Abstract
Background/Objectives
Older adults in sub‐Saharan Africa (SSA) are at greatest risk of an impending noncommunicable diseases epidemic, of which cardiac disease is the most prevalent contributor. Thus, it is essential to establish electrocardiographic reference values for a population that is likely to differ genetically and environmentally from others where reference values are established.
Methods
Two thousand two hundred thirty‐two apparently healthy community‐based participants without known cardiac disease aged 70+ in rural Tanzania underwent 12‐lead electrocardiography. Electrocardiograms were digitally analyzed and gender‐specific reference values for P duration (PD), P amplitude (PAMP), P area (PAREA), P terminal negative force (V1) (PTNF), PR interval, QRS duration (QRSD), QT/QTc, R amplitude (II, V5) (RAMP) LVH index (LVHI), R axis and R/S ratio (V1) reported, following univariate analysis of covariance using a multiple linear regression model, adjusting for age, systolic blood pressure (SBP), body mass index (BMI), and RR interval.
Results
Data from 1824 subjects were suitable for analysis. Adjusted mean values for men/women were: PD 115/110 ms, PAMP (avg) 123/114 μV, PAMP (II) 203/190 μV, PAREA (avg) 5.3/4.6 mV*s, PAREA (II) 9.3/8.1mV*s, PTNF 1.7/1.4 mV*s, PR 158/152 ms, QRSD 89/84 ms, QT 370/375 ms, QTc 421/427 ms, RAMP (II) 805/854 μV, (V5) 2022/1742 μV, LVHI 3.0/2.8 mV (Sokolow‐Lyon), 1.293/1.146 mV (Cornell), R axis 51/49°, R/S 0.2/0.2. Excluding PTNF, R axis and R/S ratio, all gender differences were significant (P < 0.001 apart from LVHI [Sokolow‐Lyon; P < 0.005)] and RAMP (II) [P < 0.05]) following adjustment for age, SBP, BMI, and RR interval.
Conclusions
Our description of comprehensive electrocardiographic parameters establishes reference values in this genetically and environmentally diverse SSA population thereby allowing identification of “outliers” with potential cardiac disease.
Keywords: Africa, epidemiology, electrocardiogram, reference values
BACKGROUND/OBJECTIVES
Reference values aid determination of an ordinal measurement by establishing cutoffs for normal‐range values in different populations.1 Once established, they help to identify outliers and the outlier's deviation from the population “norm.” Within electrocardiography, values determined for a population are an essential yardstick for evaluating risk factors for cardiovascular disease, and cardiovascular disease itself.2 There have been reports of reference values for populations in high‐ and middle‐income countries2, 3, 4, but to our knowledge, there have yet to be reference values established for electrocardiographic (ECG) parameters in a sub‐Saharan African (SSA) population of older adults.
The number of older adults in SSA is rising rapidly5 and with it, the risk of various noncommunicable disease epidemics in this neglected, vulnerable portion of society.6 Despite this, little attention is paid to this population group, with scarce resources and funding often diverted to younger age groups, and to fighting communicable disease.7 Although there are more sensitive and specific diagnostic modalities in high‐income countries, the ECG remains an essential, inexpensive, noninvasive technique for assessing various cardiovascular diseases and their potential impact on a resource‐poor population. However, before identifying disease (i.e., abnormalities) it is imperative that reference values are established so that it is known within a population what is “normal.” From research in this population, we know, that although for instance the prevalence of hypertension is very similar to that of an age‐matched high income population.8 the prevalence of atrial fibrillation is far lower.9 This suggests diverse genetic and environmental influences on SSA populations with different risk factor profiles as compared to older populations in high‐income countries, and the application of reference values derived from high‐income countries are likely to be of little value.
Our aim for this study therefore was to establish reference ECG values for an apparently healthy, community‐dwelling SSA population of older adults, and examine the influence of gender and other clinical variables on important ECG measures.
METHODS
Ethical approval for the study was obtained locally from Tumaini University ethics committee and nationally from the Tanzanian National Institute of Medical Research. All study participants provided their informed consent.
Setting
Data were collected between November 1, 2009 and July 31, 2010. The Hai district of northern Tanzania is located on the southern slopes of Mount Kilimanjaro and includes a Demographic Surveillance Site, established by the Tanzanian Ministry of Health, the UK Department for International Development, and the University of Newcastle upon Tyne. There are regular population censuses within the Hai DSS: data collection for the most recent census was completed by the end of May 2009. On the June 1, 2009 the population of all 52 villages of the Hai DSS was 161,119, of whom 8869 were aged 70 years and above.
Study Population
The study population was identified via census details of those aged 70 years and over, and through village enumerators, who invited people known to have reached 70 years between the census and our data collection period. Collecting accurate information on patient age can be difficult in SSA, as few people have a birth certificate.10 Age was calculated from birth year and confirmed using memory prompts (e.g., age at independence) where necessary. This method has been previously validated.11
We planned to assess approximately one‐quarter of the 8869 people aged 70 years and over in the DSS. We visited 12 villages, with a total census population of 2419. Villages were chosen using a random number generator, with stratification to allow a representative spread of upland and lowland villages. To be included in the study, participants had to have had no history of cardiac disease, and were deemed apparently healthy based on their community‐dwelling status and a basic assessment of demographic and clinical variables. Following adjustment for people who were ineligible, had moved, died, or refused to be in the study, our final population totaled 2232.
ECG Data Acquisition
All study participants underwent 12‐lead ECG using a GE MAC 1200 (GE Healthcare, Hertfordshire, United Kingdom) machine. The standard 10‐second, 12‐lead ECG was acquired and processed using GE Medical Systems CardioSoft and 12‐SL analysis programs. The ECG data were sampled at 500 samples/s with an amplitude resolution of 5 μV. The raw ECG data were stored together with the ECG measurements provided by the software, for further offline processing. All ECG parameters were determined automatically ensuring that reproducibility of measurements was 100%. Digital records were visually assessed for technical errors and inadequate quality, and following exclusion of these records (n = 381) along with those without P wave annotations (n = 27) from the 12‐SL program, the number of ECG records suitable for further analysis totaled 1824. Patients with AF or atrial flutter on 12‐lead ECG were excluded, but because of the nature of the single recording it was possible we included patients with paroxysmal AF/flutter who were in sinus rhythm at the time of ECG acquisition.
Other Data
Age, sex, and body mass index (BMI) were recorded for all study participants.
Seated blood pressure (BP) was recorded (three measurements taken 1 minute apart after 5 minutes resting quietly, with an average taken of the last two readings) using an A&D UA‐767 (A&D Instruments Ltd, Abingdon, United Kingdom) BP monitor.12
ECG Parameters Computation
The P wave annotations (P onset, P offset, and QRS onset) of median beats produced by 12‐SL were adopted for further offline P wave parameter computation, as were R and S wave annotations to calculate the LVH index by Sokolow‐Lyon and Cornell methods.
P onset (PON) was annotated as the earliest across the twelve leads, P offset (POFF) as the latest. The QRS onset (QRSON) was also automatically annotated as the earliest across the twelve leads. These measurements were adopted for further offline P wave parameter computation.
Based on the fiducial point markers (PON, POFF), P wave amplitude (PAMP) and area (PAREA) were computed for each lead. PAMP was calculated as the peak‐to‐nadir amplitude difference on individual leads. Mean PAMP across 12 leads (PAMP [avg]) and PAMP in lead II (PAMP [II]) were considered for statistical analysis. Defining a baseline reference as the horizontal line intercepting the ECG at PON, PAREA was computed on individual leads as the area underneath the curve with respect to the baseline, as shown in Figure 1 (grey area). Mean PAREA across 12 leads (PAREA [avg]) and PAREA in lead II (PAREA [II]) were considered for statistical analysis. For biphasic P waves (Fig. 1D) the positive and negative contributions to the area were summed. In lead V1, the P wave terminal negative force (PTNF) was also computed as the product between the (right‐most) negative lobe amplitude (Fig. 1D, vertical arrow) in units of μV and its time interval (Fig. 1D, dotted horizontal arrow) in units of seconds between the (right‐most) negative zero‐crossing of the baseline and POFF. P wave duration (PD) was computed as the time interval between PON and POFF. P wave dispersion was not calculated because of the global definition of PON and POFF by the 12‐SLTM program. The PR interval was calculated as the time interval between PON and the annotated QRSON. Mean RR interval was also calculated. LVH index according to Sokolow‐Lyon criteria was calculated as the sum of the amplitude of the S wave in V1 and the tallest R wave in either V5 or V6, and by Cornell criteria as the sum of the amplitude of the S wave in V3 and the R wave amplitude in aVL, these values being read by 12‐SL.
Figure 1.
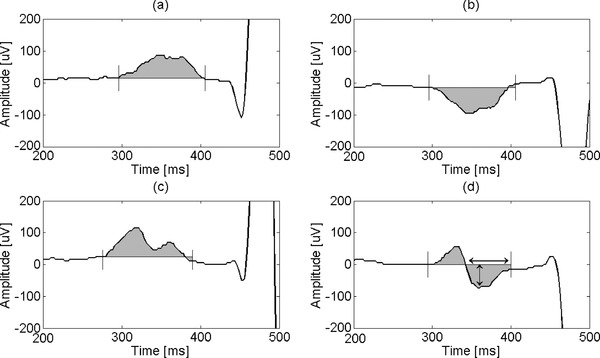
P wave area computation for different P wave morphologies: positive (a), negative (b), positive notched (c), biphasic (d). P wave area is total shaded area. Arrows in (d) show duration and amplitude for PTNF computation. Solid vertical lines show PON and POFF.
All other parameters including RR interval, QT interval and corrected QT (using Bazett's formula), R axis, QRS duration, and R/S ratio in lead V1 were read from the measurements made by 12‐SL.
Statistical Analysis
ECG parameters were assessed for normal distribution by calculating skewness and adopting the thumb rule proposed by Bulmer.13 which considers absolute value of skewness ≤ 1 a reasonable approximation to a normal distribution. This condition was not met only for PTNF (highly positively skewed) for which a logarithmic transformation was applied. Univariate analysis of covariance (ANCOVA) was performed on all measured ECG parameters individually using a multiple linear regression model (MLR) to assess for gender differences, adjusting for demographic (age) and clinical (systolic blood pressure (SBP), BMI, RR interval) covariates which were treated as continuous variables.
RESULTS
The distributions of the gender differences in ECG parameters and covariates are shown in Table 1.
Table 1.
Gender‐Specific ECG Parameters and Covariates Distribution (Mean ± SD)
| Parameter | All (n = 1824) | Male (n = 820) | Female (n = 1004) | P Value* |
|---|---|---|---|---|
| Age (years) | 77 ± 7 | 78 ± 7 | 77 ± 7 | N.S. |
| Systolic BP (mmHg) | 162 ± 34 | 155 ± 32 | 167 ± 34 | <0.001 |
| BMI (kg/m2) | 21 ± 4 | 20 ± 4 | 22 ± 5 | <0.001 |
| RR (ms) | 771 ± 145 | 812 ± 149 | 739 ± 134 | <0.001 |
| PD (ms) | 112 ± 14 | 114 ± 13 | 111 ± 14 | <0.001 |
| PAMP (Avg; μV) | 118 ± 36 | 119 ± 37 | 117 ± 35 | N.S. |
| PAMP (II) (μV) | 196 ± 76 | 197 ± 77 | 195 ± 76 | N.S. |
| PAREA(Avg; mV*s) | 4.9 ± 1.7 | 5.1 ± 1.7 | 4.7 ± 1.7 | <0.001 |
| PAREA (II; mV*s) | 8.6 ± 3.4 | 9.0 ± 3.5 | 8.3 ± 3.3 | <0.001 |
| PTNF (V1; mV*s) | 1.7 ± 1.6 | 1.5 ± 1.4 | 1.8 ± 1.7 | N.S. |
| (335/157/178)c | ||||
| PR (ms) | 155 ± 25 | 159 ± 23 | 152 ± 25 | <0.001 |
| QRS duration (ms) | 86 ± 15 | 88 ± 16 | 84 ± 14 | <0.001 |
| QT (ms) | 373 ± 34 | 377 ± 35 | 369 ± 32 | <0.001 |
| QTc Bazett (ms) | 424 ± 25 | 418 ± 24 | 429 ± 24 | <0.001 |
| LVH Indexa(mV) | 2.9 ± 1.0 | 3.0 ± 1.1 | 2.8 ± 0.9 | <0.05 |
| (1209/523/686)c | ||||
| LVH indexb (mV) | 1.2 ± 0.6 | 1.3 ± 0.7 | 1.2 ± 0.6 | N.S. |
| (1566/688/878)c | ||||
| RAMP (II) (μV) | 832 ± 426 | 803 ± 435 | 856 ± 417 | <0.005 |
| (1815/813/1002)c | ||||
| RAMP (V5; μV) | 1868 ± 810 | 2049 ± 851 | 1720 ± 742 | <0.001 |
| (1823/820/1003)c | ||||
| R‐axis (°) | 50 ± 27 | 53 ± 29 | 48 ± 26 | <0.001 |
| (1425/615/810)c | ||||
| R/S in V1 | 0.2 ± 0.3 | 0.2 ± 0.4 | 0.1 ± 0.3 | N.S. |
| (1209/523/686)c |
*Mann‐Whitney test, α = 0.05 (two‐tailed), comparing Male versus Female groups.
Sokolow‐Lyon index.
Cornell Index.
Numbers of observations in brackets for individual values (total/male/female).
Gender differences of ECG parameters, adjusted for demographic (age) and clinical (RR interval, SBP, BMI) covariates are shown in Table 2. All P wave indices except PTNF were significantly different (higher) in men (P < 0.001). PTNF was present (value greater than zero) only in a limited number of cases (157 male, 178 female patients). QRS duration was higher in men (<0.001), QT and QTc (Bazett) were longer in women (P < 0.001). Both LVH Sokolow‐Lyon index (P < 0.005) and Cornell index (P < 0.001) were higher in men. The mean cardiac axis (R‐axis) was not significantly related to gender.
Table 2.
Gender‐Specific ECG Parameters: Adjusted Mean* (Confidence Interval [CI])
| Parameter | Male (n = 820) | Female (n = 1004) | P Value |
|---|---|---|---|
| PD (ms) | 115 (114, 116) | 110 (109.3, 111.0) | <0.001 |
| PAMP (Avg; μV) | 123 (121, 125) | 114 (111.5, 115.6) | <0.001 |
| PAMP (II) (μV) | 203 (198, 208) | 190 (185.2, 194.0) | <0.001 |
| PAREA(Avg; mV*s) | 5.3 (5.2, 5.4) | 4.6 (4.5, 4.7) | <0.001 |
| PAREA (II) (mV*s) | 9.3 (9.0, 9.5) | 8.1 (7.9, 8.3) | <0.001 |
| PTNF (V1) (mV*s) | 1.7 (1.4, 2.0) | 1.4 (1.1, 1.6) | N.S. |
| (335/157/178)c | |||
| PR (ms) | 158(157, 160) | 152 (151, 154) | <0.001 |
| QRS duration (ms) | 89 (88,90) | 84 (83, 85) | <0.001 |
| QT (ms) | 370 (368, 371) | 375 (374, 376) | <0.001 |
| QTc Bazett (ms) | 421 (419, 422) | 427 (425, 428) | <0.001 |
| LVH Indexa [mV] | 3.0 (3.0, 3.1) | 2.8 (2.8, 2.9) | <0.005 |
| (1209/523/686)c | |||
| LVH Indexb (mV) | 1.3 (1.25, 1.34) | 1.1 (1.11, 1.19) | <0.001 |
| (1566/688/878)c | |||
| RAMP (II) (μV) | 805 (776, 834) | 854 (828, 881) | <0.05 |
| (1815/813/1002)c | |||
| RAMP (V5; μV) | 2022 (1969, 2075) | 1742 (1694, 1789) | <0.001 |
| (1823/820/1003)c | |||
| R‐axis (°) | 51 (49, 54) | 49 (47, 51) | N.S. |
| (1425 /615/810)c | |||
| R/S in V1 | 0.2 (0.1, 0.2) | 0.2 (0.1, 0.2) | N.S. |
| (1209/523/686)c |
*Mean values adjusted for age, systolic blood pressure, body mass index, and R‐R interval.
Sokolow‐Lyon index.
Cornell Index.
Numbers in brackets for individual values (total/male/female).
The MLR model was also used to quantify the contribution of individual covariates (predictors) to each ECG parameter (MLR model response). For a 1‐SD change in a given covariate (holding other covariates constant), the corresponding change in the ECG parameter was calculated. The results are shown in Table 3 for those ECG parameters for which the explanatory power (coefficient of determination, R2) of the MLR model was higher than an arbitrary threshold of 10%.
Table 3.
Relationship between Clinical Covariates and ECG Parameters (Mean [95% CI])
| MLR | Age | SBP | BMI | RR | |||||
|---|---|---|---|---|---|---|---|---|---|
| Parameter | R2 (%) | Mean (95% CI) | P Value | Mean (95% CI) | P Value | Mean (95% CI) | P Value | Mean (95% CI) | P Value |
| PAMP (Avg; μV) | 19.9 | −5.8 | <0.001 | 3.5 | <0.001 | −6.4 | <0.001 | −14.4 | <0.001 |
| (−7.3, −4.3) | (1.9, 5.0) | (−7.9 −4.9) | (−16.0, −12.9) | ||||||
| PAMP (II) (μV) | 16.5 | −13.3 | <0.001 | 4.0 | <0.05 | −13.7 | <0.001 | −27.0 | <0.001 |
| (−16.5, −10.0) | (0.7, 7.3) | (−17.0,−10.4) | (−30.4, −23.7) | ||||||
| PAREA (Avg; mV*s) | 14.9 | −0.3 | <0.001 | 0.3 | <0.001 | −0.2 | <0.001 | −0.5 | <0.001 |
| (−0.3, −0.2) | (0.2, 0.3) | (−0.3, −0.1) | (−0.6, −0.5) | ||||||
| PAREA (II) (mV*s) | 12.3 | −0.6 | <0.001 | 0.3 | <0.001 | −0.4 | <0.001 | −0.9 | <0.001 |
| (−0.8, −0.5) | (0.2, 0.5) | (−0.6, −0.3) | (−1.1, −0.8) | ||||||
| QT (ms) | 67.1 | 1.6 | <0.005 | 2.5 | <0.001 | 0.5 | N.S. | 28.2 | <0.001 |
| (0.7, 2.5) | (1.6, 3.5) | (−0.4, 1.4) | (27.3, 29.2) | ||||||
| QTc Bazett (ms) | 17.0 | 1.9 | <0.001 | 3.5 | <0.001 | 0.6 | N.S. | −7.4 | <0.001 |
| (0.9,3.0) | (2.4, 4.6) | (−0.4, 1.7) | (−8.6, −6.4) | ||||||
| LVH Indexa (mV) | 15.4 | −0.1 | <0.05 | 0.4 | <0.001 | −0.2 | <0.001 | 0.1 | <0.005 |
| (−0.4, −0.0) | (0.3, 0.4) | (−0.3, −0.2) | (0.0, 0.1) | ||||||
| RAMP (V5; μV) | 15.2 | −42.7 | <0.05 | 191.0 | <0.001 | −206.2 | <0.001) | 106.5 | <0.001 |
| (−77.3, −8.1) | (155.5, 226.5) | (−241.5,−170.8) | (70.9, 142.1) | ||||||
Sokolow‐Lyon
PAMP and PAREA decreased with age, BMI and RR (all P < 0.001), and increased with SBP (all P < 0.001, except PAMP(II) with P < 0.05). The highest absolute change was given by RR. The QT interval increased with age (P < 0.005), SBP, and RR (both P < 0.001), with the largest contribution by RR (28 ms). QTc (QT corrected by Bazett's formula) also increased with age and SBP (P < 0.001), but decreased with RR (P < 0.001). Both the Sokolow‐Lyon index of LVH and RAMP in V5 decreased with age (P < 0.05) and BMI (P < 0.001), and increased with SBP (P < 0.001) and RR (P < 0.005 LVH index, P < 0.001 for RAMP [V5]); the highest absolute change was given by SBP for LVH index and by BMI for RAMP (V5).
DISCUSSION
This study has determined ECG reference values using an accurate and highly reproducible digital analysis program.14, 15, 16 in a large community‐dwelling population of older adults in rural SSA. Using the population studied we have been able to make several observations about the influences on ECG parameters across this population.
Differences in ECG Parameters with Gender
Mean analysis of variance adjusted demographic and clinical variables (Table 2) of P wave indices showed significantly higher values in men (P < 0.001, two‐tail) for all indices except PTNF. These results, are consistent with those reported by Magnani and coworkers in a US population.2 The P wave has previously been described as an “intermediate phenotype” and “surrogate” of atrial size.2 and men are known to have echocardiographically larger atrial diameters than women in some studies.17 Greater height and weight have previously correlated well with atrial size and volume.18 and men in our cohort were significantly (P < 0.001) taller (164 vs 154 cm) and heavier (55 vs 50 kg) than their female counterparts, despite lower BMIs overall. Furthermore, gender differences in chest wall impedance because of subcutaneous/breast tissue may contribute to differences in surface P wave size between genders. Mean PR interval was longer (P < 0.001) in men than women, consistent with a previous large multicenter study.19
QRS duration was greater in men than women (P < 0.001) in our study, and again is in line with studies done both within and outside SSA.3, 19, 20 including a study in a community‐based Nigerian population.21 A possible reason supporting this reproducible finding is that men have larger hearts compared with women and thus depolarization takes longer to move through increased muscle mass; this may also explain why men have higher LVH indices values than women, despite a significantly lower mean SBP. QT and QTc were both longer in women than men, a finding that whilst well accepted in the literature.22, 23, 24 remains incompletely understood.
Changes in ECG Parameters with Clinical Covariates
PAMP and PAREA decreased significantly with age (P < 0.001; Table 3). It has been previously suggested that autonomic tone may be related to P wave area, and that during periods of sleep when autonomic tone is reduced, this is reflected by decreased P wave area.25 Autonomic tone is known to reduce with age26 and this could be a potential explanation for a decrease in PAREA seen with age in our population. Age‐related loss of atrial myocytes and thus decreased volume of electrically active atrial tissue, reflected in smaller P waves, might also explain the decline in values with age.
Both age and BMI reduced the LVH index, probably for opposing reasons. Myocyte atrophy is likely to reduce R wave size with age, whereast increased subcutaneous tissue and hence impedance is likely to be the reason for reduced R wave amplitude in those with a higher BMI (and also the reason for reduced PAMP and PAREA.
P wave indices tended to increase with SBP. PAMP and PAREA increased significantly with SBP (P < 0.001, except PAMP(II) with P < 0.05, covariate analysis). Increased afterload caused by increased SBP is known to increase hypertrophy (both ventricular and atrial) and subsequent dilatation, and is a possible reason for the larger P wave indices, and the likely reason why the Sokolow‐Lyon LVH index increases with increasing SBP (0.4 mV per SD of mean SBP). As left ventricular muscle mass increases with hypertrophy, so the mean frontal axis is likely to become more negative (i.e., more leftwards).
Comparison with Other Populations
Reference values reported in high‐income countries are unlikely to be representative of a SSA population of older adults. There are likely to be significant genetic and environmental differences influencing cardiac depolarization and repolarization. Studies from elsewhere in the world are not directly comparable to ours as reference values have either been reported as median values, used different analysis methodology, or not reported in a 70+ population. Magnani et al.2 reported P wave reference values from the Framingham cohort, with a median averaged P wave duration in the 76–85 year group (n = 39) of 89 ms in men and 88 ms in women, and a PR interval of 180 ms in men and 160 ms in women. Wu et al.4 reported values for a Chinese population and found in 696 men and 161 women ≥60 years of age median values of 118/113 ms respectively for PD, 159/152 ms for PR interval, 93/86 ms for QRSD, and 416/427 ms for QTc. Growing literature surrounding genetic polymorphisms of sodium and potassium channels and the influence they have on the subsequent development of AF.27, 28 suggest the potential for population‐based differences. There are well established differences in the renin‐angiotensin‐aldosterone system encoding genes between black Africans and Caucasian populations.29 High levels of angiotensin present in this population promotes fibrosis in the myocardium, thus potentially affecting electrical activity within the heart. Furthermore, the risk factor profile for cardiac disease is likely to be significantly different from a high‐income country population. Ischemic heart disease is thought to be less common in black Africans.30 and recent studies in this older SSA population have reported a strikingly low prevalence of AF.9 in spite of an epidemic of poorly controlled hypertension.8 This suggests a different pattern of risk factors influencing cardiac electrical signals to previously studied populations, and thus reference values are likely to be different.
Strengths and Limitations
A major strength is that we assessed a large sample size that had been randomly selected within a well‐defined rural African population. All study variables were ascertained uniformly, and of particular note, the ECGs were analyzed by reliable computer algorithms.14, 15, 16 thus ensuring 100% reproducibility, and we reported an ‘averaged’ beat aimed at minimizing noise and inaccurate measurements, a key feature in the machine's use when in a challenging environment in a rural SSA community.
Limitations include the possibility of sample bias (despite the randomly‐selected nature of our population), as we only surveyed approximately 25% of the 70+ population. Our population of community‐dwelling older adults was assumed to be healthy based on a very basic initial assessment of clinical and demographic variables, but we cannot rule out the presence of disease beyond this as a detailed history was not undertaken because of logistical limitations. Furthermore, the mean differences with age and gender were all fairly small in a clinical sense. Although many were statistically significant, the differences are unlikely to be clinically relevant. However, our primary aim for this study was not to look at clinical impact of the changes, but to report values and trends that can be interpreted as reference ranges for normal ECG parameters in a community‐dwelling population of older adults in rural SSA. We anticipate that if a “normal range” is accepted for this population, then abnormal ECG parameters can subsequently be identified to see if they predict clinical disease entities such as AF and stroke in a similar way to studies in high‐income countries.
CONCLUSIONS
To our knowledge, this is the first attempt at developing reference values for a rural population of older adults in SSA for comprehensive ECG parameters. Establishing reference values for a population is not only necessary to define the epidemiology of “normal” ECG parameters in that population, but also essential to determine how clinical disease processes may subsequently be identified through “abnormal” ECG parameters, all the more important in a resource‐poor setting. Established reference values for older adults in high‐income countries are likely to be of limited use in this, and other, SSA populations because of diverse genetic and environmental factors, and thus we hope our reference values will be of use for physicians in SSA to aid assessment of a patient's cardiac status in the future.
Acknowledgments
We wish to acknowledge the help of all health care workers, officials, carers, and family members who assisted in examination, assessment, data collection and input. We are very grateful to Peter Macfarlane at Glasgow University and Willi Kaiser at GE in Germany for their help with ECG file conversion.
Funding: This work was supported by a grant from the Peel Medical Research Trust, Northumbria Healthcare NHS Foundation Trust, and the National Institute for Health Research Newcastle Biomedical Research Centre based at Newcastle Hospitals Foundation Trust and Newcastle University.
Conflicts of Interest: There are no conflicts of interest.
REFERENCES
- 1. Hyltoft PP, Rustad P. Prerequisites for establishing common reference intervals. Scand J Clin Lab Invest 2004;64:285–292. [DOI] [PubMed] [Google Scholar]
- 2. Magnani JW, Johnson VM, Sullivan LM, et al. P wave indices: derivation of reference values from the Framingham Heart Study. Ann Noninvasive Electrocardiol 2010;15:344–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Macfarlane PW, Lawrie TDV. The normal electrocardiogram and vectorcardiogram In: Macfarlane PW, Lawrie TDV. (eds.): Comprehensive Electrocardiology, Oxford (UK): Pergamon Press, 1989, pp. 407–57. [Google Scholar]
- 4. Wu J, Kors J, Rijnbeck P, van Herpen G, et al. Normal limits of the electrocardiogram in Chinese subjects. Int J Cardio 2003;87:37–51. [DOI] [PubMed] [Google Scholar]
- 5. United States Census Bureau . International Data Base, 2000. http://www.census.gov/ipc/www/idbnew.html. (accessed May 14, 2011)
- 6. Unwin N, Mugusi F, Aspray T, et al. Tackling the emerging pandemic of non‐communicable diseases in sub‐Saharan Africa: The essential NCD health intervention project. Public Health 1999;113:141–146. [PubMed] [Google Scholar]
- 7. Nugent R, Feigl AB. Where Have all the Donors Gone? Scarce Funding for Non‐Communicable Disease Working Paper 228. Washington, Center for Global Development, 2010. http://www.cgdev.org/content/publications/detail/1424546 [Google Scholar]
- 8. Dewhurst MJ, Dewhurst F, Gray WK, et al. The high prevalence of hypertension in rural‐dwelling Tanzanian older adults and the disparity between detection, treatment and control: A rule of sixths? J Hum Hypertens 2013;27:374–380. [DOI] [PubMed] [Google Scholar]
- 9. Dewhurst MJ, Adams PC, Gray WK, et al. Strikingly low prevalence of atrial fibrillation in elderly Tanzanians. J Am Geriatr Soc 2012;60:1135–1140. [DOI] [PubMed] [Google Scholar]
- 10. Adult Morbidity and Mortality Project (AMMP) . Policy Implications of Adult Morbidity and Mortality; final report. 2004. [cited; Available from: http://research.ncl.ac.uk/ammp/finrep/ (accessed May 22, 2011).
- 11. Ogunniyi A, Osuntokun BO. Determination of ages of elderly Nigerians through historical events: validation of Ajayi‐Igun 1963 listing. West Afr J Med 1993;12:189–190. [PubMed] [Google Scholar]
- 12. Rogoza AN, Pavlova TS, Sergeeva MV. Validation of A&D UA‐767 device for the self‐measurement of blood pressure. Blood Press Monit 2000;5:227–231. [DOI] [PubMed] [Google Scholar]
- 13. Bulmer MG. Principles of Statistics, p.63, NY, Dover Publications Inc, 1979. [Google Scholar]
- 14. Reddy B, Elko P, Christianson D, Rowlandson G. Detection of P Waves in Resting ECG: A Preliminary Study, Los Alamitos, CA, IEEE Computer Society Press, 1992. [Google Scholar]
- 15. Reddy S, Elko P, Swirin S. Proceedings of the XXIII International Congress on electrocardiology, J L, editor. Singapore, World Scientific, 1996. [Google Scholar]
- 16. Farrell R, Xue J, Young BJ. Enhanced rhythm analysis using spectral and time‐domain techniques. Computers in Cardiology 2003;30:733–736. [Google Scholar]
- 17. Nikitin NP, Witte KKA, Thackray SDR, et al. Effect of age and sex on left atrial morphology and function. Eur J Echocardiography 2003;4:36–42. [DOI] [PubMed] [Google Scholar]
- 18. Pritchett AM, Jacobsen SJ, Mahoney DW, et al. Left atrial volume as an index of left atrial size: A population based study. J Am Coll Cardiol 2003;41:1036–1043. [DOI] [PubMed] [Google Scholar]
- 19. Mason JW, Ramseth DJ, Chanter DO, et al. Electrocardiographic reference ranges derived from 79,743 ambulatory subjects. J Electrocardiol 2007;40:228–234. [DOI] [PubMed] [Google Scholar]
- 20. Chen CY, Chiang B, Macfarlane PW. Normal limits of the electrocardiogram in a Chinese population. J Electrocardiol 1989;22:1–15. [DOI] [PubMed] [Google Scholar]
- 21. Katibi I, Clark E, Devine B, et al. Comparison of QRS Duration in African Blacks and European Caucasians. Comput Cardiol 2010;37:701–704. [Google Scholar]
- 22. Bazett HC. An analysis of the time‐relations of electrocardiograms. Heart 1920;7:353–370. [Google Scholar]
- 23. Stramba‐Badiale M, Locati EH, Martinelli A, et al. Gender and the relationship between ventricular repolarization and cardiac cycle length during 24‐h Holter recordings. Eur Heart J 1997;18:1000–1006. [DOI] [PubMed] [Google Scholar]
- 24. Merri M, Benhorin J, Alberti M, et al. Electrocardiographic quantitation of ventricular repolarization. Circulation 1989;80:1301–1308. [DOI] [PubMed] [Google Scholar]
- 25. Dilaveris PE, Farbom P, Batchvarov V, et al. Circadian behaviour of P wave duration, P‐wave area, and PR interval in healthy subjects. Ann Noninvasive Electrocardiol 2001;6:92–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Seals DR, Monahan KD, Bell C, et al. The aging cardiovascular system: Changes in autonomic function at rest and in response to exercise. Int J Sport Nutr Exerc Metab 2001;11 (Suppl):S189–S195. [DOI] [PubMed] [Google Scholar]
- 27. Tsai CT, Lai LP, Hwang JJ, et al. Molecular genetics of atrial fibrillation. J Am Coll Cardiol 2008;52:241–250. [DOI] [PubMed] [Google Scholar]
- 28. Shreieck J, Dostal S, von Beckerath N, et al. C825T polymorphism of the G‐protein beta3 sub‐unit gene and atrial fibrillation: Association of the TT phenotype with reduced risk for atrial fibrillation. Am Heart J 2004;148:545–550. [DOI] [PubMed] [Google Scholar]
- 29. Tiago AD, Badenhorst D, Nkeh B, et al. Impact of renin‐angiotensin‐ aldosterone system gene variants on the severity of hypertension in patients with newly diagnosed hypertension. Am J Hypertens 2003;16:1006–1010. [DOI] [PubMed] [Google Scholar]
- 30. Walker ARP, Sareli P. Urbanisation and health in transition: South Africa: paradox of coronary heart disease. Lancet 1997;349(Suppl III):1–32.8988107 [Google Scholar]


