Abstract
Background: Systolic time intervals measured by echocardiography and carotid artery tracings are validated methods of assessing left ventricular function. However, the clinical utility of phonoelectrocardiographic systolic time intervals for predicting heart failure using newer technology has not been evaluated.
Methods: We enrolled 100 adult patients undergoing left heart catheterization. Participants underwent computerized phonoelectrocardiographic analysis, left ventricular end‐diastolic pressure (LVEDP) measurement, transthoracic echocardiographic measurement of left ventricular ejection fraction (LVEF), and B‐type natriuretic peptide (BNP) testing. The heart rate‐adjusted systolic time intervals included the time from the Q wave onset to peak S1 (electromechanical activation time, EMAT), Q wave onset to peak S2 (electromechanical systole, Q‐S2), and peak S1 to peak S2 (left ventricular systolic time, LVST). Left ventricular dysfunction was defined as the presence of both LVEDP >15 mmHg and LVEF <50%.
Results: EMAT (r =−0.51; P < 0.0001), EMAT/LVST (r =−0.41; P = 0.0001), and Q‐S2 (r =−0.39; P = 0.0003) correlated with LVEF, but not with LVEDP. An abnormal EMAT ≥15 (odds ratio 1.38, P < 0.0001) and EMAT/LVST ≥0.40 (OR 1.13, P = 0.002) were associated with left ventricular dysfunction. EMAT ≥15 had 44% sensitivity, 94% specificity, and a 7.0 likelihood ratio for left ventricular dysfunction, while EMAT/LVST ≥0.40 had 55% sensitivity, 95% specificity, and a 11.7 likelihood ratio. In patients with an intermediate BNP (100–500 pg/mL), the likelihood ratio increased from 1.1 using the BNP result alone to 11.0 when adding a positive EMAT test for predicting left ventricular dysfunction.
Conclusions: Phonoelectrocardiographic measures of systolic time intervals are insensitive but highly specific tests for detecting abnormalities in objective markers of left ventricular function. EMAT and EMAT/LVST provide diagnostic information independent of BNP for detecting patients with left ventricular dysfunction.
Keywords: heart failure, phonocardiography, hemodynamics, echocardiography, natriuretic peptides
Heart failure is the primary diagnosis in over one million hospitalizations annually in the United States. 1 The physical examination for the diagnosis of heart failure is inexact with considerable interobserver variability. Despite the recent incorporation of B‐type natriuretic peptide (BNP) measurement into clinical practice, the bedside diagnosis of left ventricular dysfunction remains a diagnostic challenge. Intermediate‐range BNP levels are not diagnostic. 2 Though echocardiography represents a noninvasive criterion standard in diagnosis, it requires a high level of skill for acquisition and interpretation, and is associated with cost and time, necessitating selective utilization of this resource. The development of a simple inexpensive point‐of‐care bedside test to aid in the diagnosis of left ventricular dysfunction could help stratify which patients would benefit from a further, more detailed, diagnostic work‐up.
A validated noninvasive method of detecting left ventricular dysfunction is the measurement of systolic time intervals (STIs), 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 combining data from electrocardiography, echocardiography, phonocardiography, and carotid pulse tracings. STIs have been extensively studied over the past 40 years, and abnormalities in STIs have been shown to correlate with left ventricular end‐diastolic volume, 3 stroke volume, 5 , 11 cardiac output, 12 and left ventricular ejection fraction. 3 While STIs hold diagnostic potential, their bedside applicability in the past has been limited due to the necessity of skilled technicians and manual calculations. With the emergence of echocardiography, diagnostic use of STIs has fallen out of favor. However, new methods of measuring STIs at the bedside using recently developed technology that requires no skill beyond placing a standard 12‐lead electrocardiogram have been created, but have not yet been clinically tested in the detection of abnormal left ventricular function.
We undertook a prospective study to correlate STIs detected by computerized phonoelectrocardiography with invasive and noninvasive measures of left ventricular function in patients referred for left heart catheterization.
METHODS
Participants and Study Design
Adult patients referred for nonemergent left heart catheterization at the University of California‐San Francisco Medical Center were eligible for enrollment. Study enrollment was prospectively set at 100 subjects using cross‐sectional sampling. All patients gave written informed consent prior to enrollment, and the protocol as approved by the UCSF Committee on Human Research. Exclusion criteria included age <18 years old, systolic blood pressure <90 mmHg, intravenous vasopressor, inotropic, or vasodilator pharmacotherapy, cardiac rhythm other than a sinus or paced atrial rhythm, severe mitral regurgitation or stenosis, constrictive pericarditis, serum creatinine ≥4.0 mg/dL, severe pulmonary hypertension, and mechanical ventilation.
Patients' primary diagnoses and significant comorbidities were determined based on a systematic review of clinical charts. These conditions included coronary artery disease (defined as at least one coronary artery with ≥70% diameter stenosis), systemic hypertension, clinical heart failure, aortic stenosis, mitral regurgitation, chronic renal insufficiency, hypertrophic obstructive cardiomyopathy, and chronic obstructive pulmonary disease.
Within a four‐hour period, cardiac catheterization, transthoracic echocardiography, computerized heart sound phonocardiographic analysis, and BNP measurement (using a membrane immunofluorescence assay, Biosite Inc, San Diego, CA) were performed. A BNP >100 pg/mL was prospectively specified as abnormal. Left ventricular dysfunction was defined as the presence of both left ventricular end‐diastolic pressure (LVEDP) >15 mmHg and left ventricular ejection fraction (LVEF) <50%.
Invasive Left Ventricular Hemodynamics
Patients underwent recording of LVEDP using a 6 French pigtail catheter and a fluid‐filled pressure transducer. Pressure was recorded using a 50 mmHg scale at 50 mm/s paper speed. A blinded physician measured the post‐A wave pressure. A minimum of five consecutive cardiac cycles were used to measure the mean LVEDP over the respiratory cycle. An LVEDP >15 mmHg was prospectively specified as abnormal. 13 , 14
Echocardiography
Transthoracic echocardiographic data was obtained by an experienced echocardiographer (Acuson Sequoia, Siemens, Malvern, PA or SONOS 5500, Philips Medical Systems, Andover, MA). Echocardiographic contrast (Optison, Amersham, Little Chalfont, United Kingdom; 0.3 to 0.5 mL injected into a peripheral vein) was administered when required to improve endocardial border detection and enhance Doppler signals. Echocardiographic data was stored on magneto‐optical disks and analyzed off‐line by a single experienced reader blinded to any clinical or study data. The average of three measurements was used for the analysis. End‐diastolic and end‐systolic volumes were calculated using the biplane method of discs, and were then indexed to body surface area. These volumes were used to calculate LVEF. An LVEF <50% was prospectively defined as abnormal. 15
Computerized Heart Sound Phonocardiographic Analysis
A 3‐minute audioelectrocardiographic tracing (Audicor, Inovise Medical, Inc., Portland, OR) was obtained. Audioelectrocardiographic leads were attached to the V3 and V4 positions and connected to a Marquette MAC 5000 (General Electric Healthcare Technologies, Waukesha, WI). The audioelectrocardiographic data was stored electronically to a CD. A 10‐second segment free of artifact was selected off‐line by a blinded technician.
Systolic Time Intervals
Several phases of the cardiac cycle were measured directly (Fig. 1). The Q‐S1 interval was measured from the initial deflection of the electrocardiographic Q wave to the peak component of the S1 phonocardiographic complex. This Q‐S1 interval was designated as the electromechanical activation time (EMAT). The left ventricular systolic time (LVST) was defined as the interval between the peak components of the S1 and S2 complexes. Q‐S2 was measured from the onset of the Q wave to the peak S2. To correct for heart rate, the EMAT, LVST, and Q‐S2 intervals were divided by the R‐R interval to determine the percent of time in the cardiac cycle occupied by each interval.
Figure 1.
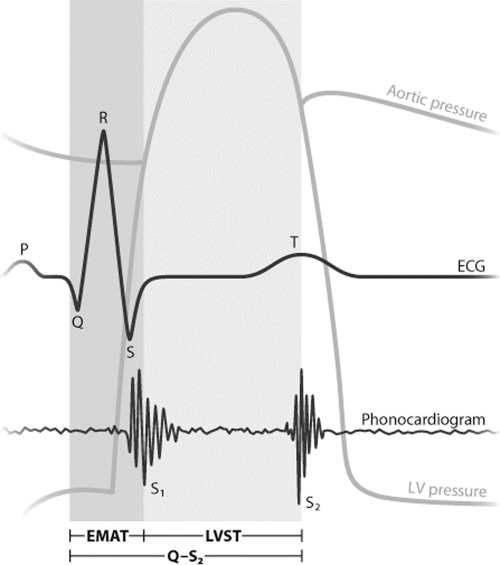
Schematic displaying the timing of the electrocardiogram, phonocardiogram, left ventricular pressure waveform, and the aortic pressure waveform (illustration by Giovanni Maki).
Statistical Analysis
Data was presented as mean values and standard deviations for continuous variables with normal Gaussian distribution. BNP had a right‐skewed distribution, and was presented as the median and interquartile range. Categorical data was presented as exact numbers and proportions. Continuous variables were compared using linear regression models and t‐tests. Categorical variables were compared using the Fisher's exact test. BNP levels were compared using the Kruskal‐Wallis test. The units for the systolic time intervals were displayed as means and standard deviations, with each unit representing 1% of the cardiac cycle. Sensitivity, specificity, area under the receiver operating curve (ROC), and likelihood ratios for the diagnostic tests were calculated as predictors of left ventricular dysfunction, defined as those with LVEDP >15 mmHg and LVEF <50%. The likelihood ratio for a positive test was sensitivity/(1 – specificity). The likelihood ratio for a negative test was (1 – sensitivity)/specificity. Multivariable linear regression with adjustment for heart rate and gender were performed to compare the STIs with measures of ventricular function. 11 , 16 , 17 Cutoff values were retrospectively determined upon inspection of the ROC curves. A 2‐tailed P < 0.05 was considered significant. All analyses were performed using STATA version 9.1 (StataCorp, College Station, TX).
RESULTS
Patient Population
One hundred patients were enrolled. Ninety‐two patients had adequate phonocardiographic data. Two additional patients with paced rhythms were excluded, as these could not be assessed by the available phonocardiographic software. Nine additional patients had inadequate echocardiographic data. A total of 81 patients had adequate assessments of the measured parameters (phonocardiographic STIs, LVEDP, and LVEF) and comprise this study's cohort. BNP data was collected for all but one of these patients.
The mean age was 61.2 ± 13.1 years (range 24–90), and 55 (68%) were male. Based on chart review, sixty‐three (78%) had systemic hypertension, 29 (36%) had a prior clinical diagnosis of heart failure, 24 (30%) had diabetes, and 14 (17%) were hospitalized for an acute coronary syndrome. Fifty‐eight (72%) patients had angiographic evidence of coronary artery disease. The mean body mass index was 28.6 ± 7.1 kg/m2, and the mean creatinine was 1.43 ± 1.27 mg/dL. Eight patients (10%) had left bundle branch block and four patients (5%) had at least mild aortic stenosis.
Of the total study population, 18 (22%) had left ventricular dysfunction by our study criteria. The prevalence of left ventricular dysfunction was higher in males (P = 0.044) and those with a previous diagnosis of heart failure (P < 0.0001; Table 1).
Table 1.
Baseline Demographic and Clinical Characteristics
| Characteristics | Total | No LV Dysfunction | LV Dysfunction | P‐Value |
|---|---|---|---|---|
| Number | 81 (100%) | 63 (78%) | 18 (22%) | |
| Age, mean ± SD (years) | 61.2 ± 13.1 | 61.7 ± 13.1 | 59.5 ± 13.2 | 0.54 |
| Male, n (%) | 55 (68%) | 39 (62%) | 16 (89%) | 0.044 |
| BMI (kg/m2) | 28.4 ± 6.9 | 28.7 ± 7.4 | 27.1 ± 4.7 | 0.40 |
| History, n (%) | ||||
| Coronary artery disease | 58 (72%) | 44 (70%) | 14 (78%) | 0.57 |
| Acute coronary syndrome | 14 (17%) | 9 (14%) | 5 (28%) | 0.29 |
| Diabetes mellitus | 24 (30%) | 18 (29%) | 6 (33%) | 0.77 |
| Hypertension | 63 (78%) | 50 (79%) | 13 (72%) | 0.53 |
| Hyperlipidemia | 65 (80%) | 52 (83%) | 13 (72%) | 0.33 |
| Clinical heart failure | 29 (36%) | 15 (24%) | 14 (78%) | <0.0001 |
LV = left ventricular; SD = standard deviation; BMI = body mass index.
Hemodynamics
Patients with left ventricular dysfunction were more likely to have a lower aortic systolic (P = 0.0047) and mean pressure (P = 0.021; Table 2). The median BNP in the left ventricular dysfunction group was higher than the normal group (872 pg/mL, interquartile range [IQR] 311–1420 versus 83, IQR 42–192; P < 0.0001).
Table 2.
Hemodynamics and Systolic Time Intervals
| No LV Dysfunction | LV Dysfunction | P‐Value | |
|---|---|---|---|
| Hemodynamics | |||
| Heart rate (beats/min) | 68 ± 11 | 73 ± 13 | 0.094 |
| Systolic aortic pressure (mmHg) | 133 ± 22 | 116 ± 23 | 0.0047 |
| Mean aortic pressure (mmHg) | 94 ± 15 | 85 ± 15 | 0.021 |
| LVEDP (mmHg) | 12.4 ± 7.0 | 22.2 ± 5.0 | <0.0001 |
| LVEF (%) | 64.9 ± 12.9 | 31.5 ± 10.2 | <0.0001 |
| BNP (pg/mL) Median (IQR) | 83 (42–192) | 872 (311–1420) | 0.0001 |
| Systolic time intervals | |||
| EMAT | 10.6 ± 2.4 | 13.8 ± 3.8 | 0.0001 |
| Q‐S2 | 47.9 ± 5.6 | 52.4 ± 8.0 | 0.0086 |
| LVST | 37.2 ± 4.3 | 38.6 ± 6.4 | 0.29 |
| EMAT/LVST | 0.29 ± 0.06 | 0.36 ± 0.11 | 0.0001 |
LV = left ventricular; LVEDP = left ventricular end‐diastolic pressure; LVEF = left ventricular ejection fraction; BNP = B‐type natriuretic peptide; IQR = interquartile range; EMAT = electromechanical activation time; LVST = left ventricular ejection time.
Markers of Left Ventricular Function and Systolic Time Intervals
Patients with heart failure had a higher EMAT (P < 0.0001), EMAT/LVST (P < 0.0001), and Q‐S2 (P = 0.0086) compared to those without heart failure (Table 2). The correlation coefficients for the systolic time intervals compared to LVEDP and LVEF are displayed in Table 3. EMAT, EMAT/LVST, and Q‐S2 showed statistically significant correlations with LVEF, while none of these STIs correlated significantly with LVEDP. When patients with LBBB and aortic stenosis were excluded, the correlation coefficients did not significantly change.
Table 3.
Correlation Coefficients of Systolic Time Intervals and Left Ventricular Ejection Fraction and Left Ventricular End‐Diastolic Pressure
| Systolic Time Intervals | LVEF | P‐Value | LVEDP | P‐Value | BNP | P‐Value |
|---|---|---|---|---|---|---|
| EMAT | −0.51 | <0.0001 | 0.16 | 0.14 | 0.16 | 0.14 |
| Q‐S2 | −0.39 | 0.0003 | 0.05 | 0.64 | 0.08 | 0.44 |
| LVST | −0.20 | 0.069 | −0.04 | 0.73 | 0.006 | 0.96 |
| EMAT/LVST | −0.41 | 0.0001 | 0.20 | 0.055 | 0.20 | 0.055 |
LVEF = left ventricular ejection fraction; LVEDP = left ventricular end‐diastolic pressure; BNP = B‐type natriuretic peptide; EMAT = electromechanical activation time; LVST = left ventricular ejection time.
In the multivariate linear regression analysis adjusting for heart rate and gender, for each 1‐unit increase in EMAT there was a 3.0% decrease in LVEF (P < 0.0001). For each 1% increase in EMAT/LVST, there was a 0.9% decrease in LVEF (P < 0.0001). There were 1.2% (P < 0.0001) and 1.1% (P = 0.008) decreases in LVEF for each every 1‐unit increase in Q‐S2 and LVST, respectively. The associations between LVEDP and the systolic time intervals were not significant.
Test Characteristics of Systolic Time Intervals
The receiver operator curves for EMAT and EMAT/LVST are shown for diagnosing patients with heart failure (Fig. 2). The area under the receiver operating curve (AUROC) c‐statistic for EMAT was 0.75 (95% CI 0.61–0.89). The c‐statistic was 0.70 for EMAT/LVST (95% CI 0.52–0.87), and was 0.67 for Q‐S2 (95% CI 0.52–0.82). The c‐statistic for LVST did not achieve significance. EMAT/LVST had an AUROC 0.70 (95% CI 0.55–0.86) for LVEF <50%, and 0.62 (95% CI 0.51–0.74) for LVEDP >15 mmHg. When patients with LBBB or aortic stenosis were excluded, the c‐statistic for EMAT was not significantly changed (c = 0.78 in the diagnosis of HF).
Figure 2.
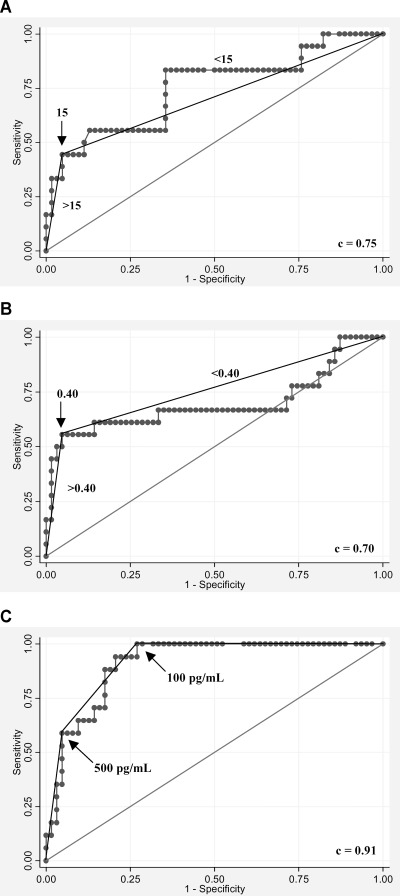
The receiver operating curves are shown for the systolic time intervals and B‐type natriuretic peptide for the diagnosis of left ventricular dysfunction: (A) electromechanical activation time (EMAT; Q‐S1), (B) electromechanical activation time divided by left ventricular systolic time (EMAT/LVST), and (C) B‐type natriuretic peptide (BNP). Arrows indicate chosen cut‐points.
Patients with an EMAT ≥15 (meaning an EMAT representing ≥15% of the cardiac cycle) were significantly more likely to have heart failure (odds ratio 1.38, 95% confidence interval CI 1.13–1.69; P < 0.0001). For those with an EMAT/LVST ≥0.40, heart failure was more common (OR 1.13, 95% CI 1.04–1.22; P = 0.002). A less robust, but still significant association was observed between Q‐S2 and heart failure (OR 1.14 95% CI 1.03–1.25; P = 0.008). LVST was not associated with heart failure.
Diagnostic Characteristics of Systolic Time Intervals Compared to BNP
The presence of an EMAT ≥15 had a sensitivity of 44%, a specificity of 94%, a positive likelihood ratio of 7.0, and an overall accuracy of 83% for diagnosing heart failure. An EMAT/LVST ratio ≥0.40 had a sensitivity of 55%, a specificity of 95%, a positive likelihood ratio of 11.7, and an accuracy of 86% (Table 4).
Table 4.
Diagnostic Test Characteristics for Detecting Left Ventricular Dysfunction Using Systolic Time Intervals and B‐Type Natriuretic Peptide Levels
| Sensitivity | Specificity | + LR | − LR | Accuracy | |
|---|---|---|---|---|---|
| EMAT ≥ 15 | 44 (22–69) | 94 (85–98) | 7.0 | 0.59 | 0.83 |
| EMAT/LVST ≥ 0.40 | 56 (31–78) | 95 (87–99) | 11.7 | 0.47 | 0.86 |
| BNP ≥ 100 pg/mL | 100 (80–100) | 57 (44–70) | 2.3 | 0 | 0.66 |
| BNP ≥ 250 pg/mL | 94 (71–100) | 79 (67–89) | 4.6 | 0.07 | 0.83 |
| BNP ≥ 500 pg/mL | 59 (33–82) | 94 (85–98) | 9.3 | 0.44 | 0.86 |
EMAT = electromechanical activation time; LVST = left ventricular ejection time; BNP = B‐type natriuretic peptide.
Overall, BNP performed well in the diagnosis of left ventricular dysfunction with an AUROC of 0.91 (95% CI 0.85–0.97), 0.80 (95% CI 0.69–0.92) for LVEF<50% alone, and 0.81 (95% CI 0.72–0.90) for LVEDP >15 mmHg alone. The likelihood of left ventricular dysfunction was very low (0) for those with a BNP <100 pg/mL and very high (9.3) for those with a BNP >500 pg/mL. However, the BNP was not helpful in diagnosing left ventricular dysfunction for intermediate values of 100–500 pg/mL (likelihood ratio 1.1). In this 31‐patient subgroup with BNP between 100 and 500 pg/mL, an EMAT ≥15 had a sensitivity of 43%, a specificity of 96%, and a likelihood ratio of 11.0 (Table 5). Conversely, an EMAT <15 had a likelihood ratio of 0.60 for the diagnosis of left ventricular dysfunction for these patients with BNP 100–500 pg/mL. An EMAT/LVST ≥0.40 performed similarly in diagnosing left ventricular dysfunction for those with an indeterminate BNP level (Table 5).
Table 5.
Likelihood Ratios for Combining the Results of B‐Type Natriuretic Peptide Levels with the Systolic Time Intervals EMAT (A) and EMAT/LVST (B).
| A | |||||||
|---|---|---|---|---|---|---|---|
| BNP | EMAT ≥15 | N | +HF, n | +HF, % | −HF, n | −HF, % | LR |
| <100 | + | 3 | 0 | 0 | 3 | 4.8 | 0 |
| <100 | − | 32 | 0 | 0 | 32 | 50.8 | 0 |
| 100–500 | + | 4 | 3 | 17.6 | 1 | 1.6 | 11.0 |
| 100–500 | − | 27 | 4 | 23.5 | 23 | 36.5 | 0.6 |
| >500 | + | 5 | 5 | 29.4 | 0 | 0 | ∞ |
| >500 | − | 9 | 5 | 29.4 | 4 | 6.3 | 4.7 |
| Total | 80 | 17 | 100 | 63 | 100 | ||
| B | |||||||
| BNP | EMAT/LVST ≥0.4 | n | +HF, n | +HF, % | −HF, n | −HF, % | LR |
| <100 | + | 3 | 0 | 0 | 3 | 4.8 | 0 |
| <100 | − | 32 | 0 | 0 | 32 | 50.8 | 0 |
| 100–500 | + | 4 | 4 | 23.5 | 0 | 0 | ∞ |
| 100–500 | − | 27 | 3 | 17.6 | 24 | 38.1 | 0.6 |
| >500 | + | 6 | 6 | 35.3 | 0 | 0 | ∞ |
| >500 | − | 8 | 4 | 23.5 | 4 | 6.3 | 3.7 |
| Total | 80 | 17 | 100 | 63 | 100 | ||
BNP = B‐type natriuretic peptide; EMAT = electromechanical activation time; HF = heart failure; LVST = left ventricular ejection time.
Under the Systolic Time Interval Test Column, a “+” Sign Denotes an Abnormally Elevated Test Result, While a “−” Sign Indicates a Normal Test Result
DISCUSSION
In this prospective study of patients undergoing left heart catheterization, echocardiography, and BNP measurement, noninvasive bedside electrophonocardiographic systolic time interval assessment had a significant association with left ventricular ejection fraction. While these intervals were specific tests for the presence of left ventricular dysfunction, there were no significant correlations with left ventricular filling pressures or BNP. The STIs that displayed the strongest associations with LVEF were EMAT and the ratio between the EMAT to the left ventricular systolic time (EMAT/LVST). While an intermediate BNP level 100–500 pg/mL is not helpful in diagnosing left ventricular dysfunction, the addition of EMAT or EMAT/LVST added independent diagnostic information that significantly changed the likelihood of disease.
STIs have previously been validated for the detection of abnormal left ventricular function. 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 These studies, mostly performed in the late 1960s through the early 1980s, showed strong correlations with direct measures of left ventricular performance. The strongest correlation (r =−0.90) was between EMAT/LVET (with left ventricular ejection time defined by carotid pulse tracing) and angiographically determined LVEF. 3 These observations held true regardless of the type of underlying heart disease. STIs with an EMAT/LVET ≥0.42 were shown in a prospective study of 112 patients to display high sensitivities (88%) and specificities (96%) for detecting abnormal LVEF. 10 It is noteworthy that this EMAT/LVET cutoff of 0.42 is extremely close to the EMAT/LVST cutoff of 0.40 from our study.
More recently, Tei and colleagues examined STIs derived from echocardiographic Doppler mitral inflow and left ventricular outflow tracings. The Tei‐index, defined as the sum of isovolumic contraction and relaxation times divided by ejection time, incorporates measures of both systolic and diastolic cardiac performance. This index was shown to be a reliable indicator of global cardiac dysfunction in patients with heart failure from either ischemic or dilated cardiomyopathy. 18 , 19 , 20
Several studies have been performed to investigate the physiologic underpinnings of the relationship between STIs and measures of left ventricular function. Studies in animal models demonstrated that changes in left ventricular stroke volume caused by alterations in left ventricular filling were accompanied by changes in the duration of STIs. 22 , 23 In human studies of heart failure, the left ventricular ejection time decreased while the pre‐ejection period (from the initial deflection of the Q wave to the initial upward deflection of the carotid pulse tracing 5 , 11 , 12 , 21 lengthened.
Preejection period prolongation was the subject of further investigation as a potential measure of myocardial contractile performance. These studies showed that LVEDP, aortic diastolic pressure, and the rate of left ventricular pressure development affect the preejection period. 24 , 25 , 26 , 27 In heart failure, the decrease in the rate of left ventricular pressure development in the pre‐ejection phase results in a prolonged isovolumic contraction time and a consequent lengthening of the pre‐ejection time interval. This decrease in LV dp/dt during the pre‐ejection phase and the consequent lengthening of the pre‐ejection period reflect ineffective force generated by the left ventricle. 4
The calculation of STIs in these early studies relied upon combining phonocardiography, electrocardiography, and carotid pulse tracings to determine the preejection period. The simultaneous acquisition of these three methods was cumbersome and required a highly trained technician, thus limiting its widespread clinical applicability. In contrast, the phonoelectrocardiography device used in our study (Audicor) provides a simple, noninvasive method of evaluating potentially useful clinical data at the bedside. This device generates a standard 12‐lead electrocardiogram, where leads V3 and V4 are replaced with phonoelectrocardiographic leads, obviating the need to perform carotid pulse recordings. These recordings do not require any special training beyond that required for a 12‐lead electrocardiogram. Moreover, these results may be immediately available upon printing the computerized heart sound detection report.
The correlations of the phonoelectrocardiographically determined STIs with LVEF in our study were considerably less robust than those reported previously. 3 , 5 , 9 , 10 , 12 The sensitivities, specificities, and likelihood ratios were likewise poorer compared to previous studies. 10 The absence of carotid pressure tracing analysis in the current study could contribute to this discrepancy. Previous investigators have attributed the lengthening of EMAT in left ventricular dysfunction almost entirely to a prolonged isovolumic contraction time, 4 , 6 a measurement that can only be obtained through carotid pressure analysis or Doppler echocardiography by trained technicians.
Inspection of the receiver operating characteristic curves for the systolic time intervals EMAT and EMAT/LVST shows a steep early portion of the curve corresponding to the true positives with the highest values of specificity. After this initial early steep portion, the remainder of the ROC curves are relatively flat, resulting in a likelihood ratio close to 1 for lower values. Therefore, the chosen cutoffs of EMAT and EMAT/LVST result in relatively insensitive, but highly, specific tests for the diagnosis of left ventricular dysfunction.
The BNP ROC curve for the diagnosis of left ventricular dysfunction in this and prior studies has a very different shape. 2 , 27 , 28 There is a steep early portion for the true positives with high BNP values, then a flat middle portion for those with intermediate BNP levels, followed by a very flat top portion corresponding to those without left ventricular dysfunction who have the lowest BNP values. For those patients with an intermediate BNP level between 100 and 500 pg/mL, this test does not change the pretest probability of left ventricular dysfunction.
Combining EMAT and EMAT/LVST results to the BNP level increases the diagnostic accuracy of predicting left ventricular dysfunction. While there is little room for improvement in diagnostic accuracy for those with very low (<100 pg/mL) or high BNP levels (>500 pg/mL), the STIs significantly improved the diagnostic accuracy for those with intermediate BNP levels. Because the likelihood ratio for those with an intermediate BNP and a positive systolic time interval was at least as high as the product of each test's individual likelihood ratio, we conclude that EMAT and EMAT/LVST provide diagnostic information independent from that obtained with BNP alone.
This study has several limitations. First, this study is limited by the sample size. In particular, the data relating to the diagnostic accuracy within different ranges of BNP results requires confirmation with a larger sample. Further studies are required before these findings could be extrapolated to patients with conditions known to independently affect STIs, including left bundle branch block, aortic valve disease, and the use of positive or negative inotropic agents. The correlation coefficients for the STIs and LVEF were unchanged when those with left bundle branch block and/or aortic stenosis were excluded. Since the cutoffs chosen for EMAT and EMAT/LVST were chosen after inspection of the ROC curves, repeating these comparisons in a validation cohort is required.
In conclusion, phonoelectrocardiographically‐measured STIs have important correlations with LVEF. An elevated EMAT and/or EMAT/LVST are relatively insensitive, but highly specific, tests for the diagnosis of left ventricular dysfunction. EMAT and EMAT/LVST offer additional independent predictive value of left ventricular dysfunction beyond that achieved from BNP alone.
Acknowledgments
Acknowledgments: We thank the patients who participated in the study; the staff in the UCSF Cardiac Catheterization Laboratory for their technical assistance; Michael Kohn for his mentoring and assistance in analysis of diagnostic testing methodology; and Patti Arand, Ph.D., Nancy Forman, R.N., B.S.N., and Robert Warner, M.D., of Inovise Medical Inc. for training and technical assistance.
Dr. Michaels has received an unrestricted educational grant from Inovise Medical Inc., Portland, OR. The study was supported by internal research funds within the University of California San Francisco, Division of Cardiology. Use of the phonocardiographic equipment and interpretation of the tracings was provided free of charge by Inovise Medical Inc, Portland, OR.
REFERENCES
- 1. Koelling TM, Chen RS, Lubwama RN, et al. The expanding national burden of heart failure in the United States: The influence of heart failure in women. Am Heart J 2004;147:74–78. [DOI] [PubMed] [Google Scholar]
- 2. Maisel AS, Krishnaswamy P, Nowak RM, et al. Rapid measurement of B‐type natriuretic peptide in the emergency diagnosis of heart failure. N Engl J Med 2002;347:161–167. [DOI] [PubMed] [Google Scholar]
- 3. Garrard CL, Weissler AM, Dodge HT. The relationship of alterations in systolic time intervals to ejection fraction in patients with cardiac disease. Circulation 1970;62:455–462. [DOI] [PubMed] [Google Scholar]
- 4. Lewis RP, Leighton RF, Forester WF, et al. Systolic time intervals In Weissler AM. (ed.): Non‐Invasive Cardiology. New York , Grune and Stratton, 1974, pp. 301–368. [Google Scholar]
- 5. Lewis RP, Boudoulas H, Welch TG, et al. Usefulness of systolic time intervals in coronary artery disease. Am J Cardiol 1976;37:787–796. [DOI] [PubMed] [Google Scholar]
- 6. Lewis RP, Rittogers SE, Froester WF, et al. A critical review of the systolic time intervals. Circulation 1977;56:146–158. [DOI] [PubMed] [Google Scholar]
- 7. Martin CE, Shaver JA, Thompson ME, et al. Direct correlation of external systolic time intervals with internal indices of ventricular function in man. Circulation 1971;44:419–431. [DOI] [PubMed] [Google Scholar]
- 8. McDonald IG, Hobson ER. A comparison of the relative value of non‐invasive techniques—echocardiography, systolic time intervals, and apexcardiography—in the diagnosis of primary myocardial disease. Am Heart J 1974;88:454–462. [DOI] [PubMed] [Google Scholar]
- 9. Stack RS, Lee CC, Reddy BP, et al. Left ventricular performance in coronary artery disease evaluated with systolic time intervals and echocardiography. Am J Cardiol 1976;37:331–339. [DOI] [PubMed] [Google Scholar]
- 10. Stack RS, Sohn YH, Weissler AM. Accuracy of systolic time intervals in detecting abnormal left ventricular performance in coronary artery disease. Am J Cardiol 1981;47:603–609. [DOI] [PubMed] [Google Scholar]
- 11. Weissler AM, Harris WS, Schoenfeld CD. Systolic time intervals in heart failure in man. Circulation 1968;37:149–159. [DOI] [PubMed] [Google Scholar]
- 12. Weissler AM, Harris WS, Schoenfeld CD. Bedside technics for the evaluation of ventricular function in man. Am J Cardiol 1969;23:577–583. [DOI] [PubMed] [Google Scholar]
- 13. Harlan WR, Oberman A, Grimm R, et al. Chronic congestive heart failure in coronary artery disease: Clinical criteria. Ann Intern Med 1977;86:133–138. [DOI] [PubMed] [Google Scholar]
- 14. Baim DS, Grossman WE. Grossman's Cardiac Catheterization, Angiography, and Intervention, 6th Edition Philadelphia , PA , Lippincott Williams & Wilkins, 2000. [Google Scholar]
- 15. Schiller NB, Shah PM, Crawford M, et al. American Society of Echocardiography Committee on Standards Subcommittee on Quantitation of Two‐Dimensional Echocardiograms. Recommendations for quantitation of the left ventricle by two‐dimensional echocardiography. J Am Soc Echocardiogr 1989;2:358–367. [DOI] [PubMed] [Google Scholar]
- 16. Willems JL, Roelandt J, DeGeest H, et al. Left ventricular ejection time in elderly subjects. Circulation 1970;42:37–42. [DOI] [PubMed] [Google Scholar]
- 17. Weissler AM, Harris LC, White GD. Left ventricular ejection time index in man. J Appl Physiol 1963;18:919–923. [DOI] [PubMed] [Google Scholar]
- 18. Tei C, Ling LH, Hodge DO, et al. New index of combined systolic and diastolic myocardial performance: A simple and reproducible measure of cardiac function—A study in normals and dilated cardiomyopathy. J Cardiol 1995;26:357–366. [PubMed] [Google Scholar]
- 19. Bruch C, Schmerund A, Marin D, et al. Tei‐index in patients with mild to moderate congestive heart failure. Eur Heart J 2000;21:1888–1895. [DOI] [PubMed] [Google Scholar]
- 20. Ono M, Tanabe K, Asanuma T, et al. Doppler echocardiography‐derived index of myocardial performance (TEI index): Comparison with brain natriuretic peptide levels in various heart disease. Jpn Circ J 2001;65:637–642. [DOI] [PubMed] [Google Scholar]
- 21. Perloff JK, Talano JV, Ronan JA. Non‐invasive techniques in acute myocardial infarction. Prog Cardiovasc Dis 1971;13:437–564. [DOI] [PubMed] [Google Scholar]
- 22. Braunwald E, Sarnoff SJ, Stainsby WN. Determinants of duration and mean rate of ventricular ejection. Circ Res 1958;6:319–325. [DOI] [PubMed] [Google Scholar]
- 23. Wallace AG, Mitchell JH, Skinner NS, et al. Duration of the phases of left ventricular systole. Circ Res 1963;12:611–619. [DOI] [PubMed] [Google Scholar]
- 24. Reeves TJ, Hefner LL, Jones WB, et al. The hemodynamic determinants of the rate of change in pressure in the left ventricle during isometric contraction. Am Heart J 1960;60:745–761. [DOI] [PubMed] [Google Scholar]
- 25. Talley RC, Meyer JF, McNay JL. Evaluation of the pre‐ejection period as an estimate of myocardial contractility in dogs. Am J Cardiol 1971;27:384–391. [DOI] [PubMed] [Google Scholar]
- 26. Diamond G, Forrester JS, Chatterjee K, et al. Mean electromechanical P‐ t: An indirect index of the peak rate of rise of left ventricular pressure. Am J Cardiol 1972;30:338–342. [DOI] [PubMed] [Google Scholar]
- 27. Agress CM, Wegner S, Forrester JS, et al. An indirect method for evaluation of left ventricular function in acute myocardial infarction. Circulation 1972;46:291–297. [DOI] [PubMed] [Google Scholar]
- 28. Morrison LK, Harrison A, Krishnaswamy P, et al. Utility of a rapid B‐natriuretic peptide assay in differentiating congestive heart failure from lung disease in patients presenting with dyspnea. J Am Coll Cardiol 2002;39:202–209. [DOI] [PubMed] [Google Scholar]


