Abstract
Background
Automatic detection of atrial fibrillation (AF) in electrocardiograms (ECGs) is beneficial for AF diagnosis, therapy, and management. In this article, a novel method of AF detection is introduced. Most current methods only utilize the RR interval as a critical parameter to detect AF; thus, these methods commonly confuse AF with other arrhythmias.
Methods
We used the average number of f waves in a TQ interval as a characteristic parameter in our robust, real‐time AF detection method. Three types of clinical ECG data, including ECGs from normal, AF, and non‐AF arrhythmia subjects, were downloaded from multiple open access databases to validate the proposed method.
Results
The experimental results suggested that the method could distinguish between AF and normal ECGs with accuracy, sensitivity, and positive predictive values (PPVs) of 93.67%, 94.13%, and 98.69%, respectively. These values are comparable to those of related methods. The method was also able to distinguish between AF and non‐AF arrhythmias and had performance indexes (accuracy 94.62%, sensitivity 94.13%, and PPVs 97.67%) that were considerably better than those of other methods.
Conclusions
Our proposed method has prospects as a practical tool enabling clinical diagnosis, treatment, and monitoring of AF.
Keywords: electrocardiogram (ECG), atrial fibrillation, detection, accuracy, real‐time
Atrial fibrillation (AF) is the most common sustained arrhythmia. AF occurs in approximately 1–2% of the population and can cause serious complications, such as heart failure and stroke.1, 2 In most cases, cardiac experts detect AF using long‐term electrocardiogram (ECG) recordings. This detection requires a burdensome search for AF characteristics in lengthy recordings. In addition, other arrhythmias have similar characteristics to AF and are difficult to distinguish from it. Thus, it is desirable to develop methods that automatically detect AF to reduce the workload of doctors and increase the accuracy of AF diagnosis.
In recent years, researchers have adopted RR interval (RRI) analysis to detect AF. Petrucci et al. performed statistical analysis of RRIs in AF ECG signals from the MIT database 3 and identified the signal asymmetry and characteristics of these RRIs. Ghodrati et al.4 developed an AF diagnosis method using the absolute deviation of RRIs and the variation of consecutive RRIs (ΔRRI). Based on the randomness, variability, and complexity of RRIs, Dash et al.5 proposed to combine the turning point ratio, the mean square root, and the Shannon entropy of ΔRRI parameters to characterize AF. Lin et al.6 proposed to detect the AF using ΔRRI and the standard deviation of the RRI (RRIstd). They compared the ECGs of 50 AF patients and 50 healthy subjects using ΔRRI and RRIstd as parameters and were able to distinguish patients from controls with an accuracy of 95.14%. Huang et al.7 applied the density histograms of RRI variation to express the distribution difference curve of RRIs and detected AF events using the peaks of the curve. Lian et al.8 drew two dimensional diagrams using the RRI and the variation of the RRI and divided the diagrams into grids with 25 ms × 25 ms resolution. AF patients and controls were distinguished by the number of nonempty grids in the diagrams. Classic pattern recognition methods have also been applied to detect AF. For example, Mohebbi et al.9, 10 used the support vector machine (SVM) to predict AF based on the characteristics of RRIs and heart rate variability (HRV). Of all the methods, the one developed by Lin et al.6 is the simplest and most effective and is capable of real‐time detection. The two methods of Ghodrati et al.4 can achieve high‐accuracy AF detection and are currently the most popular real‐time detection methods. All of the methods described can effectively distinguish the ECG signals of AF patients from those of healthy controls with more than 90% accuracy. However, these methods usually perform poorly in discriminating AF from arrhythmias that have similar ECG characteristics to AF, such as premature ventricular contraction (PVC).
There are two important AF characteristics in ECGs. The first is an irregular ventricular response rate. The second is the disappearance of the P wave in the ECG and the appearance of an f wave between the T wave and the Q wave of a cardiac cycle. The RRI is a critical parameter that reflects an irregular cardiac rhythm of AF and so it has good discrimination power between sinus heart rate and AF. However, some other arrhythmias like premature atrial contraction and atrial flutter have similar RRI characteristics with AF. RRI‐based methods are sometimes not able to differentiate AF and other arrhythmias (our subsequent experiments verified this analysis).
In this article, a novel method to detect AF in single lead ECG recordings is introduced. We proposed to define ECG parameters that are specific to AF to support our method, including the average number of f waves in a TQ interval (NfTQ). This is defined as the distance between T‐wave offset and Q‐wave onset. The maximum deviation is defined as the difference between the maximum and minimum values of the RRI in a TQ interval (ΔRRImax). Three types of ECG data were adopted to validate our method. Test data and independent data included the recordings of healthy subjects, AF patients, and patients with other arrhythmias but no AF. The results showed that the proposed method can detect AF with a high accuracy and can effectively differentiate between healthy subjects, AF patients, and non‐AF arrhythmia patients.
MATERIALS AND METHODS
ECG Data
The used ECG data in this manuscript were divided into the testing data set and the independent data set. Each data set further included data from normal subjects, patients with AF, and patients with arrhythmias other than AF. The testing data were used to select and adjust the parameters in the proposed algorithm. The independent data set were used to validate the parameters and proposed algorithm. Data from normal subjects was downloaded from the MIT‐BIH Normal Sinus Rhythm Database.11 This group in the testing data set includes 2‐minute ECG recordings from each of 10 healthy people totaling 20 minutes of recordings and in the independent data set it includes 20 minutes of recordings from other 10 healthy people. Several forms of AF have been classified, including paroxysmal AF, persistent AF, and permanent AF. Therefore, data for the AF group were collected from multiple databases,11 such as MIT‐BIH AF database, and were used to verify that our proposed method is suitable for detecting different types of AF. In total, there were 80 minutes of ECG data from 30 AF patients in the AF data group of the testing data set. This group in the independent data set includes 5‐minute recordings from each of 16 AF patients totaling 80 minutes of recordings. In ECGs, the arrhythmias that are often mistaken for AF include PVC, ventricular flutter, atrial flutter, etc. The group of complex arrhythmias was selected to illustrate the robustness of our proposed method and was downloaded from the intracardiac AF database and MIT‐BIH Arrhythmia Database.11 This group of the testing data set included PVC, fusion of ventricular and normal beat, left or right bundle branch block, different types of AV nodal block and broad complex tachycardia, and was composed of 30‐minute ECG signals from eight arrhythmia patients. Except those arrhythmias types mentioned in this group of the testing data set, we added nonconducted P wave, ventricular flutter, and atrial flutter into this group of independent data set, which was composed of 40.8‐minute ECG signals from nine arrhythmia patients different with those patients in the testing data set.
To illustrate that the proposed method can distinguish between AF and normal ECGs as well as AF and non‐AF arrhythmia ECGs, we defined experimental groups for each data set as follows: The combination of ECGs from the normal and AF groups is group A, and the combination of ECGs from the AF and non‐AF arrhythmia groups is group B. For real‐time analysis, the proposed method evenly divides the ECG signals into 6‐second segments. The number of 6‐second segments for group A of the testing and independent data sets is both 1000. The number is 1100 and 1208 for group B of the testing and independent data sets, respectively.
Methods
Usually RRI‐based methods depend on only a few parameters to detect AF and are consequently easy to implement in real‐time applications. However, these parameters lack the capacity to represent the characteristics of f wave during AF. A method that combines RRI analysis and f‐wave analysis could potentially be better for AF detection.
AF Characteristic Parameters
In this section, two new AF characteristic parameters, NfTQ, and ΔRRImax, are defined. These parameters, combined with a classical parameter (RRIstd), are used to comprehensively represent the characteristics of AF.
NfTQ is defined as the average number of f‐wave fluctuations in the TQ interval in an ECG segment. The f wave is an important feature of AF, and the NfTQ was used to represent the dynamic features of the f wave. In the ECG recordings of healthy subjects, there is an obvious P wave in the TQ interval of an RRI. Some of non‐AF arrhythmia patients have no P wave due to cardiac disease. In either case, NfTQ ⩽ 1. In AF ECG recordings, the P wave disappears and is replaced by an f wave. In this situation, the number of f‐wave fluctuations in a TQ interval is more than 1 and varies with each beat. To decrease the influence of differences between beats, the average number of f‐wave fluctuations in adjacent TQ intervals is used as an AF characteristic parameter. NfTQ > 1 indicates potential AF.
The parameters ΔRRImax and RRIstd reflect the dynamic changes of the RRI. Here, ΔRRImax, or the maximum deviation, is defined as the difference between the maximum and minimum values of RRIs in an ECG segment. RRIstd describes the degree of variation of the RRI.6 Because ΔRRImax and RRIstd can reflect two different aspects of the heart rhythm, both are adopted to detect AF. The reasonable thresholds of ΔRRImax and RRIstd for AF events are able to balance between false positive (FP) and false negative (FN) AF detection rates. We derived the two thresholds from a large amount of experimental evidence and the existing RRI‐based methods.4, 6
In summary, the novel AF characteristic parameters we defined include NfTQ, ΔRRImax, and RRIstd. These parameters account for irregular cardiac rhythms and the dynamic variation of the f wave in AF ECGs.
Calculation of AF Characteristic Parameters
Preprocessing of ECG signals is necessary before calculating AF characteristic parameters. A bandpass filter with a bandwidth of 0.5–40 Hz was used to eliminate interference in each 6‐second segment, after which the QRST ECG characteristics were located.12, 13, 14, 15, 16
NfTQ
A large amount of baseline drift noise was eliminated with bandpass filtering, but there was residual noise in each ECG segment. The f wave can be identified from noise due to its higher amplitude and relative regular frequency range.17 However, the residual noise in the segment still hindered recognition of the f wave. To avoid misidentifying noise as the f wave, an adaptive calculation method of NfTQ was developed. We intercept TQ intervals from the RRIs in 6‐second ECG segments. A moving average filter with the window length of 4‐second was used to eliminate residual noise and keep the baseline of the ECG stable.18
An adaptive threshold was set to identify the f wave. The amplitude of ECG signals varies from beat to beat due to the randomness of noise and the f wave. The adaptive threshold () is defined as follows:
| (1) |
where mean(VTQ) and are the average amplitude and the T‐wave peak amplitude in a TQ interval, respectively.
Next, f‐wave fluctuations were identified in each TQ interval. The difficulty in this step lies in how to distinguish fluctuations caused by f waves from noise. Normally, the f wave has a larger amplitude and fluctuation cycle than noise. The width of the fluctuation is used here to denote the difference between f‐wave fluctuations and noise fluctuations. A fluctuation with a large width tends to be an f‐wave fluctuation.
The width of a fluctuation is defined as the distance between the two intersection points of a fluctuation and the threshold line. Assuming that there are K fluctuations whose amplitudes exceed the threshold in a TQ interval (i.e., qualified fluctuations), the width is denoted by . If the width of a qualified fluctuation matches the following condition:
| (2) |
where denotes the maximum value of , the fluctuation is identified as an f‐wave fluctuation. This means that an f‐wave fluctuation corresponds to a qualified fluctuation with a width that is larger than the threshold. A is a weight coefficient and its value is derived from a large amount of experimental evidence. A larger coefficient value means a higher accuracy, a lower FP rate, and a higher FN rate.
Finally, NfTQ, the average number of f waves in a TQ interval, was calculated. Assuming that there are N RRIs and the number of f waves is fTQ(i) in the i‐th TQ interval of a 6‐second ECG segment, the total number of f waves in the ECG segment is calculated as follows:
| (3) |
The number of TQ intervals is the same as that of RRIs in a 6‐second ECG segment. Thus, the average number of f waves in a TQ interval can be obtained as follows:
| (4) |
ΔRRImax and RRIstd
The RRIs in a 6‐second ECG segment can be identified by locating the R peaks. Assuming the RRI of the j‐th cardiac cycle is denoted by RRI(j), the maximum deviation and the standard deviation for a 6‐second ECG segment can be calculated as follows:
| (5) |
| (6) |
where the RRImax and the RRImin are the maximum and minimum values of RRIs in the ECG segment, respectively. The RRImean is the average value of the RRI in the ECG segment.
Detection of AF
An ECG segment is identified as an AF segment when the characteristic parameter conditions in the segment, including , and , are satisfied simultaneously, where θ max and θ std are, respectively, the thresholds of and RRIstd. Otherwise, the ECG segment is identified as a normal or non‐AF arrhythmia segment.
The framework of the proposed AF detection algorithm is shown in Figure 1.
Figure 1.
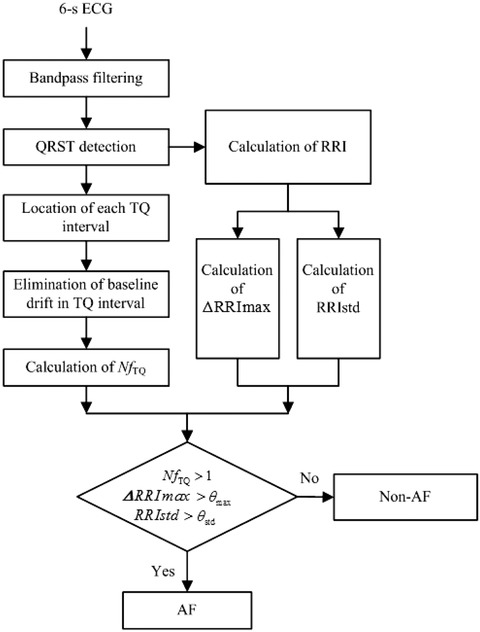
The framework of the proposed algorithm.
RESULTS
To evaluate the performance of our proposed method, we calculated the accuracy, sensitivity, and positive predictive value (PPV). Here true positive (TP) is the number of 6‐second ECG segments that are correctly identified as AF, FP is the number of 6‐second ECG segments that are incorrectly identified as AF, FN is the number of 6‐second ECG segments that are incorrectly identified as the normal or non‐AF arrhythmia and true negative (TN) is the number of 6‐second non‐AF (normal or non‐AF arrhythmia) ECG segments that are correctly identified.
Testing Data Set
In the Methods section, we have analyzed the reason that NfTQ > 1 indicates potential AF. Through the repeated experiments on the testing data set and inspired by existing RRI‐based methods,4, 6 we found that, respectively, selecting 0.5, 70 ms, and 50 ms as the value of A, θmax, and θstd is an appropriate trade‐off and makes the performance of proposed method optimal. Thus, we set the thresholds of ΔRRImax and RRIstd for AF events as follows: ΔRRImax > 70 ms and RRIstd > 50 ms. The detailed test results were shown as below.
Detection of AF Using Single Rules
Three rules, defined as (Rule 1), (Rule 2), and (Rule 3), were independently applied to detect AF and demonstrate the effectiveness of each parameter. Groups A and B were both analyzed. The test results are shown in Table 1, and the performance of each of the three rules is shown in Table 2.
Table 1.
Test Results for Different Rules in Group A and B of Testing Data Set
| Test | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Normal | AF(+) | Normal(−)/Other arrhythmias (−) | |||||||||||||||
| Method | Nf TQ |
|
|
Nf TQ |
|
|
|||||||||||
| Rule | Rule | Rule | Rule | Rule | Rule | ||||||||||||
| A | B | A | B | A | B | A | B | A | B | A | B | ||||||
| Truth | AF(+) | 781 | 781 | 791 | 791 | 776 | 776 | 19 | 19 | 9 | 9 | 24 | 24 | ||||
| N (−)/O (−) | 150 | 6 | 55 | 293 | 3 | 273 | 50 | 294 | 145 | 7 | 197 | 27 | |||||
A = group A; B = group B.
Table 2.
Performance of Each of the Three Rules in Groups A and B of Testing Data Set (Because the Amount of AF Data in Group A and Group B Is the Same, the Sensitivities of the Two Groups Are Equal)
| Accuracy (%) | Sensitivity (%) | PPV (%) | ||||
|---|---|---|---|---|---|---|
| A | B | A | B | A | B | |
| Rule 1 | 83.1 | 97.73 | 97.62 | 97.62 | 83.89 | 99.24 |
| Rule 2 | 93.6 | 72.55 | 98.88 | 98.88 | 93.50 | 72.97 |
| Rule 3 | 97.3 | 73.00 | 97.00 | 97.00 | 99.61 | 73.98 |
The results in Table 2 show that Rule 2 and Rule 3 are better at distinguishing AF ECGs from normal ECGs than Rule 1. However, Rule 1 outperforms the other two in distinguishing between AF and non‐AF arrhythmias. These data indicate that Rule 1 is able to discriminate AF from other arrhythmias well, while Rule 2 and Rule 3 are good at discriminating AF ECGs from normal ECGs. One can infer that by combining the three rules, the weaknesses of one rule can be overcome by the strengths of the others, thereby achieving optimal AF detection performance.
Detection of AF Using the Proposed Method
Group A was used to demonstrate that the method can discriminate AF ECGs from normal ECGs, while group B was used to test discrimination between different arrhythmias.
Using the selected parameters and thresholds, our approach achieves the optimal performance at distinguishing AF ECGs from normal ECGs, with accuracy, sensitivity, and PPVs of 96%, 95%, and 100%, respectively, for group A of the testing data set. These performance indexes of our method are comparable to those of other related methods4, 6 for discriminating between AF ECGs and normal ECGs.
A major concern in our study was whether our method could discriminate between AF ECGs and non‐AF arrhythmia ECGs. The results of testing our method on group B ECGs are shown in Table 3, and thus the accuracy, sensitivity, and PPV of our method are 95.91%, 95.00%, and 99.35%, respectively. These values illustrate that RRI‐related and f‐wave characteristic parameters and thresholds in our proposed method are reasonable and our method is able to detect AF in complex case.
Table 3.
Test Results of Our Method for Distinguishing between AF and Other Arrhythmias on Testing Data Set
| Test | |||
|---|---|---|---|
| Normal | AF(+) | Other Arrhythmias (−) | |
| Truth | AF(+) | 760 | 40 |
| O (‒) | 5 | 295 | |
Detection of AF Using the Proposed Method on Independent Data Set
To validate the method and parameters, we further applied it to the independent data set and compared it with popular real‐time AF detection methods, including the method described in Ref. 6 and methods 1 and 2 described in Ref. 4 (denoted Ref. 4 M.1 and Ref. 4 M.2, respectively).
As shown previously, Ref. 6, Ref. 4 M.1, and Ref. 4 M.2 are able to successfully discriminate AF ECGs from normal ECGs. The sensitivity values for Ref. 6, Ref. 4 M.1, and Ref. 4 M.2 were 94.56%, 85%, and 87%, respectively, and the PPVs were 99.39%, 96%, and 94%, respectively. For the independent data set in group A, the accuracy, sensitivity values, and the PPVs for our proposed method were 93.67%, 94.13%, and 98.69%, respectively. The performance of our method in group A of independent data set is slightly worse than that in group A of the testing data set. However, it is better than that of Ref. 4 M.1 and Ref. 4 M.2, and is close to that of Ref. 6 at distinguishing AF ECGs from normal ECGs. Thus, our method is effective for discriminating between AF ECGs and normal ECGs.
The results of testing each method on group B ECGs are shown in Table 4, and the performance of the four methods is shown in Table 5. Table 5 shows that the accuracy, sensitivity, and PPV of our method are 94.62%, 94.13%, and 97.67%, respectively. Although the performance indexes of our method tested on an independent data set are a little bit lower than those on group B of the testing data set, these values are higher than those of the other methods. This illustrates that our proposed method, which utilizes RRI‐related and f‐wave characteristic parameters, is a powerful tool for detecting AF in complex cases.
Table 4.
The Results of Different Methods for Distinguishing between AF and Other Arrhythmias on Independent Data Set
| Test | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Normal | AF(+) | Other arrhythmias (−) | |||||||
| Method | Our Method | Ref. 6 | Ref. 4 M. 1 | Ref. 4 M. 2 | Our Method | Ref. 6 | Ref. 4 M. 1 | Ref. 4 M. 2 | |
| Truth | AF(+) | 753 | 469 | 732 | 507 | 47 | 331 | 68 | 293 |
| O (‒) | 18 | 48 | 65 | 58 | 390 | 360 | 343 | 350 | |
Table 5.
Performances of the Four Methods in Distinguishing between AF and Other Arrhythmias on Independent Data set
| Accuracy (%) | Sensitivity (%) | PPV (%) | |
|---|---|---|---|
| Ref. 6 | 68.63 | 58.63 | 90.72 |
| Ref.4.M.1 | 88.99 | 91.50 | 91.84 |
| Ref.4.M.2 | 70.94 | 63.38 | 89.73 |
| Our article | 94.62 | 94.13 | 97.67 |
DISCUSSION
Detection of T‐wave ends is difficult in sinus rhythm and becomes even more so in AF. Thus, averaging and adaptive strategies are adopted for calculating NfTQ, which helps reduce the impact of misleading T‐wave ends on the identification of f waves. In consideration of the blur of inaccurate location of T wave ends, we set a relatively relaxed threshold of NfTQ > 1 to detect AF. By employing the three described strategies, the impact of incorrect localization of T‐wave ends on NfTQ values was minimized. We performed experiments to identify f waves using 500 TQ intervals from the ECGs of normal, AF, and non‐AF arrhythmia subjects and achieved an identification accuracy of 97% using NfTQ. Figure 2 illustrates the f‐wave identification capacity of our method using three typical TQ intervals. Because the fluctuations of the TQ interval of the three types of ECGs are different, different sampling frequencies were used for each. Figure 2(A) shows a normal TQ interval with an obvious P wave. The NfTQ algorithm adaptively modulated the threshold to 50 mV. Thus, the number of fluctuation in this TQ interval is equal to 1 based on the threshold, meaning that no f wave appears in the TQ interval. Figure 2(B) shows a TQ interval from an AF patient, in which the P wave disappears while the f wave appears. Our algorithm adaptively modulated the threshold to 20 mV. The number of fluctuations in this TQ interval is more than 1, which indicates that there is an f wave in the TQ interval. In Figure 2(C), which shows the TQ interval of a non‐AF arrhythmia patient, the P wave disappears but there is no appearance of the f wave. These results show that non‐AF arrhythmia, and not AF, causes the disappearance of the P wave. In this case, our algorithm adaptively modulated the threshold to 62.5 mV, and the number of fluctuations is equal to 0 based on this threshold. Our experiments also illustrated that the impact of incorrect localization of T‐wave ends on NfTQ is trivial.
Figure 2.
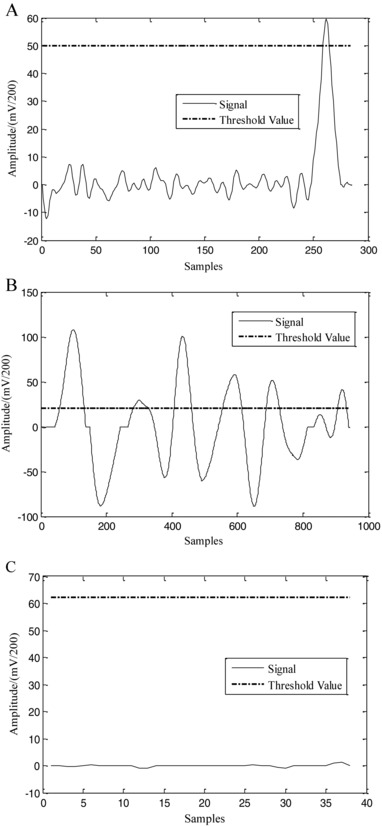
The typical TQ intervals for the three groups of patients. (A) Normal (the sampling frequency is 500 Hz), (B) AF (1920 Hz), and (C) other non‐AF arrhythmias (128 Hz).
To date, several computational methods have been proposed to detect AF, but most are not suitable for processing only 6‐second ECG segments at one time. For example, SVM‐based methods9, 10 require longer ECG inputs as training data. Although these methods can identify important features in an ECG, they are not appropriate for use as real‐time AF detection methods. Thus, an impartial and reasonable approach for this study was to compare our method with real‐time methods such as Ref. 6, Ref. 4 M.1, and Ref. 4 M.2. The effectiveness of these three methods has been well demonstrated in the literature, where they were shown to differentiate between AF and normal ECGs with a high accuracy. The accuracy, sensitivity, and PPVs of our method (93.67%, 94.13%, and 98.69%, respectively) are comparable to the performance values of these methods. However, when the four methods were tested using the independent data set from group B, the complex disease group, our method (accuracy 94.62%, sensitivity 94.13%, and PPV 97.67%) significantly outperformed the control methods in its ability to discriminate between AF and other arrhythmias (Table 5). Thus we can conclude that using only RRI characteristic parameters for AF detection is not sufficient to distinguish different arrhythmias, which is an important requirement for AF detection methods in practice.
Of the AF characteristic parameters investigated here, the standard deviation (RRIstd) has being extensively used in AF detection methods. This work described the use of the maximum deviation (ΔRRImax) and the average number of f waves within a TQ interval (NfTQ) for the first time. Our study demonstrates the effectiveness of using a combination of parameters that considers irregular heart rhythm and the dynamic features of the f wave to detect AF. In addition, setting the thresholds of AF characteristic parameters has been a challenge in the past. It is well known that a high threshold could fail to identify AF events, while a low threshold could result in high false detection rates. Our results demonstrate that the thresholds used for the three AF characteristic parameters are reasonable.
The proposed method has real‐time capabilities and can be applied in clinical practice. In future work, we will apply our method to a wireless AF monitoring system.
This work was supported in part by the National Natural Science Foundation of China (NSFC), grant nos. 81171411 and 30900318.
REFERENCES
- 1. Healey JS, Connolly SJ, Gold MR, et al. Subclinical atrial fibrillation and the risk of stroke. N Engl J Med 2012;366:120–129. [DOI] [PubMed] [Google Scholar]
- 2. Harris K, Edwards D, Mant J. How can we best detect atrial fibrillation? J R Coll Physicians Edinb 2012; 42(Suppl 18):5–22. [DOI] [PubMed] [Google Scholar]
- 3. Petrucci E, Balian V, Filippini G, et al. The use of sequential RR distributions to detect atrial fibrillation episodes in very long term ECG monitoring. Comput Cardiol 2006:945–948. [Google Scholar]
- 4. Ghodrati A, Murray B, Marinello S. RR interval analysis for detection of atrial fibrillation in ECG monitors. Proc 2008 30th Annu Int Conf IEEE Eng Med Biol Soc, Vancouver, Canada. 2008;18:601–604. [DOI] [PubMed] [Google Scholar]
- 5. Dash S, Chon KH, Lu S, et al. Automatic real time detection of atrial fibrillation. Ann Biomed Eng. 2009;37(9):1701–1709. [DOI] [PubMed] [Google Scholar]
- 6. Lin CT, Chang KC, Lin CL, et al. An intelligent telecardiology system using a wearable and wireless ECG to detect atrial fibrillation. IEEE Trans Inf Technol Biomed 2010;14(3):726–733. [DOI] [PubMed] [Google Scholar]
- 7. Huang C, Ye S, Chen H, et al. A novel method for detection of the transition between atrial fibrillation and sinus rhythm. IEEE Trans Biomed Eng 2011;58(4):1113–1119. [DOI] [PubMed] [Google Scholar]
- 8. Lian J, Wang L, Muessig D. A simple method to detect atrial fibrillation using RR intervals. Am J Cardiol 2012;107(10):1494–1497. [DOI] [PubMed] [Google Scholar]
- 9. Mohebbi M, Ghassemian H.Prediction of paroxysmal atrial fibrillation using recurrence plot‐based features of the RR‐interval signal. Physiol Measure 2011;32:1147–1162. [DOI] [PubMed] [Google Scholar]
- 10. Mohebbi M, Ghassemian H.. Prediction of paroxysmal atrial fibrillation based on non‐linear analysis and spectrum and bispectrum features of the heart rate variability signal. Comput Methods Progr Biomed 2012;105(1):40–49. [DOI] [PubMed] [Google Scholar]
- 11. Goldberger AL, Amaral LAN, Glass L et al. PhysioBank, PhysioToolkit, and PhysioNet: Components of a new research resource for complex physiologic signals. Circulation 2000;101(23):215–220. [DOI] [PubMed] [Google Scholar]
- 12. Kohler BU, Hennig C, Orglmeister R. The principles of software QRS detection. Eng Med Biol Mag IEEE 2002;21(1):42–57. [DOI] [PubMed] [Google Scholar]
- 13. Portet F, Hernandez A, Carrault G. Evaluation of real‐time QRS detection algorithms in variable contexts. Med Biol Eng Comput 2005;43(3):379–385. [DOI] [PubMed] [Google Scholar]
- 14. Arzeno NM, Deng ZD, Poon CS. Analysis of first‐derivative based QRS detection algorithms. IEEE Trans Biomed Eng 2008;55(2):478–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang Q, Manriquez AI, Medigue C, et al. An algorithm for robust and efficient location of T‐wave ends in electrocardiograms. IEEE Trans Biomed Eng 2006;53(12):2544–2552. [DOI] [PubMed] [Google Scholar]
- 16. Elgendi M, Jonkman M, Boer FD. Recognition of T waves in ECG signals 2009 Bioengineering Conference IEEE 35th Annual Northeast, Boston, MA: 2009:1–2. [Google Scholar]
- 17. Martin S, Sornmo L. Spatiotemporal QRST cancellation techniques for analysis of atrial fibrillation. IEEE Trans Biomed Eng 2001;48(1):105–111. [DOI] [PubMed] [Google Scholar]
- 18. Chouhan VS, Mehta SS. Total removal of baseline drift from ECG signal In 2007 ICCTA ‘07 International Conference on Computing: Theory and Applications, Kolkata, India; 2007;512–515. [Google Scholar]


