Abstract
Objective: To determine whether menopausal hormone therapy alters the QT interval in primarily healthy postmenopausal women.
Background: Despite well‐known gender differences in myocardial repolarization that include a longer heart‐rate‐corrected QT interval (QTC) in women compared to men, the effects of menopausal hormone therapy on myocardial repolarization in women have not been well characterized.
Methods: We studied 34,378 postmenopausal women participating in the dietary intervention component of the Women's Health Initiative. Cross‐sectional associations were examined to assess possible effects of estrogen + progesterone on myocardial repolarization. Women who reported that they were never treated with menopausal hormone therapy (n = 12,451) were compared to women with a past use of menopausal hormone therapy (n = 3891), currently taking unopposed estrogen therapy (n = 9987), or combined current estrogen and progesterone therapy (n = 8049).
Results: Using analysis of covariance, the mean (±SEM) QTC interval was 423.1 ± 0.2 milliseconds (ms) in those never treated with menopausal hormone therapy, 423.9 ± 0.3 ms in past menopausal hormone therapy users, 425.6 ± 0.2 ms in those currently on estrogen alone, and 424.0 ± 0.2 ms in women currently on combined estrogen–progesterone therapy. Differences in mean QTC between those on estrogen alone and the other three groups were statistically significant. Comparisons of JT intervals, QT intervals, and linear corrected QT intervals among the groups yielded similar results.
Conclusion: These results suggest that unopposed estrogen in menopausal women mildly prolongs myocardial repolarization, and the effect is reversed by progesterone. Whether these findings have clinical significance requires further study.
Keywords: electrocardiology, sex hormones
In the initial description of a formula for the QT interval corrected for heart rate, Bazett noted that women had a longer corrected QT interval than men. 1 Although this observation has been reproduced several times, the mechanism of this gender difference in the QT interval is unclear. 2 , 3 , 4 , 5 , 6 , 7 The gender difference appears at puberty and decreases but does not disappear later in life. 4 In vitro studies have reached discrepant conclusions, but the weight of evidence does not support an acute electrophysiologic effect of estrogen, or androgens, on action potential duration. 8 , 9 Studies also suggest that corrected QT interval (QTC) in the absence of antiarrhythmic drugs does not vary significantly throughout the menstrual cycle. These findings support the concept that acute electrophysiologic effects of estrogen and progesterone are not responsible for the gender difference in the QT interval. 10 In contrast, several reports suggest that estrogens and androgens can alter action potential duration during long‐term administration, possibly through the induction of changes in ion channel expression or other chronic effects. 11 , 12 , 13 , 14 In addition, recent studies suggest that testosterone has significant effects on myocardial repolarization. 15 Therefore, the issue of whether exogenous estrogen or progesterone alters QT interval is not resolved.
The use of menopausal hormone therapy in women who have very low levels of endogenous estrogen and progesterone serves as a clinical model for examining the electrophysiologic effects of exogenous hormone use. The purpose of this study is to conduct a cross‐sectional assessment to investigate whether exogenous hormones alter the QT interval by examining raw (observed) and heart‐rate‐corrected QT intervals (QTC) in a large sample of postmenopausal women being treated with a variety of menopausal hormone therapy regimens compared to women not taking menopausal hormone therapy.
METHODS
Population and Inclusion Criteria
The Women's Health Initiative is a large study of postmenopausal women sponsored by the National Institutes of Health. Details of the Women's Health Initiative have been published previously. 16 , 17 Forty clinical centers began enrollment into the clinical trials and observational study in 1993. The main goal of the Women's Health Initiative is to study risks and benefits of strategies that could potentially reduce the incidence of heart disease, cancers, and fractures in postmenopausal women. Between 1993 and 1998, the Women's Health Initiative enrolled 161,809 postmenopausal women between the ages of 50 and 79 years into a set of clinical trials and an observational study at 40 clinical centers in the United States, as previously reported. 16 , 17 The Women's Health Initiative trials include two menopausal hormone therapy arms (estrogen alone vs placebo in women with prior hysterectomy and combined estrogen–progesterone vs placebo in women with intact uterus). 17 At the time this study was performed, unblinded ECG data from the randomized arms were not available for analysis. The data reported here are, therefore, restricted to women in the dietary trial arm of the Women's Health Initiative who underwent baseline ECGs and medical evaluations and who reported on their current and past hormone use.
Exclusion criteria for the present study were:
-
1
Overt heart disease defined as major electrocardiographic abnormalities, a history of congenital heart disease, or coronary artery disease. Abnormalities were defined based on the Novacode system. 18
-
2
Subjects taking medications known to alter the QT interval (including antiarrhythmic drugs, phenothiazines, tricyclic antidepressants, terfenadine).
-
3
QRS duration of more than 120 ms.
-
4
The use of vaginal estrogen creams.
Data Collection
The following measures were collected: age, blood pressure, weight, height, time since menopause, and presence and type of menopausal hormone therapy. The heart rate, raw QT interval, QRS duration, and heart‐rate‐corrected QT interval were determined in standard computerized fashion and extracted from the Women's Health Initiative ECG database. ECGs were obtained at the enrollment visit. A total of 48,837 participants enrolled in the dietary modification study of the Women's Health Initiative. The exclusions listed above eliminated 13,893 women enrolled in Women's Health Initiative. Fifty‐five women were subsequently excluded from the present analysis because of missing information on menopausal hormone therapy use, and 511 women were excluded because of missing or technically poor ECG data. The remaining 34,378 women formed the study sample. These subjects were divided into four groups as follows:
-
1
Women who never took menopausal hormone therapy (no menopausal hormone therapy),
-
2
Women who had used menopausal hormone therapy in the past, but are not users at the present time (past menopausal hormone therapy),
-
3
Women currently taking hormone therapy with estrogen alone, and
-
4
Women currently taking hormone therapy with combined estrogen and progesterone compounds. Data on specific doses of estrogen or combined hormone therapy were not available.
Strict quality control procedures were used for the ECG acquisition in the core laboratory. QT measurements were made using two different ECG programs, the Marquette 12SL program (GE Marquette, Milwaukee, WI) and the Dalhousie ECG program. Both of these programs measure the QT interval as a global interval using algorithms based on derived spatial magnitude functions. QT measures differ by 40 ms or more and 2.6% of the ECGs, in most instances due to partially overlapping T waves and U waves. An algorithm was used for the ECGs to select QT measurements that were closer to the rate‐adjusted normal median values. In all the cases the QT measurement by the Marquette 12SL program was retained for analysis because overall measurement variability was smaller than the other program. All ECG program and QT measurements were verified using interactive graphics terminals with manual overreading.
Statistical Analysis
The primary endpoints in the study were differences in raw and corrected QT interval and JT interval among the four groups. The JT interval was obtained by subtracting the QRS interval from the QT interval. The corrected QT interval was calculated using Bazett's method. 1 Despite some limitations, Bazett's correction has been the most widely used formula for correcting the QT interval and appears to perform well, except at extremes of heart rate. 1 , 19 , 20 Thus, we also used a two‐stage linear correction for QT intervals (QTLC) based upon a method initially described by Sagie. 21 Since fewer than 0.5% of subjects had heart rates greater than 100 beats per minute, and because in a prior study we demonstrated that a two‐stage linear correction performed as well as a three‐stage correction, we utilized the two‐stage linear correction. 19 After separate linear fits were performed at heart rates less than 60 and a heart rate of 60 to 100 beats per minute (bpm), the QT interval was corrected to a heart rate of 60 bpm as previously described. 19
In addition to comparing QT intervals among the four groups, we also performed a heart‐rate‐independent analysis. 19 The large number of subjects included in the present study allowed us to divide heart rate into 5‐bpm bins from 50 to 95 bpm and perform separate comparisons among the four groups in each heart rate bin. There were too few subjects with heart rates lower than 50 bpm and above 95 bpm to allow analysis in those heart rate ranges. These comparisons do not require any correction of the QT interval. Analysis of variance and Bonferroni's method were used to compare differences among the four groups. For these comparisons, P < 0.05 was considered significant.
Data are presented as mean ± SEM. Analysis of variance was used to compare differences in QRS, QT, QTC, QTLC, and JT intervals. Small differences in demographic and clinical characteristics between the four groups were detected at P < 0.05. Therefore, analysis of covariance was also used to assess the effects of menopausal hormone therapy on QRS, QT, QTLC, and JT intervals; the covariates were age, systolic and diastolic blood pressure, and body mass index. One hundred fifty‐nine subjects (<0.5%) were missing either height, weight, systolic blood pressure, or diastolic blood pressure values. The covariate‐adjusted comparisons were made in the remaining 34,219 subjects. Bonferroni's method was used to compare differences in the unadjusted and covariate‐adjusted means among the groups. All statistical tests were two tailed; P < 0.05 was regarded as statistically significant.
RESULTS
Baseline Characteristics
Characteristics of the study population are shown in Table 1. Due to the large numbers of women in the study sample, relatively small differences in several of the baseline variables were statistically significant. These differences were taken into account in subsequent statistical analyses to adjust for potential confounding effects on the ECG variables of interest.
Table 1.
Demographic Characteristics of 34,378 Postmenopausal Women in the Dietary Arm of the Women's Health Initiative
| Characteristic | No MHT | Past MHT | EST | COMB |
|---|---|---|---|---|
| Age (year) | 63.2 ± 0.1 | 63.7 ± 0.1* | 61.5 ± 0.1*, † | 59.8 ± 0.1*, †, ‡ |
| Weight (kg) | 77.8 ± 0.2 | 75.8 ± 0.3* | 75.0 ± 0.2* | 72.8 ± 0.2*, †, ‡ |
| Height (cm) | 161.8 ± 0.1 | 162.0 ± 0.1 | 162.3 ± 0.1* | 163.0 ± 0.1*, †, ‡ |
| BMI (kg/m2) | 29.6 ± 0.1 | 28.8 ± 0.1* | 28.3 ± 0.1*, † | 27.3 ± 0.1*, †, ‡ |
| BSA (m2) | 1.82 ± 0.002 | 1.80 ± 0.003* | 1.79 ± 0.002* | 1.78 ± 0.002*, †, ‡ |
| SBP (mm Hg) | 128.2 ± 0.2 | 127.6 ± 0.3* | 127.5 ± 0.2 *, † | 123.9 ± 0.2 *, †, ‡ |
| DBP (mm Hg) | 76.1 ± 0.1 | 75.6 ± 0.2* | 76.0 ± 0.1 | 75.0 ± 0.1 *, †, ‡ |
| HR (bpm) | 66.2 ± 0.1 | 66.0 ± 0.2 | 65.4 ± 0.1 *, † | 65.7 ± 0.1* |
BMI = body mass index; BSA = body surface area; COMB = combined estrogen and progesterone therapy; DBP = diastolic blood pressure; EST = estrogen therapy; HR = heart rate; MHT = menopausal hormone therapy; SBP = systolic blood pressure. Data are expressed as mean ± SEM.
* P < 0.05 versus no MHT, † P < 0.05 versus past MHT, ‡ P < 0.05 versus EST.
Raw (Non–Heart‐Rate–Corrected) QT Interval
Unadjusted QT intervals among the groups are shown in Table 2, and covariate‐adjusted raw QT intervals are shown in Table 3. Women currently taking estrogen therapy alone had a longer raw QT duration than those never on menopausal hormone therapy, past users of hormone therapy, and those currently taking menopausal hormone therapy. The difference between means of the longest and shortest QT duration was 4.0 ms or approximately 1%. Except for the estrogen‐only group, there were no significant differences among the remaining groups in either the unadjusted or adjusted raw QT intervals. Thus, the group being treated with estrogen in combination with progesterone had QT intervals similar to the no previous or current estrogen groups.
Table 2.
Unadjusted Repolarization‐Related ECG Variables by Hormone Use Category
| ECG Interval | No MHT | Past MHT | EST | COMB |
|---|---|---|---|---|
| QRS (ms) | 85.6 ± 0.1 | 85.5 ± 0.1 | 86.0 ± 0.1 | 85.0 ± 0.1 |
| QT (ms) | 406.5 ± 0.3 | 407.5 ± 0.5 | 410.3 ± 0.3* | 406.5 ± 0.3 |
| QTC (ms) | 423.8 ± 0.2 | 424.3 ± 0.3 | 425.6 ± 0.2* | 422.7 ± 0.2*,†,‡ |
| QTLC | 415.8 ± 0.2 | 416.3 ± 0.3 | 417.9 ± 0.2* | 415.0 ± 0.2 |
| JT (ms) | 321.0 ± 0.3 | 322.0 ± 0.5 | 324.3 ± 0.3* | 321.5 ± 0.3 |
EST = estrogen therapy; COMB = combined estrogen and progesterone use; MHT = menopausal hormone therapy.
* P < 0.05 versus no MHT, † P < 0.05 versus past MHT, ‡ P < 0.05 versus EST.
Table 3.
Repolarization‐Related ECG Variables by Hormone Use Category, Adjusted for Multiple Covariates
| ECG Interval | No MHT | Past MHT | EST | COMB |
|---|---|---|---|---|
| QRS (ms) | 85.5 ± 0.1 | 85.6 ± 0.1 | 86.0 ± 0.1† | 85.2 ± 0.1†,‡,§ |
| QT (ms) | 406.8 ± 0.3 | 407.4 ± 0.5 | 410.3 ± 0.3† | 406.3 ± 0.3 |
| QTC (ms) | 423.1 ± 0.2 | 423.9 ± 0.3 | 425.6 ± 0.2† | 424.0 ± 0.2 |
| QTLC | 415.8 ± 0.2 | 416.3 ± 0.3 | 417.9 ± 0.2† | 415.0 ± 0.2 |
| JT (ms) | 321.3 ± 0.3 | 321.8 ± 0.5 | 324.3 ± 0.3† | 321.1 ± 0.3 |
Covariates used were age, systolic and diastolic blood pressure, and body mass index. COMB = combine estrogen and progesterone use; EST = estrogen use alone; MHT = menopause hormone therapy.
† P < 0.05 versus no MHT, ‡ P < 0.05 versus EST, § P < 0.05 versus Past MHT.
Heart‐Rate–Adjusted QT Interval (QTC and QTLC)
The unadjusted and covariate‐adjusted QTC intervals according to menopausal hormone therapy usage are shown in Tables 2 and 3, respectively. As with raw QT intervals, estrogen‐alone users had a longer QTC interval than the other menopausal hormone therapy groups for both unadjusted and covariate‐adjusted QTC intervals (Fig. 1 and Table 3). The unadjusted heart‐rate–corrected QTC interval was significantly shorter in patients on combined menopausal hormone therapy compared to women never treated with menopausal hormones or past users of menopausal hormone therapy. However, QTC differences were no longer detected after covariate adjustment. Thus, QTC differences paralleled raw QT differences. Using Bazett's correction, 5.0% of the subjects not taking menopausal hormone therapy, 5.6% of past menopausal hormone therapy users, 5.4% of estrogen users, and 4% of combined menopausal hormone therapy used had QT intervals ≥460 ms.
Figure 1.
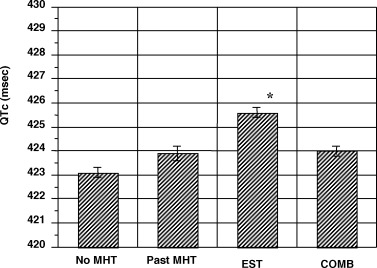
Comparisons of QTC intervals (using Bazett's correction) in four menopausal hormone therapy groups. The figure shows QTC intervals after adjustments for covariates. Subjects taking unopposed estrogen therapy had a QTC interval that was longer than that in the three other groups (P < 0.05). Data are shown as mean ± SEM. COMB = combine estrogen and progesterone use; EST = estrogen use alone; MHT = menopause hormone therapy.
Utilizing the linear correction of QT interval rather than Bazett's correction resulted in similar observations. When adjustment for covariates was not performed, the corrected QTLC interval was 415.8 ± 18.5 ms in women who were not taking menopausal hormone therapy, 416.3 ± 18.4 in past menopausal hormone therapy users, 417.9 ± 18.8 in estrogen users and 415.0 ± 17.3 in those who took combined menopausal hormone therapy. After adjustment for covariates, similar results were obtained (Fig. 2). In both adjusted and unadjusted analyses, subjects not taking menopausal hormone therapy and past users of menopausal hormone therapy had similar QTLC intervals. Patients taking estrogen had QT intervals that were significantly longer than the other three groups (P < 0.01), and those taking combined menopausal hormone therapy had QTLC that tended to be shorter than the other two groups (P = 0.06; Fig. 2). Using the linear correction, 1.7% of the subjects not taking menopausal hormone therapy, 1.9% of past menopausal hormone therapy users, 2.0% of estrogen users, and 1.3% of combined menopausal hormone therapy used had QT intervals ≥460 ms.
Figure 2.
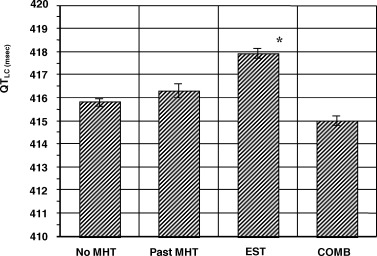
Comparisons of linear corrected QT intervals among four menopausal hormone therapy groups. Subjects taking unopposed estrogen therapy had a QTLC that was significantly longer than those not taking menopausal hormone therapy or are past menopausal hormone therapy users. Subjects taking combined HRT had a QTLC that tended to be shorter than the other three groups (P = 0.06). Data are shown as mean ± SEM. *P < 0.05. COMB = combine estrogen and progesterone use; EST = estrogen use alone; MHT = menopause hormone therapy.
Heart‐Rate–Independent Analysis
Heart‐rate–independent analysis of the QT interval was also performed both before and after correcting for covariates. The QT interval in women taking menopausal hormone therapy ranged from 1.0 to 3.5 ms, longer than in the other groups (P < 0.05 for each comparison) except in the bin with heart rates between 80 and 84 bpm. In this bin, there were no significant differences among the four groups. After correction for covariates, the corrected QT interval in women taking estrogen was 0.9 to 3.5 ms, longer than any other group (P < 0.05 for each comparison) except in the heart beat range of 80 to 84 bpm (Fig. 3). Subjects taking combined menopausal hormone therapy had QT intervals similar to those not taking menopausal hormone therapy and past users.
Figure 3.
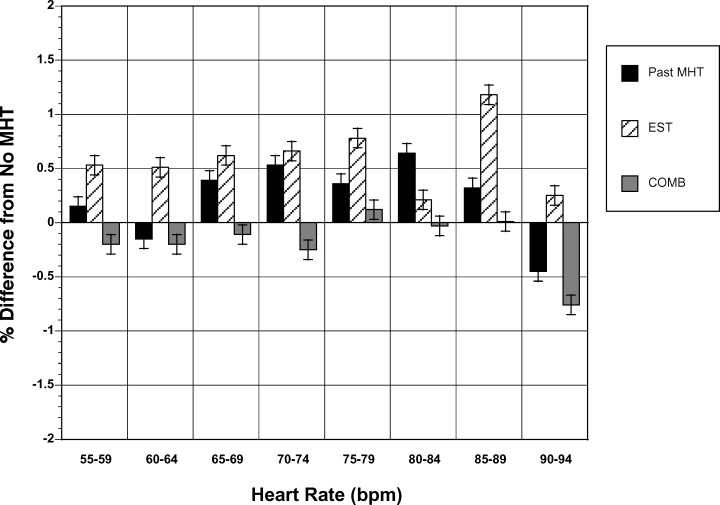
Heart‐rate–independent analysis. Mean difference in QT interval (in percent) between the three other groups and those who had never taken menopausal hormone therapy. Data are shown in heart rate bins of 5 bpm from 55 to 94 bpm. In almost all bins, subjects taking unopposed estrogen therapy had QT intervals that were longer than each of the other groups. See text for details. Data are shown as mean ± SEM. COMB = combine estrogen and progesterone use; EST = estrogen use alone; MHT = menopause hormone therapy
QRS Duration
The unadjusted mean QRS duration for each of the groups is shown in Table 2. There was a 1% difference in QRS duration among the groups. Women taking combined estrogen and progesterone therapy had the shortest QRS duration and those on estrogen alone had the longest QRS duration. Those taking estrogen alone also had significantly longer QRS durations than those taking no menopausal hormone therapy or past users of menopausal hormone therapy (Table 2). In addition, the QRS duration was significantly shorter in those currently taking combined estrogen and progesterone than either past users of menopausal hormone therapy or those taking no menopausal hormone therapy.
The covariate‐adjusted mean QRS duration for each of the groups is shown in Table 3. Again, those taking estrogen alone had significantly longer QRS durations than the other three groups. However, differences between those taking combined estrogen and progesterone and either past or no menopausal hormone therapy use were not significant.
JT Interval
The JT interval was also examined because of differences in baseline QRS duration. The mean JT intervals according to menopausal hormonal therapy usage are shown in Table 2 (unadjusted) and Table 3 (covariate adjusted). The differences were similar to those for the raw QT interval in both the unadjusted and covariate‐adjusted comparisons. Estrogen‐alone users had JT intervals that were 2.2 to 3.3 ms longer than each of the three other menopausal hormonal therapy groups and, in all cases, the differences were significant (Table 3). There were no significant differences among the remaining groups in either the unadjusted or adjusted JT intervals. Thus, differences in JT intervals were similar to those seen in QT intervals.
DISCUSSION
Main Findings
The present study suggests that unopposed estrogen therapy has a small but measurable effect in prolonging myocardial repolarization in postmenopausal women. This effect was present and similar whether the QT interval, corrected QT interval, or JT interval was examined. It was also similar using Bazett's correction, a linear correction, or heart‐rate‐independent analysis. Adjusting for covariates of age, blood pressure, and body mass index did not alter the effects associated with estrogen alone. In contrast, women taking combined menopausal hormone therapy had similar QT intervals to those not taking menopausal hormones, and the QT interval was shorter than that in women taking estrogen alone. Although the effects were small, the data also suggest that progesterone antagonizes the effects of estrogen on myocardial repolarization and may block prolongation of repolarization seen with estrogen. A small, but statistically significant, difference was also detected with estrogen use on the QRS duration both before and after covariate adjustment. The QRS was slightly longer in current estrogen users compared to the other groups of women. However, since JT intervals were also longer in the estrogen therapy group, the differences in QT interval noted were not solely due to differences in QRS duration.
Prior Studies
In previous work, menstrual‐cycle‐associated variability in the QT interval was studied, and there were no effects seen on the QT interval in the baseline state although there was a tendency for the QT interval to be shorter in the luteal phase. 10 Although Rodriguez showed a differential response to the potassium channel blocking drug ibutilide at different times in the menstrual cycle, they confirmed observations on the lack of menstrual cycle variability in the QT interval in the absence of medication. 22 These data suggest that naturally occurring short‐term fluctuations in estrogen and progesterone in premenopausal women do not significantly alter the QT interval. However, the trends seen in our prior data and the lesser QT prolongation response to ibutilide in the luteal phase of the menstrual cycle 22 , 23 do indicate a possible effect of progesterone on myocardial repolarization.
Prior data regarding the effect of menopausal hormone therapy on myocardial repolarization have not been entirely consistent. In an observational study of approximately 400 postmenopausal women, we examined the QT interval in patients on estrogen‐only therapy, the combination of estrogen and progesterone therapy, and on no hormone therapy. We found no significant differences among the QT interval in the three groups. However, based on the sample size in that study, we indicated that the study was powered to detect differences of 8 ms or more. 6
A small study by Haseroth suggested that estrogen usage in postmenopausal women resulted in longer QTC intervals than in postmenopausal women taking no hormones or taking progesterone in combination with estrogen. This study only included approximately 20 subjects in each group and found a 30‐ms difference in the QTC interval between the control group and those on estrogen therapy alone without inclusion of covariate adjustment. 24 However, these results were not found in prior studies. 25 While it is well known that women are at a greater risk for torsades des pointes than men, it has been suggested that this difference is primarily due to the “protective” effects of testosterone 15 , 26 , 27 rather than the effects of estrogen or progesterone on myocardial repolarization. However, an experimental study by Drici et al. suggested that estrogen could also exert effects on myocardial repolarization. 12
Present Study
The present study includes a larger number and more diverse group of women than any other of the prior reports, and demonstrates that postmenopausal use of both estrogen and progesterone appear to have small but measurable effects on myocardial repolarization. Although there were some differences in baseline demographic characteristics among the groups, the large number of subjects and the analysis of covariance to control for these differences strongly suggest that estrogens prolong myocardial repolarization by 0.5% to 1% in postmenopausal women without overt evidence of structural heart disease. While the difference was small, it persisted despite adjustment for a number of potentially confounding covariates. In addition, women who had received past hormone therapy and who had similar demographic characteristics to those on current hormone therapy had corrected QT intervals that were similar to those women who never used hormones. These observations suggest that differences in characteristics among the groups cannot explain the effects of estrogen. In contrast, women taking combined hormone therapy with estrogen and progestins had QT intervals that were similar to those who were not on hormone therapy (despite the use of estrogen). This suggests that progesterone protects against the QT‐prolonging effects of estrogen as it does against the QT‐prolonging drug ibutilide, although the precise ionic mechanism responsible for these effects cannot be determined from the results of the present study. However, the magnitude of the effect (3–4 ms) is not adequate to completely explain gender differences in the QT interval that usually average 10 ms or more. 1 , 4 , 7 , 10 , 19 The QRS duration was also larger in subjects taking estrogen. The difference persisted after adjustment for covariates. The mechanism of this difference remains to be determined.
Limitations
No prior studies have carefully examined the effect of menopausal hormone therapy on QRS duration. Although the effects on QRS duration were small, they persisted despite covariate adjustment. The mechanism of this effect remains to be determined. One significant limitation to the present study is that it is a cross‐sectional study of a sample of apparently healthy postmenopausal women at a single point in time. Thus, the results are potentially subject to differences in the demographic characteristics of the different groups of women. However, the large number of subjects in covariate analysis should minimize this limitation. The randomized hormone therapy portions of Women's Health Initiative will provide the opportunity to test this observation by using pre‐ and postintervention ECGs in women randomized to either estrogen alone or estrogen plus progesterone. Although the combined menopausal hormone therapy arm of the study has been unblinded, 17 the estrogen‐only arm continues and unblinded data are not available.
Implications
While the effects noted were small and not adequate to explain the gender differences reported previously in corrected QT interval between men and women, they do indicate effects of estrogen and progesterone on myocardial repolarization that could potentially affect the use of hormone therapy in large populations of postmenopausal women. In particular, women whose baseline QT interval is borderline prolonged may be at greater risk (even though the QT interval is “normal”) from unopposed estrogen therapy than women whose QT interval is shorter. The finding of QT intervals during combined estrogen and progesterone administration that were indistinguishable from QT intervals of women not taking any hormones would also lead us to speculate that combined menopausal hormone therapy did not have a QT‐prolonging effect in the randomized portion of the Women's Health Initiative and, therefore, QT effects could not explain any of the observed differences in cardiac event rates between placebo and combined menopausal hormone therapy. 17 Women have a higher risk of torsades de pointes that men. 27 It is possible that sensitivity to antiarrhythmic drugs could be affected by hormonal concentrations. Finally, future studies of menopausal hormone therapy use should be aware that only small levels of QTC changes should be expected and that the type of menopausal hormone therapy may alter the response of myocardial repolarization to menopausal hormone therapy.
Acknowledgments
Acknowledgment: The authors wish to acknowledge the following Women's Health Initiative Investigators.
Program Office: (National Heart, Lung, and Blood Institute, Bethesda, MD) Jacques E. Rossouw, Linda Pottern, Shari Ludlam, Joan McGowan, Nancy Morris.
Clinical Coordinating Center: (Fred Hutchinson Cancer Research Center, Seattle, WA) Ross Prentice, Garnet Anderson, Andrea LaCroix, Ruth Patterson, Anne McTiernan; (Bowman Gray School of Medicine, Winston‐Salem, NC) Sally Shumaker, Pentti Rautaharju; (Medical Research Labs, Highland Heights, KY) Evan Stein; (University of California at San Francisco, San Francisco, CA) Steven Cummings; (University of Minnesota, Minneapolis, MN) John Himes; (University of Washington, Seattle, WA) Susan Heckbert.
Clinical Centers: (Albert Einstein College of Medicine, Bronx, NY) Sylvia Wassertheil‐Smoller; (Baylor College of Medicine, Houston, TX) Jennifer Hays; (Brigham and Women's Hospital, Harvard Medical School, Boston, MA) JoAnn Manson; (Brown University, Providence, RI) Annlouise R. Assaf; (Emory University, Atlanta, GA) Lawrence Phillips; (Fred Hutchinson Cancer Research Center, Seattle, WA) Shirley Beresford; (George Washington University Medical Center, Washington, DC) Judith Hsia; (Harbor‐UCLA Research and Education Institute, Torrance, CA) Rowan Chlebowski; (Kaiser Permanente Center for Health Research, Portland, OR) Cheryl Ritenbaugh; (Kaiser Permanente Division of Research, Oakland, CA) Bette Caan; (Medical College of Wisconsin, Milwaukee, WI) Jane Morley Kotchen; (Medstar Research Institute, Washington, D.C.) Barbara V. Howard; (Northwestern University, Chicago/Evanston, IL) Linda Van Horn; (Rush‐Presbyterian St. Luke's Medical Center, Chicago, IL) Henry Black; (Stanford Center for Research in Disease Prevention, Stanford University, Stanford, CA) Marcia L. Stefanick; (State University of New York at Stony Brook, Stony Brook, NY) Dorothy Lane; (The Ohio State University, Columbus, OH) Rebecca Jackson; (University of Alabama at Birmingham, Birmingham, AL) Cora Beth Lewis; (University of Arizona, Tucson/Phoenix, AZ) Tamsen Bassford; (University at Buffalo, Buffalo, NY) Maurizio Trevisan; (University of California at Davis, Sacramento, CA) John Robbins; (University of California at Irvine, Orange, CA) Allan Hubbell; (University of California at Los Angeles, Los Angeles, CA) Howard Judd; (University of California at San Diego, La Jolla/Chula Vista, CA) Robert D. Langer; (University of Cincinnati, Cincinnati, OH) Margery Gass; (University of Florida, Gainesville/Jacksonville, FL) Marian Limacher; (University of Hawaii, Honolulu, HI) David Curb; (University of Iowa, Iowa City/Davenport, IA) Robert Wallace; (University of Massachusetts, Worcester, MA) Judith Ockene; (University of Medicine and Dentistry of New Jersey, Newark, NJ) Norman Lasser; (University of Miami, Miami, FL) Mary Jo O'Sullivan; (University of Minnesota, Minneapolis, MN) Karen Margolis; (University of Nevada, Reno, NV) Robert Brunner; (University of North Carolina, Chapel Hill, NC) Gerardo Heiss; (University of Pittsburgh, Pittsburgh, PA) Lewis Kuller; (University of Tennessee, Memphis, TN) Karen C. Johnson; (University of Texas Health Science Center, San Antonio, TX) Robert Schenken; (University of Wisconsin, Madison, WI) Catherine Allen; (Wake Forest University School of Medicine, Winston‐Salem, NC) Gregory Burke; (Wayne State University School of Medicine/Hutzel Hospital, Detroit, MI) Susan Hendrix.
Supported by the Women's Health Initiative, NHLBI, NIH, and by grant HL075382 from the NIH, NHLBI.
REFERENCES
- 1. Bazett H. An analysis of the time‐relations of electrocardiograms. Heart 1920;7: 353–370. [Google Scholar]
- 2. Ashman R. Essentials of electrocardiography. New York , Macmillian, 1945. [Google Scholar]
- 3. Goldberg RJ, Bengtson J, Chen ZY, et al Duration of the QT interval and total and cardiovascular mortality in healthy persons (The Framingham Heart Study experience). Am J Cardiol 1991;67: 55–58.DOI: 10.1016/0002-9149(91)90099-7 [DOI] [PubMed] [Google Scholar]
- 4. Rautaharju PM, Zhou SH, Wong S, et al Sex differences in the evolution of the electrocardiographic QT interval with age. Can J Cardiol 1992;8: 690–695. [PubMed] [Google Scholar]
- 5. Makkar RR, Fromm BS, Steinman RT, et al Female gender as a risk factor for torsades de pointes associated with cardiovascular drugs. JAMA 1993;270: 2590–2597. [DOI] [PubMed] [Google Scholar]
- 6. Larsen JA, Tung RH, Sadananda R, et al Effects of hormone replacement therapy on QT interval. Am J Cardiol 1998;82: 993–995.DOI: 10.1016/S0002-9149(98)00523-2 [DOI] [PubMed] [Google Scholar]
- 7. Kadish AH, Larsen JA. Effects of gender on cardiac arrhythmias. J Cardiovasc Electrophysiol 1998;9: 655–664. [DOI] [PubMed] [Google Scholar]
- 8. Prinzmental M, Nakashima M, Oishi H, et al Estrogens and the heart. Am J Obstet Gynecol 1967;1967: 575–576. [DOI] [PubMed] [Google Scholar]
- 9. Gimeno A, Webb J. Action of sex steriods on the electrical and mechanical properties of rat atrium. Am J Physiol 1963;1963: 198–200. [DOI] [PubMed] [Google Scholar]
- 10. Burke JH, Ehlert FA, Kruse JT, et al Gender‐specific differences in the QT interval and the effect of autonomic tone and menstrual cycle in healthy adults. Am J Cardiol 1997;79: 178–181.DOI: 10.1016/S0002-9149(96)00707-2 [DOI] [PubMed] [Google Scholar]
- 11. Knollman B, Franz M. Acute effects of 17 B estradiol and dihydrotestosterone on action potential duration and QY‐interval in isolated rabbit hearts. J Investig Med 1996; 44: 209A. [Google Scholar]
- 12. Drici MD, Burklow TR, Haridasse V, et al Sex hormones prolong the QT interval and downregulate potassium channel expression in the rabbit heart. Circulation 1996;94: 1471–1474. [DOI] [PubMed] [Google Scholar]
- 13. Pham TV, Sosunov EA, Anyukhovsky EP, et al Testosterone diminishes the proarrhythmic effects of dofetilide in normal female rabbits. Circulation 2002;106: 2132–2136. [DOI] [PubMed] [Google Scholar]
- 14. Shuba YM. Testosterone‐mediated modulation of HERG blockade by proarrhythmic agents. Biochem Pharmacol 2001;62: 41–49.DOI: 10.1016/S0006-2952(01)00611-6 [DOI] [PubMed] [Google Scholar]
- 15. Bidoggia H, Maciel JP, Capalozza N, et al Sex differences on the electrocardiographic pattern of cardiac repolarization: possible role of testosterone. Am Heart J 2000;140: 678–683. [DOI] [PubMed] [Google Scholar]
- 16. Prentice R, Rossouw JE, Johnson S, et al The Role of randomized controlled trials in assessing the benefits and risks of long‐term hormone replacement therapy: example of the women's health initiative. Menopause: JN Am Menopause Soc 1996;3: 71–76. [Google Scholar]
- 17. Investigators WGftWsHI . Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the women's health initiative randomized controlled trial. JAMA 2002;288: 321–333. [DOI] [PubMed] [Google Scholar]
- 18. Rautaharju PM, Park LP, Chaitman BR, et al The Novacode criteria for classification of ECG abnormalities and their clinically significant progression and regression. J Electrocardiol 1998;31: 157–187.DOI: 10.1016/S0022-0736(98)90132-7 [DOI] [PubMed] [Google Scholar]
- 19. Mayuga KA, Parker M, Sukthanker ND, et al Effects of age and gender on the QT response to exercise. Am J Cardiol 2001;87: 163–167.DOI: 10.1016/S0002-9149(00)01309-6 [DOI] [PubMed] [Google Scholar]
- 20. Stramba‐Badiale M, Locati EH, Martinelli A, et al Gender and the relationship between ventricular repolarization and cardiac cycle length during 24‐h Holter recordings. Eur Heart J 1997;18: 1000–1006. [DOI] [PubMed] [Google Scholar]
- 21. Sagie A. An improved method for adjusting the QT interval for heart rate (the Framingham Heart Study). Am J Cardiol 1992;70: 797–801.DOI: 10.1016/0002-9149(92)90562-D [DOI] [PubMed] [Google Scholar]
- 22. Rodriguez I. Drug‐induced QT prolongation in women during the menstrual cycle. JAMA 2001;285: 1322–1326. [DOI] [PubMed] [Google Scholar]
- 23. Challapalli S, Lingamneni R, Horvath G, et al Twelve‐leadQT dispersion is smaller in women then in men. Noninvasive Electrophysiol 1998;3: 25–31. [Google Scholar]
- 24. Haseroth K. Effects of progestin‐estrogen replacement therapy on QT‐dispersion in postmenopausal women. Int J Cardiol 2000; 75: 161–165; discussion 165–166.DOI: 10.1016/S0167-5273(00)00317-X [DOI] [PubMed] [Google Scholar]
- 25. Sbarouni E, Zarvalis E, Kyriakides ZS, et al Absence of effects of short‐term estrogen replacement therapy on resting and exertional QT and QTC dispersion in postmenopausal women with coronary artery disease. Pacing Clin Electrophysiol 1998;21: 2392–2395. [DOI] [PubMed] [Google Scholar]
- 26. Bidoggia H, Maciel JP, Capalozza N, et al Sex‐dependent electrocardiographic pattern of cardiac repolarization. Am Heart J 2000;140: 430–436. [DOI] [PubMed] [Google Scholar]
- 27. Wolbrette D. Gender differences in the proarrhythmic potential of QT‐prolonging drugs. Curr Women Health Rep 2002; 2: 105–109. [PubMed] [Google Scholar]


