundle branch block, right bundle branch block, and nonspecific intraventricular conduction delay (defined by standard ECG criteria) were defined as the presence of >2 R waves, >2 notches in the R wave, or >2 notches in the downstroke or upstroke of the S wave (Fig. 1). Fragmented premature ventricular complexes were defined as the presence of >2 R′ waves, >2 notches in the S wave, or only two notches in the R wave but where the notches were >40 ms apart. Fragmented‐paced QRS complexes were defined as the presence of >2 R′ waves or >2 notches in the S wave.
Figure 1.
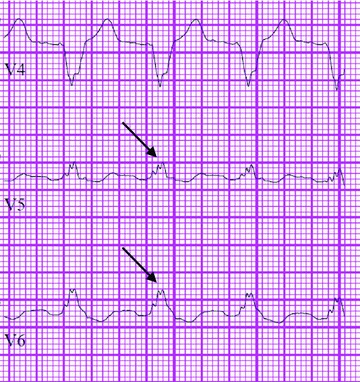
Example of QRS fragmentation in a patient with a left bundle branch block.
CRT DEVICE IMPLANTATION
CRT device implantations were performed transvenously in the vast majority of patients by electrophysiologists targeting a lateral or posterolateral vein for the left ventricular lead position. In instances when a transvenous lead could not be placed due to procedural difficulty, a minimally invasive epicardial lead was placed by a staff cardiothoracic surgeon. CRT devices were commonly programmed with an atrioventricular sensed delay of 100 ms and paced delay of 130 ms, with optimization performed according to the standard protocols of the Cleveland Clinic. Medications were recorded immediately prior to implantation of the CRT device with subsequent titration of medications made at the discretion of patients’ outpatient physicians.
DATA COLLECTION
Clinical data were gathered via retrospective chart review. Echocardiograms were reanalyzed by two board‐certified cardiologists specializing in cardiac imaging blinded to the current study. Left ventricular end‐diastolic (LVEDV) and systolic (LVESV) volumes were manually recorded and LVEFs calculated from the volumetric data. Remodeling end points after CRT included changes in LVEDV, LVESV, and LVEF. All‐cause mortality was assessed using the US Social Security Death Index. Lead position was analyzed using a postimplant PA and lateral chest x‐ray by two cardiologists blinded to the current study using a standardized technique that has been described previously. 9
STATISTICAL ANALYSIS
Continuous variables are presented as a mean ± standard deviation and dichotomous variables as an absolute number with percentage. Comparisons between continuous variables were made using student's t‐tests for parametric variables and Mann‐Whitney tests for nonparametric variables. Dichotomous variables were compared using Fisher's exact tests. Variables thought to impact outcomes in patients with heart failure, including fQRS in contiguous leads, were entered into a forward stepwise Cox multivariate regression model to determine factors significantly associated with all‐cause mortality. Kaplan‐Meier curves compared time to long‐term mortality in patients based on the presence of fQRS. 10 A log rank test was used to compare time to death between the two groups. A two‐sided P ≤ 0.05 was considered statistically significant. All analyses were done using SPSS software (Rel. 17.0, 2007., SPSS Inc., Chicago, IL).
RESULTS
Two hundred thirty‐two patients met inclusion criteria and comprised the final cohort. Two patients who lacked US social security numbers were excluded from the analysis of mortality. Of the 232 patients, 50 (21.6%) had fQRS. An example of fQRS is shown in Figure 1.
Patient Characteristics
Baseline characteristics of the study population are listed in Table 1. In the total population, 73.7% were male, 52.6% had ischemic cardiomyopathy, and 43.1% had native left bundle branch block. Baseline characteristics were similar between patients with and without fQRS except that patients with fQRS were more likely to be female (38.0% vs 23.1%, P = 0.045) and have a wider baseline QRS duration (174.5 ± 27.2 vs 158.3 ± 29.2 ms, P = 0.004), a higher incidence of native left bundle branch block (64.0% vs 37.4%, P = 0.001), and a trend toward lower incidences of right bundle branch block (0 vs 6.6%, P = 0.07) and nonspecific intraventricular conduction delay (14.0% vs 26.9%, P = 0.06). There was also a nonsignificant trend toward more ischemic cardiomyopathy (62.0% vs 50.0%, P = 0.15) and myocardial infarctions (40.0% vs 28.6%, P = 0.12) in patients with fQRS than patients without fQRS. Of the 50 patients in the fragmented QRS cohort, fQRS occurred in the following distributions: 18 (36.0%) inferior, 13 (26.0%) inferior and lateral, 15 (30.0%) lateral, 3 (6%) anterior, and 1 (2%) anterior and inferior.
Table 1.
Baseline Patient Characteristics
| Total (n = 232) | QRS Fragmentation in Contiguous Leads (n = 50) | QRS Fragmentation Not Present in Contiguous Leads (n = 182) | p Value | |
|---|---|---|---|---|
| Age (years) | 64.2 ± 11.8 | 64.5 ± 11.1 | 64.1 ± 12.0 | 0.88 |
| Male gender | 171 (73.7%) | 31 (62.0%) | 140 (76.9%) | 0.045 |
| Ischemic cardiomyopathy | 122 (52.6%) | 31 (62.0%) | 91 (50.0%) | 0.15 |
| ICD* | 217 (93.5%) | 47 (94.0%) | 170 (93.4%) | 1.0 |
| Epicardial left ventricular lead | 25 (10.8%) | 8 (16.0%) | 17 (9.3%) | 0.20 |
| Diabetes mellitus | 83 (35.8%) | 18 (36.0%) | 65 (35.7%) | 1.0 |
| COPD | 36 (15.5%) | 9 (18.0%) | 27 (14.8%) | 0.66 |
| Hypertension | 134 (57.8%) | 29 (58.0%) | 105 (57.7%) | 0.87 |
| History of myocardial infarction | 72 (31.0%) | 20 (40.0%) | 52 (28.6%) | 0.12 |
| Hyperlipidemia | 126 (54.3%) | 23 (46.0%) | 103 (56.6%) | 0.20 |
| Serum creatinine (mg/dL) | 1.3 ± 0.5 | 1.3 ± 0.6 | 1.3 ± 0.5 | 0.99 |
| Serum Hemoglobin (g/dL) | 13.0 ± 1.7 | 13.0 ± 1.6 | 13.0 ± 1.8 | 0.55 |
| Serum BNP (pg/mL) † | 597.2 ± 738.4 | 582.9 ± 706.7 | 601.0 + 748.7 | 0.99 |
| QRS duration (ms) pre | 161.8 ± 29.5 | 174.5 ± 27.2 | 158.3 ± 29.2 | 0.0004 |
| QRS duration (ms) post CRT | 158.6 ± 20.4 | 162.7 ± 21.7 | 157.4 ± 20.0 | 0.09 |
| Lateral lead placement‡ | 53 (26.6%) | 11 (25.6%) | 42 (26.9%) | 1.0 |
| Anterior lead placement‡ | 8 (16.7%) | 3 (7.0%) | 5 (3.2%) | 0.37 |
| Posterior lead placement‡ | 138 (69.3%) | 29 (67.4) | 109 (70.0%) | 0.85 |
| Left bundle branch block | 100 (43.1%) | 32 (64.0%) | 68 (37.4%) | 0.001 |
| Right bundle branch block | 12 (5.2%) | 0 | 12 (6.6%) | 0.07 |
| IVCD§ | 56 (24.1%) | 7 (14.0%) | 49 (26.9%) | 0.06 |
| Paced rhythm | 50 (21.6%) | 10 (20.0%) | 40 (22.0%) | 0.85 |
| Narrow (<120 ms) | 14 (6.0%) | 1 (2.0%) | 13 (7.1%) | 0.31 |
| Beta blocker | 168 (76.4%) | 38 (79.2%) | 130 (75.6%) | 0.70 |
| Ace inhibitor or angiotensin receptor blocker | 177 (80.5%) | 37 (77.1%) | 140 (78.7%) | 0.54 |
| Aspirin | 115 (52.3%) | 24 (50.0%) | 91 (52.9%) | 0.75 |
| Diuretic | 181 (82.3%) | 40 (83.3%) | 141 (82.0%) | 1.0 |
| Nitrates | 77 (35.0%) | 21 (43.8%) | 56 (32.6%) | 0.17 |
| Hydralazine | 31 (14.1%) | 4 (8.3%) | 27 (15.7%) | 0.25 |
| Statin | 106 (48.2%) | 26 (54.2%) | 80 (46.5%) | 0.41 |
| Antiarrhythmic medication | 58 (26.4%) | 14 (29.2%) | 44 (25.6%) | 0.71 |
| Baseline NYHA functional class II | 15 (6.5%) | 2 (4.0%) | 13 (7.1%) | 0.53 |
| Baseline NYHA functional class III | 209 (90.1%) | 45 (90.0%) | 164 (90.1%) | 1.0 |
| Baseline NYHA functional class IV | 8 (3.4%) | 3 (6.0%) | 5 (2.7%) | 0.37 |
| Time from implant to follow‐up echocardiogram (months) | 11.6 ± 9.0 | 12.4 ± 8.5 | 11.3 ± 9.1 | 0.22 |
*History of implantable cardiac defibrillator implantation.
†Serum brain natriuretic peptide prior to CRT.
‡Available in 199 patients; 43 contiguous and 156 noncontiguous.
§Nonspecific intraventricular conduction delay.
Medications available in 220/232 total; 48/50 fragmented; 172/182 nonfragmented.
Remodeling and Mortality Outcomes after CRT
The follow‐up echocardiogram was performed at a mean of 11.6 ± 9.0 months following device implantation. Patients with contiguous fQRS had similar improvements in LVEF (7.9 ± 12.9 vs 6.8 ± 11.0%, P = 0.60) and reductions in LVEDV (−30.1 ± 57.2 vs −15.7 ± 47.6 mL) and LVESV (−33.7 ± 58.1 vs −22.7 ± 50.6 mL, P = 0.40) compared to those without fQRS, respectively (Table 2). To evaluate the relationship between fQRS and remodeling further, patients with fQRS present in at least one lead or in three or greater leads were compared to all others (Table 3). In these two subgroups, no significant differences were noted in any of the remodeling end points.
Table 2.
Changes in Remodeling End Points in Patient with and without QRS Fragmentation in Contiguous Leads
| Total (n = 232) | Fragmented QRS (n = 50) | Nonfragmented QRS (n = 182) | p Value | |
|---|---|---|---|---|
| LVEF pre‐CRT (%) | 23.7 ± 7.5 | 22.7 ± 6.8 | 23.9 ± 7.7 | 0.41 |
| LVEF post‐CRT (%) | 30.6 ± 12.8 | 30.2 ± 14.0 | 30.7 ± 12.6 | 0.70 |
| LVEF difference (%) | 7.0 ± 11.4 | 7.9 ± 12.9 | 6.8 ± 11.0 | 0.60 |
| LVEDV pre (mL) | 250.4 ± 83.7 | 253.6 ± 82.9 | 249.6 ± 84.2 | 0.53 |
| LVEDV post (mL) | 231.6 ± 95.0 | 223.5 ± 93.8 | 233.9 ± 95.5 | 0.57 |
| LVEDV difference (mL) | −18.8 ± 50.0 | −30.1 ± 57.2 | −15.7 ± 47.6 | 0.14 |
| LVESV pre (mL) | 194.2 ± 76.8 | 198.0 ± 74.3 | 193.1 ± 77.6 | 0.40 |
| LVESV post (mL) | 169.1 ± 91.0 | 164.3 ± 90.6 | 170.4 ± 91.3 | 0.85 |
| LVESV difference (mL) | −25.1 ± 52.3 | −33.7 ± 58.1 | −22.7 ± 50.6 | 0.40 |
LVEF = left ventricular ejection fraction; LVEDV = left ventricular end‐diastolic volume; LVESV = left ventricular end‐systolic volume; pre = pre–biventricular pacemaker implantation; post = at follow‐up post–biventricular pacemaker implantation.
Table 3.
Remodeling End Points and QRS Fragmentation
| No QRS Fragmentation (n = 146) | QRS Fragmentation any lead (n = 86) | p Value | QRS Fragmentation in >3 leads (n = 33) | p Value | |
|---|---|---|---|---|---|
| LVEF pre | 23.9 ± 7.7 | 23.3 ± 7.0 | 0.66 | 21.8 ± 6.6 | 0.22 |
| LVEF post | 31.4 ± 13.0 | 29.2 ± 12.5 | 0.29 | 28.5 ± 12.9 | 0.25 |
| LVEF difference | 7.6 ± 11.4 | 6.1 ± 11.6 | 0.39 | 7.4 ± 11.1 | 0.78 |
| LVEDV pre | 242.4 ± 81.1 | 264.1 ± 86.7 | 0.05 | 240.4 ± 88.2 | 0.79 |
| LVEDV post | 227.7 ± 94.5 | 238.3 ± 96.1 | 0.36 | 226.5 ± 100.3 | 0.92 |
| LVEDV difference | −14.7 ± 44.3 | −25.8 ± 58.1 | 0.41 | −13.9 ± 46.5 | 0.92 |
| LVESV pre | 187.9 ± 75.6 | 204.9 ± 78.0 | 0.06 | 189.9 ± 80.3 | 0.89 |
| LVESV post | 164.7 ± 89.8 | 176.6 ± 93.1 | 0.27 | 169.4 ± 96.1 | 0.69 |
| LVESV diff | −23.2 ± 45.7 | −28.3 ± 62.2 | 0.72 | −20.5 ± 42.6 | 0.60 |
LVEF = left ventricular ejection fraction; LVEDV = left ventricular end‐diastolic volume; LVESV = left ventricular end‐systolic volume: pre = pre–biventricular pacemaker implantation; post = at follow‐up post–biventricular pacemaker implantation.
At a mean follow‐up of 4.4 ± 1.9 years, there were a total of 89 deaths (38.7%), 22 in the fragmented QRS group (44.9%) and 67 in the nonfragmented QRS group (37.0%). Kaplan‐Meier analysis demonstrated no significant difference between the two groups (log rank P = 0.31) (Fig. 2). In multivariate forward stepwise analysis, fQRS was not part of the final model describing variables associated with all‐cause mortality (Table 4).
Figure 2.
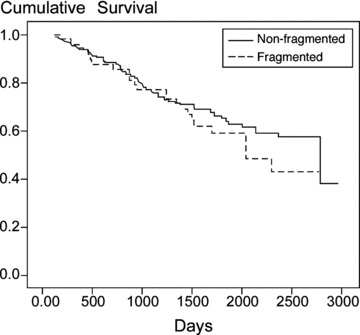
Kaplan‐Meier curves comparing all‐cause mortality in patients with and without QRS fragmentation in contiguous leads.
Table 4.
Cox Multivariate Hazard Model for All‐Cause Mortality
| HR (95% CI) | p Value | |
|---|---|---|
| Ischemic cardiomyopathy | 2.2 (1.3–3.7) | 0.002 |
| History of atrial fibrillation | 2.2 (1.3–3.7) | 0.002 |
| Serum creatinine pre‐CRT (mg/dL) | 2.0 (1.5–2.8) | <0.0001 |
| Red cell distribution width pre‐CRT | 1.2 (1.1–1.3) | 0.005 |
DISCUSSION
Identifying patients prior to or early after device implantation who are at heightened risk of nonresponse to CRT continues to be an important clinical question in determining appropriate advanced treatment options in patients with progressive heart failure. Despite the intense research focus this topic has garnered, accurately predicting nonresponse and poor long‐term outcomes in heart failure patients undergoing CRT has remained a challenge. The presence of fQRS on a standard 12‐lead ECG has been shown to be a moderately sensitive and specific sign for myocardial scar in patients with both ischemic and nonischemic cardiomyopathy. 2 , 5 , 6 Based on this finding, we sought to determine whether fQRS was associated with progressive remodeling, indicating poor responsiveness to CRT, and increased all‐cause mortality in heart failure patients undergoing CRT. In our study, however, we found no differences in indices of left ventricular remodeling or rates of all‐cause mortality between patients with and without fQRS.
fQRS has been associated with adverse events in patients with both ischemic and nonischemic cardiomyopathies. 4 In patients with known or suspected coronary artery disease, fQRS is associated with the presence of myocardial scar and higher rates of myocardial infarction, cardiac death, need for revascularization, arrhythmic events, and all‐cause mortality. 11 In patients with a history of myocardial infarction, fQRS may be a better marker of regional perfusion abnormalities than a Q wave. 12 fQRS is also associated with increased rates of recurrent cardiac death, nonfatal myocardial infarction, or unstable angina in patients who suffered a Q‐wave myocardial infarction in whom the Q wave resolved. 13
While substantially less is known with regard to fQRS in patients with nonischemic cardiomyopathy, a relationship has been demonstrated between fQRS and the presence of scar on cardiac MRI. 5 In patients with suspected sarcoidosis, fQRS was shown to correlate with cardiac involvement based on an assessment of gadolinium delayed enhancement images. 6 Patients with nonischemic dilated cardiomyopathies and fQRS have also been shown to have increased arrhythmic events and a trend toward higher rates of all‐cause mortality. 4 Finally, fQRS has been shown to be an independent predictor of arrhythmic events in patients with the Brugada syndrome and to be a more sensitive marker than an epsilon wave in the diagnosis of arrhythmogenic right ventricular dysplasia. 14 , 15
While the precise mechanism is still under investigation, fQRS is thought to be due to inhomogenous ventricular activation due to myocardial scar and/or ischemia. 16 Wide band recording in patients with coronary artery disease reveals greater QRS notching in patients with a myocardial scar. 17 A correlation, however, between fQRS and scar burden has yet to be established. Therefore, it is quite possible that patients with fQRS in our study may have had scar but not to a sufficient degree as to preclude the favorable effects of CRT.
Recently, Morita and colleagues demonstrated the presence of fQRS in the absence of structural heart disease. 14 Using a canine model of the Brugada syndrome, the authors successfully produced fQRS using delayed pacing of excised epicardial tissue treated with medications to induce Brugada physiology. 14 The authors concluded that fQRS may be a marker of an arrhythmogenic substrate independent of the presence of myocardial scar. 14 Given that the presence of myocardial scar has been well documented to be a predictor of progressive remodeling in patients undergoing CRT, it is this latter mechanism that may also explain why fQRS was not associated with impaired outcomes in our study. Given the retrospective nature of this study, many of the patients in our cohort had no study to assess myocardial scar. Therefore, it is possible that the fQRS seen in some patients in this cohort was the result of electrical abnormalities independent of scar. These abnormalities may not have precluded the reverse remodeling effects of CRT. In addition, the large majority of patients in this cohort had an internal cardioverter defibrillator, which presumably would have been protective against arrhythmic deaths, thus, potentially explaining why no difference in mortality between the two groups was observed.
Patients with fQRS in our study were more likely to be female, have higher incidences of left bundle branch block, lower incidences of right bundle branch block and nonspecific intraventricular conduction delay, and have a wider baseline QRS duration compared to those without fQRS. In the recently published large MADIT‐CRT study, female gender, a wider baseline QRS duration, and the presence of a left bundle branch block were associated with improved outcomes following CRT. 18 Therefore, it is possible that these factors, which portend favorable outcomes, may have overcome the negative influence that fQRS may have had.
One final reason why our study failed to show progressive remodeling in the fQRS population may be due to the definition or functional significance of fQRS in patients with a wide QRS complex. In our cohort, 94% of the patients had a QRS of ≥120 msec. To our knowledge, only one study has evaluated outcomes in a large cohort of patients with fQRS with wide QRS complexes. 2 Based on the definition of fQRS in patients with wide QRS duration derived by Das and colleagues, fQRS had a sensitivity and specificity for myocardial scar of 86.8% and 92.5%, respectively. 2 It does not appear the correlation between scar and fQRS in patients with wide QRS complexes has been validated in other studies.
Our study has several limitations. The retrospective nature cannot account for all confounders despite our best efforts to identify important baseline differences. Patients in our cohort come from a single tertiary care center and, therefore, may not be representative of patients presenting to other centers. There may be a selection bias since all patients in the cohort had available, high‐quality pre‐ and post‐CRT echocardiograms. There is no reason to believe, however, that inclusion of patients with lower quality echocardiograms would have changed our results. This study did not include an assessment of myocardial scar, which would have been useful to determine the relationship between fQRS and scar in this population. Although our study could have been underpowered to identify a statistically significant difference in left ventricular remodeling indices between patients with and without fQRS, assuming a clinically significant LVEF difference of 10% and a standard deviation of 10 based on prior studies of CRT, for an alpha level of 0.05 we would need only 16 patients in each group to achieve a power of 80%. Our cohort had 50 patients with and 182 without fQRS, which appears to be an adequate population size for comparison.
CONCLUSIONS
In this study of patients undergoing CRT, the presence of fragmented QRS complexes on the 12‐lead ECG was not associated with progressive remodeling, indicating CRT unresponsiveness or increased all‐cause mortality following CRT. Future studies to determine the relationship between total myocardial scar burden and fragmented QRS complexes in patients with wide QRS complexes may be useful in determining the significance of fQRS in patients considered for CRT.
Disclosures: John Rickard MD: No disclosures. Omeed Zardkoohi MD: No disclosures. Zoran Popovic MD: No disclosures. David Verhaert MD: No disclosures. Dan Sraow MD: No disclosures. Bryan Baranowski MD: No disclosures. David O. Martin MD: Boston Scientific Research support, major. Richard A. Grimm DO: No disclosures. Mina K. Chung, MD: Medtronic, St. Jude Medical, Boston Scientific, Biotronik: Research support. Patrick Tchou MD: No disclosures. Bruce A. Lindsay: Minor educational Boston Scientific. Bruce L. Wilkoff MD: Medtronic, St. Jude Medical and Boston Scientific: consulting, modest; research support, major.
REFERENCES
- 1. Das MK, Zipes DZ. Fragmented QRS: A predictor of mortality and sudden cardiac death. Heart Rhythm 2009;6:S8‐S14. [DOI] [PubMed] [Google Scholar]
- 2. Das MK, Suradi H, Maskoun W, et al Fragmented wide QRS on a 12‐lead ECG: A sign of myocardial scar and poor prognosis. Circ Arrhythmia Electrophysiol 2008;1:258–368. [DOI] [PubMed] [Google Scholar]
- 3. Das MK, Saha C, El Masry H, et al Fragmented QRS on a 12‐lead ECG: A predictor of mortality and cardiac events in patients with coronary artery disease. Heart Rhythm 2007;4:1385–1392. [DOI] [PubMed] [Google Scholar]
- 4. Das MK, Maskoun W, Shen C, et al Fragmented QRS on twelve‐lead electrocardiogram predicts arrhythmic events in patients with ischemic and nonischemic cardiomyopathy. Heart Rhythm 2010;7:74–80. [DOI] [PubMed] [Google Scholar]
- 5. Homsi M, Alsayed L, Das MK, et al Fragmented QRS complexes on a 12‐lead ECG is a marker of greater myocardial scarring related to non‐coronary artery diseases by magnetic resonance imaging. J Am Coll Cardiol 2009;53:A140. [Google Scholar]
- 6. Homsi M, Alsayed L, Safadi B, et al Fragmented QRS complexes on 12‐lead ECG: A marker of cardiac sarcoidosis as detected by gadolinium cardiac magnetic resonance imaging. Ann Noninvasive Electrocardiol 2009;14:319–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bleeker GB, Kaandorp TA, Lamb HJ, et al Effect of posterolateral scar tissue on clinical and echocardiographic improvement after cardiac resynchronization therapy. Circulation 2006;113:969–976. [DOI] [PubMed] [Google Scholar]
- 8. Adelstein EC, Saba S. Scar burden by myocardial perfusion imaging predicts echocardiographic response to cardiac resynchronization therapy in ischemic cardiomyopathy. Am Heart J 2007;153:105–112. [DOI] [PubMed] [Google Scholar]
- 9. Wilton SB, Shibata MA, Sondergaard R, et al Relationship between left ventricular lead position using a simple radiographic classification scheme and long‐term outcome with resynchronization therapy. J Interv Card Electrophysiol 2008;23:219–227. [DOI] [PubMed] [Google Scholar]
- 10. Kaplan ER, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958;53:457–481 [Google Scholar]
- 11. Das MK, El Masry H. Fragmented QRS and other depolarization abnormalities as a predictor of mortality and sudden cardiac death. Curr Opin Cardiol 2010;25:59–64. [DOI] [PubMed] [Google Scholar]
- 12. Das MK, Khan B, Jacob S, et al Significance of a fragmented QRS complex versus a Q wave in patients with coronary artery disease. Circulation 2006;113:2495–2501. [DOI] [PubMed] [Google Scholar]
- 13. Pietrasik G, Goldenberg I, Zdzienicka J, et al Prognostic significance of fragmented QRS complex for predicting the risk of recurrent cardiac events in patients with Q‐wave myocardial infarction. Am J Cardiol 2007;100:583–586. [DOI] [PubMed] [Google Scholar]
- 14. Morita H, Kusano KF, Miura D, et al Fragmented QRS as a marker of conduction abnormality and a predictor of prognosis of Brugada Syndrome. Circulation 2008;18:1697–1704. [DOI] [PubMed] [Google Scholar]
- 15. Peters S, Truemmel M, Koehler B. QRS fragmentation in standard ECG as a diagnostic marker of arrythmogenic right ventricular dysplasia‐cardiomyopathy. Heart Rhythm 2008;5:1417–1421. [DOI] [PubMed] [Google Scholar]
- 16. Das MH, Zipes DP. Fragmented QRS: A predictor of mortality and sudden cardiac death. Heart Rhythm 2009;6:S8–S14. [DOI] [PubMed] [Google Scholar]
- 17. Langner PH Jr, Geselowitz DB, Briller SA. Wide band recording of the electrocardiogram and coronary heart disease. Am Heart J 1973;86:308–317. [DOI] [PubMed] [Google Scholar]
- 18. Moss AJ, Hall WJ, Cannom DS, et al Cardiac resynchronization therapy for the prevention of heart‐failure events. N Engl J Med 2009;361:1329–1338. [DOI] [PubMed] [Google Scholar]


