Abstract
Background: Although the association of repolarization alterations to the development of life‐threatening ventricular arrhythmias has received considerable research attention, there is paucity of data regarding what may be considered as normal, especially in children.
Methods: To define electrocardiographic (ECG) and vectorcardiographic (VCG) descriptors of ventricular repolarization in healthy school‐age children, 12‐lead digital ECGs were obtained from 646 children (348 males/298 females, mean age 8.54 ± 1.86 years). All QT intervals were measured manually using the digitally stored ECGs. Orthogonal X, Y, and Z leads were reconstructed from the standard 12‐lead ECGs and the maximal amplitudes of the spatial QRS and T vectors were calculated, as well as the spatial QRS‐T angle.
Results: The mean heart rate was 95.3 ± 15.8 bpm and the QRS duration was 83.4 ± 9.3 ms. Mean QT interval was 334.1 ± 24.2 ms and the corrected QT interval was 436.5 ± 23.8 ms (Bazzet) and 404.3 ± 19.4 ms (Fridericia). Although the uncorrected maximum and mean QT intervals were significantly higher in boys (P values 0.011 and 0.009, respectively), there was no difference in the rate‐corrected QT interval. The spatial QRS and T‐vector amplitudes were 1512.0 ± 365.7 μV and 478.8 ± 149.3 μV, respectively. The spatial QRS‐T angle was 14.1 ± 8.0 degrees. Although the mean QT interval showed significant increase with age (P = 0.014), all VCG parameters did not show significant variance with age.
Conclusions: A range of ECG and VCG descriptors of ventricular repolarization was determined in a large sample of healthy school‐age children to provide a data basis of normal values for future reference.
Ann Noninvasive Electrocardiol 2011;16(1):49–55
Keywords: electrocardiography, vectorcardiography, ventricular repolarization, QT interval, healthy children
Although the repolarization phase of the cardiac electrical cycle has received considerable research attention, as regards to its association with the arrhythmic potential, there is paucity of data regarding what may be considered as normal. 1 This is particularly true in healthy children. 2 , 3 , 4 Moreover, children with inherited channelopathies, or those on medication that may influence the repolarizing potassium channels, or children post cardiac surgery may have altered spatial dispersion of repolarization. 4 QT dispersion has been used to quantify the dispersion of ventricular refractoriness from the standard 12‐lead electrocardiogram (ECG) in healthy children. 3 However, not only the accuracy and reproducibility of the “dispersion” indexes, 5 but also the presence of a direct link between the heterogeneity of ventricular repolarization and QT dispersion 6 has been challenged previously. Several studies have now focused on the spatial T‐loop morphology features as a more accurate measure of the repolarization heterogeneity. 1 , 7 , 8 However, there are no adequate data on the determinants of the spatial vectorcardiographic (VCG) descriptors of ventricular repolarization in healthy children. The objective of this study was to assess the clinical determinants of the ECG and spatial VCG descriptors of ventricular repolarization in a population of healthy, school‐age children.
METHODS
The study population consisted of 646 consecutively recruited children from the primary schools of Santorini Island, Cyclades, Greece. None of the children had history of any cardiovascular or other disease and received any medications. All children had normal physical examination and a normal 12‐lead surface ECG in the supine resting position. Children with left or right bundle branch block, atrioventricular block, ventricular preexcitation, or atrial fibrillation were excluded from the study. The study was approved by the Ministry of National Education and by our hospital's ethics committee. Informed consent was obtained from all children's parents. All study participants underwent a 12‐lead digital ECG recording, by previously described techniques. 1
To optimize the QT intervals determination from 12‐lead surface ECGs, we used a commercially available computer‐based ECG system (Cardioperfect version 1.1; Cardio Control NV, Delft, The Netherlands), which enabled us to record all 12 ECG leads simultaneously at a sampling rate of 1200 Hz and a 12 bit A/D conversion rate. 1 From each lead, the average complex was calculated by the MEANS (Modular ECG Analysis) system. 9 Individual average complexes were stored digitally and displayed on a high‐resolution computer screen. Each lead was separately magnified with a magnification of 160 mm/s and 60 mm/mV. 1 All QT intervals were measured manually using the digitally stored ECGs displayed on a high‐resolution computer screen. 1 To correct for possible heart rate effects on the maximum QT interval among the 12‐ECG leads (QTc), we applied the Bazett formula (QT/✓RR) 10 and the Fridericia formula (QT/RR1/3). 11 Intra‐ and interobserver relative errors were determined for all the manually measured ECG indexes in 100 randomly selected study participants. 1
To derive VCG descriptors of ventricular repolarization, orthogonal X, Y, and Z leads were reconstructed from the standard 12‐lead ECGs. 12 The projections of the maximum QRS and T vectors on the frontal (xy), horizontal (xz), and right sagittal (yz) planes were automatically calculated by our analysis system. 1 , 8 According to previously published equations 13 and by use of the Pythagorean theorem, we calculated the amplitude of the maximum spatial T vector (Spatial T‐vector amplitude) from the formula: Spatial T‐vector amplitude=[(Txy 2+Txz 2+Tyz 2)/2]1/2, the amplitude of the maximum spatial QRS vector (Spatial QRS vector amplitude) from the formula: Spatial QRS vector amplitude=[(QRSxy 2+QRSxz 2+QRSyz 2)/2]1/2, and the angle (θ˚) between the maximum spatial QRS and T vectors (Spatial QRS‐T angle) from the formula: cos θ˚= (QRSxTx+QRSyTy+QRSzTz)/|QRS||T|. 1 , 8
Statistical Analysis
Continuous variables are summarized as mean ± standard deviation and were compared using the 2‐sided t‐test (equality of variances of compared variables was tested by the Levene's test of homoscedasticity and pooled or discrete variance was used accordingly). Categorical variables are expressed as counts and percentages and were compared using the chi square test. The Kolmogorov‐Smirnov test was used to examine the deviation of parameter distributions from the Gaussian distribution. Univariate analysis of variance (ANOVA) was applied to test for the age‐dependence of the normally distributed repolarization variables. For variables significantly deviating from the Gaussian distribution nonparametric tests were applied (Mann‐Whitney U‐test, Kruskal‐Wallis, and Jonckheere‐Terpstra tests), accordingly. Statistical significance was signified by a P value of <0.05. Analyses were made using the SPSS 15.0 software package (SPSS Inc., Chicago, IL).
RESULTS
Population
Basic demographic characteristics are presented in Table 1. In short, the studied population consisted of 646 children, with ages ranging from 6 to14 years old (a vast majority among them, 98.2%, were aged 6–11 years old, corresponding to the 6 grades of primary school, as shown in Table 1).
Table 1.
Population Characteristics
| Parameter | |
|---|---|
| N | 646 |
| Age (years) | 8.54 ± 1.86 |
| Gender (male) | 348 (53.9%) |
| Age distribution (N) | |
| 6–7 years | 150 (23.2%) |
| 8–9 years | 213 (33.0%) |
| ≥10 years | 283 (43.8%) |
| Heart rate (beats per minute) | 95.3 ± 15.8 |
| QRS duration (ms) | 83.4 ± 9.3 |
Continuous variables are expressed as mean ± standard deviation. Categorical ones are summarized as counts (%).
Repolarization Descriptors
The overall descriptive statistics of the studied repolarization parameters are summarized in Table 2. The 10th and 90th percentile values are also provided, since these are frequently used as reference values in pediatric populations. According to the Kolmogorov‐Smirnov test the distributions of Maximum QT, Mean QT, QTc (Bazzet), QTc (Fridericia) were statistically similar to the normal (Gaussian) distribution, as opposed to heart rate, QRS duration and minimum QT distributions, which differed from the normal (P < 0.01 for all). The VCG descriptors, spatial QRS vector amplitude, spatial T‐vector amplitude and spatial QRS‐T angle, also showed distributions that differed statistically from the normal (P = 0.029, 0.007, and 0.001, respectively). The frequency distributions of selected parameters are illustrated in Figure 1.
Table 2.
Repolarization Descriptors in the Studied Pediatric Population
| Heart Rate (Beats Per Minute) | QRS Duration (ms) | Spatial QRS Vector Amplitude (μV) | Spatial T‐Vector Amplitude (μV) | Spatial QRS‐T Angle (degrees) | |
|---|---|---|---|---|---|
| Mean | 95.3 | 83.4 | 1512.0 | 478.8 | 14.1 |
| Std. Deviation | 15.7 | 9.3 | 365.7 | 149.3 | 8.0 |
| Median | 92.0 | 83.0 | 1498.0 | 454.5 | 12.7 |
| Percentiles 10th | 76.0 | 73.0 | 1059.5 | 300.7 | 5.0 |
| 90th | 118.0 | 97.0 | 2008.8 | 677.5 | 24.3 |
| Minimum QT (ms) | Maximum QT (ms) | Mean QT (ms) | QTc (Bazzet) (ms) | QTc (Fridericia) (ms) | |
| Mean | 313.9 | 349.1 | 334.1 | 436.5 | 404.3 |
| Std. Deviation | 26.2 | 25.2 | 24.2 | 23.8 | 19.4 |
| Median | 315.0 | 348.0 | 333.8 | 435.6 | 403.6 |
| Percentiles 10th | 280.0 | 315.0 | 304.0 | 407.6 | 380.0 |
| 90th | 348.0 | 383.0 | 365.9 | 465.7 | 428.5 |
Figure 1.
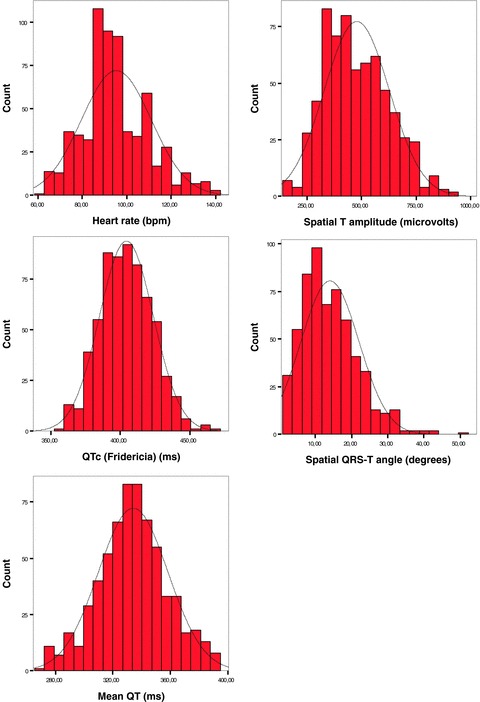
Frequency distributions of selected electrocardiographic parameters and repolarization descriptors in the studied pediatric population (646 children aged 6–14 years). The graphs illustrate (as confirmed by the Kolmogorov‐Smirnov test) that heart rate, spatial T‐vector amplitude and spatial QRS‐T angle distributions differed from the normal (indicated by the curve in the graphs).
In the gender‐specific analysis, the uncorrected QT interval was significantly higher in boys than in girls (average mean QT interval 336.4 ± 23.8 ms vs 331.4 ± 24.5 ms; P = 0.009), but this difference was completely attributable to the lower heart rate of boys (93.6 ± 15.3 bpm vs 97.2 ± 16.1 bpm; P = 0.003), since the RR‐interval‐corrected QT interval (both with the Bazzet and the Fridericia correction formula) was similar between genders (Table 3). The absolute voltages of the spatial QRS and T vectors were also higher in boys, which is explainable, since these parameters are dependent on body size and habitus. The spatial QRS‐T angle also differed significantly between genders (P = 0.031) (Table 3).
Table 3.
Gender‐Specific Analysis
| Parameter | Gender | Mean | Std. Deviation | Std. Error Mean | P |
|---|---|---|---|---|---|
| Age (years) | Male | 9.0 | 1.8 | 0.1 | 0.302 |
| Female | 8.9 | 1.8 | 0.1 | ||
| Minimum QT (ms) | Male | 315.7 | 25.5 | 1.4 | 0.069 |
| Female | 311.9 | 27.0 | 1.6 | ||
| Maximum QT (ms) | Male | 351.5 | 25.0 | 1.3 | 0.011 |
| Female | 346.4 | 25.3 | 1.5 | ||
| Mean QT (ms) | Male | 336.4 | 23.8 | 1.3 | 0.009 |
| Female | 331.4 | 24.5 | 1.4 | ||
| QTc (Bazzet) (ms) | Male | 435.7 | 25.4 | 1.4 | 0.348 |
| Female | 437.5 | 21.9 | 1.3 | ||
| QTc (Fridericia) (ms) | Male | 404.7 | 20.3 | 1.1 | 0.544 |
| Female | 403.8 | 18.2 | 1.1 | ||
| Spatial QRS‐vector amplitude (μV) | Male | 1545.4 | 370.9 | 20.1 | 0.014 |
| Female | 1473.7 | 356.4 | 20.7 | ||
| Spatial T‐vector amplitude (μV) | Male | 508.6 | 153.6 | 8.2 | <0.001 |
| Female | 444.1 | 136.2 | 7.9 | ||
| Spatial QRS‐T angle (degrees) | Male | 13.4 | 7.2 | 0.4 | 0.031 |
| Female | 14.8 | 8.8 | 0.5 | ||
| Heart rate (bpm) | Male | 93.6 | 15.3 | 0.8 | 0.003 |
| Female | 97.2 | 16.1 | 0.9 | ||
| QRS duration (ms) | Male | 84.6 | 9.7 | 0.5 | <0.001 |
| Female | 81.9 | 8.7 | 0.5 |
As regards to the age‐dependence of the repolarization variables one‐way ANOVA of the normally distributed variables, with age group as grouping criterion (3 age groups were formed: 6 to 7, 8 to 9, ≥10 years old), showed significant increase of mean QT interval with age (329.9 ± 23.9 vs 333.4 ± 21.5 vs 336.9 ± 26.0; P = 0.014). The RR‐corrected QT interval was similar across age groups, which is explained by the fact that heart rate decreased with age (P = 0.013, standardized Jonckheere‐Terpstra statistic =−2.488). The 2 VCG repolarization indices (spatial T vector amplitude and spatial QRS‐T angle) did not differ significantly with age (P = 0.114 and P = 0.07, respectively) (Fig. 2).
Figure 2.
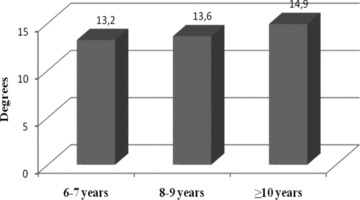
Mean spatial QRS‐T angle according to age group (the increasing tendency was not significant; P = 0.07).
Both VCG repolarization descriptors (spatial T‐vector amplitude and spatial QRS‐T angle) were significantly correlated to heart rate. The correlations (negative for the former and positive for the latter) were modest, but significant (standardized coefficient =−0.305 and 0.120; P = 10−6 and 0.003, respectively). See Figure 3 for the regression equations.
Figure 3.
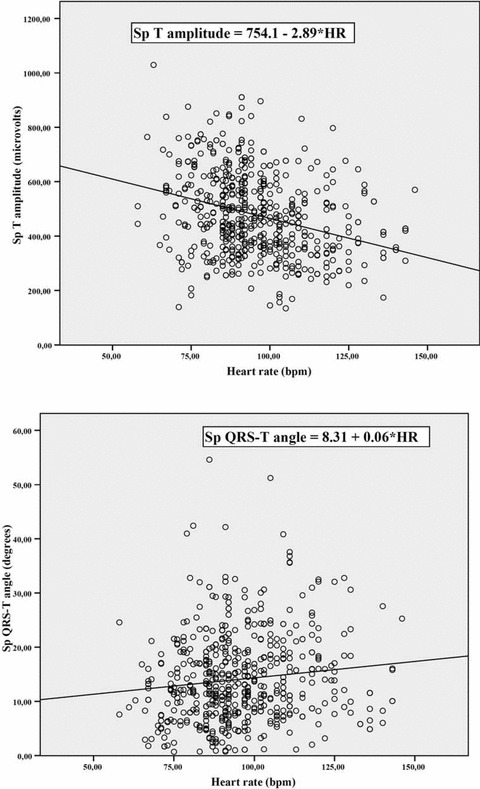
Scatterplot graphs of the 2 major repolarization vectorcardiographic indices according to heart rate, with the corresponding regression equations.
The intraobserver relative errors for QT maximum, QT minimum, and mean QT interval were 1.8 ± 0.2%, 4.3 ± 1.8%, and 1.3 ± 0.1%, respectively. The interobserver relative errors for the same indexes were 2.5 ± 0.2%, 4.8 ± 1.6%, and 2.0 ± 0.1%, respectively.
DISCUSSION
In this study, we assessed the clinical determinants of ECG and VCG descriptors of ventricular repolarization in healthy school‐age children. We found that, although the uncorrected maximum and mean QT intervals were significantly higher in boys than in girls, the rate‐corrected QT interval was similar between genders. Moreover, the mean QT interval showed significant increase with age, while the rate‐corrected QT interval was similar among age groups. Concerning the VCG variables of ventricular repolarization, both differed significantly between genders and showed a modest, but significant correlation to heart rate.
The higher uncorrected maximum and mean QT intervals in boys than in girls, in this study, should be attributed to the lower heart rate of boys as compared to girls, since the rate‐corrected QT interval did not differ between genders. Moreover, the increase in the mean QT interval with age, while the rate‐corrected QT interval was similar among age groups, could be explained by the fact that heart rate decreased significantly with age. Similar rate‐dependence of QT intervals has been reported previously by Romano et al. in a group of children of similar age. 14 In children older than 12 years, higher rate‐corrected QT intervals in girls than in boys have been reported. 2 , 3 This finding could be attributed to the presence of similar heart rates between genders, in the age group of 12 to 18 years, while the differential effects of sex hormones are ensuing. However, no gender differences in the T peak‐T end intervals have been reported recently in healthy children, although a rate–dependence of the T peak‐T end interval has been encountered. 4
Concerning the VCG descriptors of ventricular repolarization, the amplitudes of the spatial QRS and T vectors were also higher in boys than in girls, which is explainable, since these parameters are dependent on body size and habitus. On the other hand, the spatial QRS‐T angle was significantly higher in girls than in boys. Both VCG repolarization indices (spatial T‐vector amplitude and spatial QRS‐T angle) showed a modest, but significant correlation (negative for the former and positive for the latter) to heart rate. Similar associations have been reported in a population of young, healthy servicemen. 1 An abnormal orientation of the T axis has long been known to provide a global measure of repolarization abnormality, 15 and the spatial angle between the direction of the repolarization and depolarization waves (spatial QRS‐T angle) has already been used to quantify ventricular repolarization. 1 , 8 , 13 Abnormalities of the T‐wave loop morphology seem to be of significant importance. 16 The hypothesis that all information concerning cardiac repolarization is contained in a single T‐wave vector most likely is an oversimplification. The VCG derivation of a common T‐wave vector from a sample number of surface ECG leads may average existing nondipolar contents within cardiac repolarization. 17 Moreover, original Frank VCGs are not commonly used nowadays to measure the VCG repolarization variables. Instead, derived VCGs from original 12‐lead ECG recordings are used, but they are obviously dependent on the inherent problems of each synthesizing matrix. Nevertheless, the derived spatial VCG descriptors of the T‐wave vector loop appear to exhibit several advantages. They can be measured easily, are not affected by observation biases, and are likely to be less susceptible to noise and problems of definition than conventional ECG indexes of ventricular repolarization. Future studies should examine the ability of these spatial repolarization descriptors to identify high‐risk patients among children with inherited channelopathies, or those on medication that may influence the repolarizing potassium channels, or children post cardiac surgery that may have altered spatial dispersion of repolarization.
In conclusion, a range of ECG and VCG descriptors of ventricular repolarization was determined in a large sample of healthy school‐age children to provide a data basis of normal values for future reference. Gender‐ and age‐related differences were marked as well as the rate‐dependence of most repolarization variables.
Conflict of interest: None.
Financial support: None.
REFERENCES
- 1. Dilaveris P, Pantazis A, Gialafos E, et al Determinants of electrocardiographic and spatial vectorcardiographic descriptors of ventricular repolarization in normal subjects. Am J Cardiol 2001;88:912–914. 11676963 [Google Scholar]
- 2. Pearl W. Effects of gender, age, and heart rate on QT intervals in children. Pediatr Cardiol 1996;17:135–136. [DOI] [PubMed] [Google Scholar]
- 3. Tutar HE, Öcal B, Imamoglu A, et al Dispersion of QT and QTc interval in healthy children, and effects of sinus arrhythmia on QT dispersion. Heart 1998;80:77–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Benatar A, Carbonez K. Behavior of the electrocardiographic T peak to end interval in childhood. Ann Noninvasive Electrocardiol 2010;15:11–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Savelieva I, Yap YG, Gang Y, et al Comparative reproducibility of QT, QT peak, and T peak‐T end intervals and dispersion in normal subjects, patients with myocardial infarction, and patients with hypertrophic cardiomyopathy. PACE 1998;21(pt. II):2376–2381. [DOI] [PubMed] [Google Scholar]
- 6. Malik M, Acar B, Gang Y, et al QT dispersion does not represent electrocardiographic interlead heterogeneity of ventricular repolarization. J Cardiovasc Electrophysiol 2000;11:835–843. [DOI] [PubMed] [Google Scholar]
- 7. Zabel M, Acar B, Klingenheben T, et al Analysis of 12‐lead T‐wave morphology for risk stratification after myocardial infarction. Circulation 2000;102:1252–1257. [DOI] [PubMed] [Google Scholar]
- 8. Dilaveris P, Gialafos E, Pantazis A, et al The spatial QRS‐T angle as a marker of ventricular repolarization in hypertension. J Hum Hypertens 2001;15:63–70. [DOI] [PubMed] [Google Scholar]
- 9. Van Bemmel JH, Kors JA, Van Herpen G. Methodology of the modular ECG analysis system MEANS. Methods Inf Med 1990;29:346–353. [PubMed] [Google Scholar]
- 10. Bazett HC. An analysis of the time‐relations of electrocardiograms. Heart 1920;7:353–370. [Google Scholar]
- 11. Fridericia L. Die systolendauer im Elektrokardiogramm bei normalen menschen und bei herzkranken. Acta Med Scand 1920;53:469–486. [Google Scholar]
- 12. Edenbrandt L, Pahlm O. Vectorcardiogram synthesized from a 12‐lead ECG: superiority of the inverse Dower matrix. J Electrocardiol 1988;21:361–367. [DOI] [PubMed] [Google Scholar]
- 13. Ishizawa K, Ishizawa K, Motomura M, et al High reliability rates of spatial pattern analysis by vectorcardiogram in assessing the severity of eccentric left ventricular hypertrophy. Am Heart J 1976;91:50–57. [DOI] [PubMed] [Google Scholar]
- 14. Romano M, Clarizia M, Onofrio E, et al Heart rate, PR, and QT intervals in normal children: a 24‐hour Holter monitoring study. Clin Cardiol 1988;11(12):839–842. [DOI] [PubMed] [Google Scholar]
- 15. Cooksey JD, Dunn M, Massie E. Clinical Vectorcardiography and Electrocardiography. Chicago : Year Book Medical Publishers, 1977. [Google Scholar]
- 16. Kors JA, de Bryne MC, Hoes AW, et al T axis as an indicator of risk of cardiac events in elderly people. Lancet 1998;352:601–604. [DOI] [PubMed] [Google Scholar]
- 17. Franz MR, Zabel M. Electrophysiological basis of QT dispersion measurements. Prog Cardiovasc Dis 2000;42:311–324. [DOI] [PubMed] [Google Scholar]


