Abstract
Background: The terminal part of the QT interval (T peak to T end; Tp‐e)—an index for dispersion of cardiac repolarization—is often prolonged in patients experiencing malignant ventricular arrhythmias after acute myocardial infarction (AMI). We wanted to explore whether high Tp‐e might predict mortality or fatal arrhythmia post‐AMI.
Methods: Tp‐e was measured prospectively in 1359/1384 (98.2%) consecutive patients with ST elevation (n = 525) or non‐ST elevation (n = 859) myocardial infarction (STEMI or NSTEMI) admitted for coronary angiography.
Results: Tp‐e was significantly correlated with age, heart rate (HR), heart failure, LVEF, creatinine, three‐vessel disease, previous AMI and QRS and QT duration. During a mean follow‐up of 1.3 years (range 0.4–2.3),109 patients (7.9%) died; 25, 45, and 39 from cardiac arrhythmia, nonarrhythmic cardiac causes and other causes, respectively. Long Tp‐e was strongly associated with increased risk of death, and Tp‐e remained a significant predictor of death in multivariable Cox analyses (RR 1.5, 95% CI[1.3–1.7]). HR‐corrected Tp‐e (cTp‐e) was the strongest predictor of death (RR 1.6 [1.4–1.9]). Tp‐e and cTp‐e were particularly strong predictors of fatal cardiac arrhythmia (RR 1.6 [1.2–2.1] and RR 1.8 [1.4–2.4]). Findings were similar in STEMI and NSTEMI. When comparing two methods for measuring Tp‐e, one including the tail of the T wave and one not, the former had markedly higher predictive power (P < 0.001).
Conclusion: Tp‐e, and in particular cTp‐e, were strong predictors of mortality during the first year post‐AMI, and should be further evaluated as prognostic factors additional to established post‐AMI risk factors.
Keywords: QTc, Tp‐e, dispersion of repolarization, acute myocardial infarction, mortality, prognosis
INTRODUCTION
In 1978, Schwartz and Wolf demonstrated an association between long QT interval and risk of sudden death after acute myocardial infarction (AMI).( 1 ) Long QT also predicts all‐cause mortality in a variety of clinical settings.( 2 , 3 ) Recent studies show that the length of the terminal part of the QT interval–defined as the distance between the top (or nadir) and the end of the T wave (Tp‐e), is an index of total spatial dispersion of cardiac repolarization.( 4 , 5 ) Increased dispersion of repolarization is one of the several factors that may promote malignant ventricular arrhythmias.( 6 ) Accordingly, long Tp‐e has been associated with malignant arrhythmias in the long QT syndrome.( 7 ) the Brugada syndrome,( 8 ) in hypertrophic cardiomyopathy,( 9 ) and post‐AMI.( 10 )
The QT interval is prolonged after myocardial infarction,( 11 ) but since Tp‐e correlates more closely with dispersion of cardiac repolarization than the entire QT interval,( 12 , 13 ) we wanted to explore whether Tp‐e might predict mortality or fatal arrhythmias post‐AMI–a so far unsettled issue.( 14 , 15 ) We especially wanted to study the prognostic significance of different definitions of the end of the T wave as given in the literature.( 16 , 17 )
METHODS
A total of 1384 consecutive patients with AMI referred to Oslo University Hospital, Rikshospitalet (RH), for coronary angiography were prospectively enrolled between September 2005 and August 2007. Until December 2006, RH served as a tertiary referral center for three counties, and from 2007 two additional counties. The study was approved by the Regional Ethics Committee.
Diagnostic criteria for AMI were: (1) typical symptoms, and (2) Troponin I or T‐values above the upper limit of normal according to laboratory standards at the referring hospitals. At RH a troponin T assay (Troponin T, Cardiac T, 04491815 190, Roche/Cobas) with reference limit <0.10 μM was applied.
ST elevation myocardial infarction (STEMI) was diagnosed if the sum of ST elevations in two adjacent ECG leads exceeded 3 mm.
Patients without ST elevation (NSTEMI, n = 859) were referred for early coronary angiography,( 18 ) with mean time of 3.0 days (median 2, range 0–19) from start of symptoms to angiography. STEMI patients (n = 525) were routinely referred directly for urgent angiography.( 19 ) Patients with cardiac arrest prior to arrival and treated with hypothermia were not included.
ECG Measurements
Tp‐e measurements
Twelve leads ECGs were registered on Schiller Cardiovit AT‐102 ECG recorders using a 50 mm/s paper speed.
In all STEMI patients and in NSTEMI patients who underwent percutaneous coronary intervention (PCI), ECGs recorded one hour after completed revascularization were used. In NSTEMI patients scheduled for bypass surgery or in whom revascularization was not found necessary, indicated or possible, the ECGs taken immediately prior to angiography were used. Tp‐e was measured in the precordial leads using two methods (Fig. 1):
Figure 1.
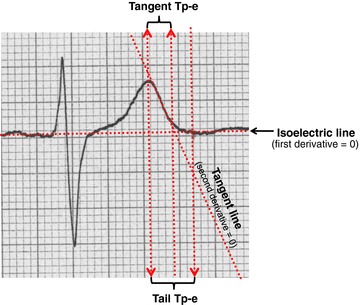
Measurement of Tp‐e according to the “tail method” (first derivative = 0) and the “tangent method” (second derivative = 0).
-
1
Method 1, the “Tangent Method”: The time in milliseconds from the peak of the T wave (or nadir if negative or biphasic T wave) and the intersection between the tangent at the steepest point of the T‐wave downslope and the isoelectric line.( 16 )
-
2
Method 2, the “Tail Method”: The time from the peak or nadir of the T wave to the point where the wave reached the isoelectric line.( 17 )
The main difference between the two methods is that the tail method also includes the terminal phase of the T wave.
All findings reported on Tp‐e in this article are based on the tail method unless otherwise specified, chosing the lead with the longest Tp‐e. In patients with atrial fibrillation (6.4%), Tp‐e was measured in five consecutive beats, and the arithmetic mean calculated. If encountering difficulties when trying to follow the definitions of Tp‐e in nontypical situations, we used a lead in which this was possible. Tp‐e in all available ECGs (n = 1359; 98.2%) was measured to the closest 0.5 mm by one cardiologist blinded to clinical outcomes. In order to assess intraobserver variability, a random sample of hundred ECGs was reread by the same observer. To assess interobserver reproducibility, Tp‐e was remeasured in two random samples of hundred ECGs by two other cardiologists blinded to all other data, analyzing one sample each.
Heart Rate Correction
Since heart rate (HR) is the principal determinant of the length of repolarization and Tp‐e reflects its terminal phase, HR correction of Tp‐e might be appropriate. No consensus exists on how such HR correction should be performed.( 20 ) A number of strategies were evaluated as suggested by Malik,( 21 ) but we decided to report HR‐corrected Tp‐e as cTp‐e = Tp‐e *2√1/RR, a method that has been used previously in HR correction of tangent Tp‐e( 22 ) and which is similar to Bazett's widely used formula for HR correction of the QT interval.( 23 )
Other ECG Data
QRS duration was calculated by the ECG recorder software. The QT interval was measured using the tail method. Presence of T wave inversions, prominent U waves and left or right bundle‐branch block (LBBB or RBBB) were recorded.
Clinical Data
Clinical information was obtained from our electronic hospital medical records and referral documents.
Acute heart failure was considered to be present in patients who had any of the following conditions:
-
•
Clinical signs of congestive heart failure necessitating loop diuretics, nitrates or CPAP/BiPAP, or
-
•
Severe hypotension treated with pressors or intravascular volume expanders, or
-
•
Treatment with intraaortic balloon pump (not for ongoing ischemia pending bypass surgery).
Echocardiography was performed in presence of acute heart failure or when significant valvular disease was suspected, and a left ventricular ejection fraction (LVEF) of <40% was noted. Other patients were judged to have normal or near normal LVEF. Coronary diameter stenoses ≥50% were labelled significant, and patients with significant stenoses in all three main coronary ateries simultaneously were labelled as having “three‐vessel disease” (3VD).
Blood Tests
Blood tests taken on RH arrival included troponin T, C‐reactive protein (CRP), erythrocyte sedimentation rate, hemoglobin, creatin kinase MB (CK‐MB) and serum glucose, creatinine, potassium, and total cholesterol. In STEMI patients, troponin T was also measured after six hours and at 08:00 AM, 2:30 PM, and 8:00 PM, using the maximum value. Since NSTEMI‐patients were initially evaluated at referring hospitals using differing troponin I or T assays which were taken at widely differing intervals prior to RH‐referrral, troponin data in NSTEMI patients have not been used for other than AMI‐diagnostic purposes.
Follow‐up
Cause specific mortality was obtained from Statistics Norway and from the hospital medical records. In patients who died outside hospital, additional information on mode of death was given by relatives contacted first by letter and then by telephone.
Deaths were categorized according to the Hinkle–Thaler classification:( 24 )
-
1
Cardiac arrhythmia (abrupt collapse without prior circulatory collapse)
-
2
Nonarrhythmic cardiac causes (cease of pulse only after peripheral circulatory collapse).
-
3
Stroke; cerebral thrombosis or hemorrhage,
-
4
Cancer, and
-
5
Other causes; pulmonary failure, renal failure, surgical complications, systemic inflammatory disease and miscellaneous diseases.
Statistical Analyses
Cox proportional hazards analyses were performed to study the relations between Tp‐e and total mortality. We initially made a multivariable model including all the variables in Table 1 except Tp‐e, HR and cTp‐e, and the variables in Table 2 that differed significantly between survivors and nonsurvivors (except drugs on discharge). After stepwise exclusion of all variables with P > 0.10, a new model was obtained that contained only significant variables. In the final analyses, Tp‐e, HR, and cTp‐e were added successively as shown in Table 3.
Table 1.
Baseline Characteristics among 1359 Survivors and Nonsurvivors with Available ECGs on Admission
| Survivors | Nonsurvivors | P | |||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Range | Mean | SD | Range | ||
| Age (years) | 64.7 | 12.4 | 22–92 | 74.8 | 9.3 | 49–91 | <0.0001 |
| Women (%) | 27.8 | 33.9 | NS | ||||
| Heart failure (%) | 11.0 | 38.5 | <0.0001 | ||||
| LVEF3 < 40% (%) | 11.6 | 32.1 | <0.0001 | ||||
| Hemoglobin (g/L) | 13.9 | 1.6 | 5.6–18.5 | 12.5 | 1.9 | 7.7–15.9 | <0.0001 |
| Creatinine (μmol/L) | 84.0 | 46.6 | 32–918 | 137.6 | 128.5 | 44–819 | <0.0001 |
| C‐reactive protein (mg/L) | 18.4 | 34.8 | 1.0–353 | 47.2 | 65.9 | 1.0–322 | <0.0001 |
| Troponin T (μg/L) | 4.6 | 4.5 | 0.1–26 | 6.6 | 6.6 | 0.1–25 | 0.0121 |
| 3 vessel disease (%) | 25.8 | 51.0 | <0.0001 | ||||
| Tp‐e (ms) | 113.6 | 19.7 | 60–260 | 122.1 | 22.0 | 80–190 | <0.0001 |
| Heart rate (bpm) | 69.6 | 14.7 | 37–132 | 80.0 | 20.7 | 45–142 | <0.0001 |
| cTp‐e (ms) | 121.4 | 22.8 | 70–267 | 139.3 | 27.2 | 79–209 | <0.0001 |
Troponin T in STEMI patients only (see text)
Table 2.
Comorbidity and Cardiovascular Drug Treatment at the Time the ECGs were Recorded, and on Discharge
| Survivors | Nonsurvivors | ||
|---|---|---|---|
| (n = 1275) | (n = 109) | ||
| Comorbidity | % | % | P* |
| • Previous PCI | 12.0 | 3.9 | 0.025 |
| • Previous CABG | 7.3 | 15.5 | 0.003 |
| • Previous AMI | 19.8 | 37.9 | <0.001 |
| • Diabetes | 14.4 | 21.4 | 0.068 |
| • Cancer | 7.7 | 15.5 | 0.006 |
| • Hypertension | 31.7 | 36.9 | 0.27 |
| Drugs on admission | |||
| • Clopidogrel | 84.4 | 67.7 | <0.001 |
| • Aspirin | 92.6 | 84.3 | 0.003 |
| • Beta‐blockers | 62.0 | 75.5 | 0.005 |
| • ACE inhibitors | 21.5 | 34.3 | 0.003 |
| • ATII blocker | 14.9 | 15.7 | 0.83 |
| • Statins | 61.3 | 54.9 | 0.007 |
| Drugs on discharge | |||
| • Clopidogrel | 93.8 | 75.3 | <0.001 |
| • Aspirin | 98.4 | 92.5 | <0.001 |
| • Beta‐blockers | 87.0 | 92.5 | 0.12 |
| • ACE inhibitors | 32.3 | 41.9 | 0.06 |
| • ATII blocker | 13.8 | 12.9 | 0.84 |
| • Statins | 93.4 | 67.7 | <0.001 |
*Wilcoxon two‐sample test
Table 3.
Univariable and Multivariable Proportional Hazards Analyses of the Relations between Selected Covariables and Mortality during 2 years of Follow‐up. Column A: Univariable Analyses. Column B: Multivariable Model Including Tp‐e and not HR, Column C: Model Including both Tp‐e and HR, and Column D: Model Including Heart Rate Corrected Tp‐e (cTp‐e)
| Univariable A RR [CI] | Multivariable (models B, C, and D) | |||||||
|---|---|---|---|---|---|---|---|---|
| P | B RR [CI] | P | C RR [CI] | P | D RR [CI] | P | ||
| Age (+10 years) | 2.1 [1.8–2.6] | <0.001 | 1.6 [1.3–2.0] | <0.001 | 1.6 [1.3–2.0] | <0.001 | 1.6 [1.3–2.0] | <0.001 |
| Heart failure (yes/no) | 5.0 [3.4–7.3] | <0.001 | 2.5 [1.6–3.8] | <0.001 | 2.0 [1.3–3.2] | 0.003 | 2.2 [1.4–3.4] | <0.001 |
| Hemoglobin (+1SD) | 0.51 [0.44–0.59] | <0.001 | 0.67 [0.55–0.81] | <0.001 | 0.70 [0.57–0.85] | <0.001 | 0.69 [0.57–0.84] | <0.001 |
| Creatinine (+1SD) | 1.3 [1.2–1.4] | <0.001 | 1.1 [1.1–1.3] | 0.010 | 1.2 [1.1–1.3] | <0.001 | 1.1 [1.1–1.3] | <0.001 |
| CRP (+1SD) | 1.4 [1.3–1.5] | <0.001 | 1.2 [1.0–1.3] | 0.008 | 1.1 [1.0–1.3] | 0.156 | 1.1 [1.0–1.3] | 0.042 |
| Previous PCI (yes/no) | 0.30 [0.11–0.81] | 0.018 | 0.23 [0.08–0.63] | 0.004 | 0.25 [0.09–0.69] | 0.008 | 0.23 [0.08–0.63] | 0.004 |
| Previous AMI (yes/no) | 2.3 [1.5–3.3] | <0.001 | 1.7 [1.1–2.6] | 0.025 | 1.7 [1.1–2.6] | 0.021 | 1.7 [1.1–2.7] | 0.015 |
| Clopidogrel* (yes/no) | 0.42 [0.28–0.63] | <0.001 | 0.43 [0.28–0.68] | <0.001 | 0.49 [0.32–0.78] | 0.002 | 0.46 [0.29–0.71] | <0.001 |
| Beta‐blocker* (yes/no) | 1.4 [1.2–1.6] | <0.001 | 1.1 [0.76–1.7] | 0.548 | 1.2 [0.81–1.6] | 0.437 | 1.1 [0.80–1.6] | 0.487 |
| Tp‐e (+1SD) | 1.4 [1.2–1.6] | <0.001 | 1.4 [1.2–1.6] | 0.003 | 1.5 [1.3–1.7] | <0.001 | ‐ | ‐ |
| Heart rate (+1SD) | 1.7 [1.5–2.0] | <0.001 | ‐ | ‐ | 1.4 [1.2–1.7] | <0.001 | ‐ | ‐ |
| cTp‐e (+1SD) | 1.7 [1.5–2.0] | <0.001 | ‐ | ‐ | ‐ | ‐ | 1.6 [1.4–1.9] | <0.001 |
RR= Relative risk; CI = 95% Confidence interval,*Drugs on admission
STEMI and NSTEMI were initially analysed separately, but differences were moderate. Supplementary analyses for specific causes of death were carried out treating other causes as censoring. The proportional hazards assumption was reasonably fulfilled for all covariables. However, since some covariables tended to have a more marked effect during the first period following the AMI, we did additional analyses separating the first 30 days and the subsequent follow‐up period. Associations between selected covariables and time to death are presented as relative risks (RRs) related to an increase of 1SD for continuous variables and to a category change for discrete variables.
Wilcoxon two sample tests were used in between‐group comparisons for continuous variables, and chi‐square tests for comparisons of dichotomous variables. Pearson correlation was used for assessing the associations between selected covariables against increasing Tp‐e. Receiver operating characteristics (ROC) curves were used to illustrate sensitivity and specificity of Tp‐e as a predictor of total mortality. Optimal cut‐off values were defined as the value of the ROC curve closest to the upper left corner.( 25 ) Confidence intervals for the area under the curve (AUC) were based on bootstrapping utilizing the R2.11 package “boot,” All other statistical analyses were performed using StatView 5.0. P‐values are two‐sided, and values <0.05 were considered significant.
RESULTS
Survivor versus Non‐survivors
Table 1 and Table 2 demonstrate significant differences in several baseline characteristics, in comorbidity and in cardiovascular medication between survivors and nonsurvivors.
Completeness of Data
Hemoglobin values were obtained in 99.6%, Troponin T (STEMI) in 99.8%, creatinine 99.9%, C‐reactive protein 98.5%, Tp‐e 98.2%, and heart failure status in 100%. Following our protocol echocardiography was performed in 38.6%.
Measurements of Tp‐e
The mean±SD difference between the first and the second reading by the same observer was 0.9 ± 9 ms. The differences between the three observers were 2.8 ± 11 ms, 1.4 ± 10 ms, and 2.8 ± 11 ms. None of these differences were statistically significant. ECG lead V2 had the longest Tp‐e in 86% of cases.
Mean ± 1SD Tp‐e was 114.2 ± 20.2, and numbers of patients in groups G1–G4 of Tp‐e in ranges 60–90 ms, 100–110 ms, 120–130 ms, and ≥140 ms were 146, 573, 434, and 206.
Mean±SD cTp‐e was 122.8 ± 23.6 ms, and interquartile ranges of increasing cTp‐e were Q1; ≤105 ms, Q2; 106–120 ms, Q3; 121–136 ms, Q4; ≥137 ms.
Tp‐e and Other Covariables
Tp‐e was significantly correlated with age, heart failure, LVEF, HR, QRS, and QT duration, creatinine, 3VD and previous AMI, but not with hemoglobin or CRP (details not shown).
Mortality
Overall Mortality
During a mean follow‐up among survivors of 1.3 years (range 0.4–2.3) 109 (7.9%) died; 45 within the first 30 days. Among NSTEMI patients 72 (8.4%) died, among STEMI 37 (7.0%). Twenty‐five died from cardiac arrhythmia; 45 from nonarrhythmic cardiac causes; 10 from stroke; 12 from cancer; 17 from miscellaneous causes. Six of the 25 patients in whom admission ECG was not taken, died. Among the 25 patients who died from cardiac arrhythmia, 17 were witnessed sudden deaths, and eight unwitnessed (the abrupt time course of death confirmed by relatives).
Mortality Related to Tp‐e and cTp‐e
Long Tp‐e was significantly associated with high total mortality;particularly short term. In groups G1–G4 of increasing Tp‐e 5.5%, 4.4%, 8.5%, and 16.0% died (Fig. 2A). The association between cTp‐e and mortality was stronger, and mortality in quartiles Q1–Q4 of increasing cTp‐e was 3%,1%, 5.0%, 6.7%, and 15.8% (Fig. 2B). Figure 3 shows a particularly strong trend for cardiac arrhythmias, but also for nonarrhythmic cardiac deaths and other causes. These patterns were similar above and below 75 years (details not shown).
Figure 2.
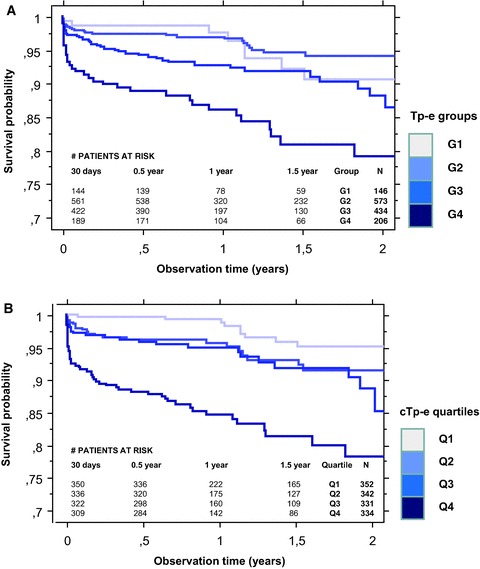
(A) Kaplan–Meier plot of cumulative survival post‐AMI among patients in groups G1–G4 with heart rate uncorrected Tp‐e 60–90 ms (G1, n = 146), 100–110 ms (G2, n = 573), 120–130 ms (G3, n = 434), and ≥140 ms (G4, n = 206). (B) Kaplan–Meier plot of cumulative survival post‐AMI among patients in quartiles Q1–Q4 of increasing heart rate corrected Tp‐e (cTp‐e) (Q1; ≤105 ms, Q2; 106–120ms, Q3; 121–136ms, Q4; ≥137 ms).
Figure 3.
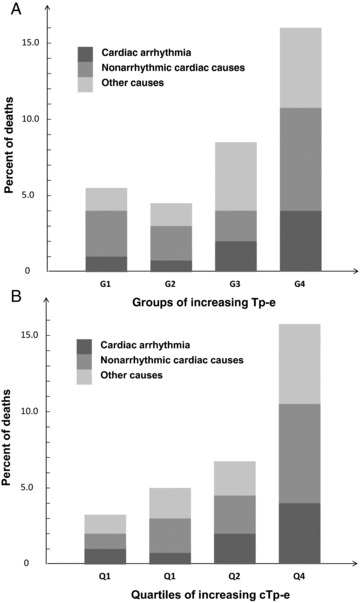
(A) Total‐ and cause‐specific mortality in groups G1–G4 with heart rate uncorrected Tp‐e 60–90 ms, (G1, n = 146), 100–110 ms (G2, n = 573), 120–130 ms (G3, n = 434), and ≥140 ms (G4, n = 206). (B) Total‐ and cause‐specific mortality according to quartiles Q1–Q4 of increasing heart rate corrected Tp‐e (cTp‐e) (Q1; ≤105 ms, Q2; 106–120 ms, Q3; 121–136 ms, Q4; ≥137 ms).
Proportional Hazards Analyses
Table 3 shows that all candidate covariables were significant predictors of mortality in univariable analyses (column A). In multivariable analyses all covariables except beta‐blocker on admission remained significant or borderline significant (columns B–D). Uncorrected Tp‐e was a significant multivariable predictor in a model not including HR (column B). When uncorrected Tp‐e and HR were included simultaneously, both were significant, and the predictive power of uncorrected Tp‐e was increased (column C). HR corrected Tp‐e (cTp‐e) was the strongest multivariable predictor in terms of chi‐square value (column D) (details not shown). LVEF was significant in models not including heart failure, but non‐significant when heart failure was included. The predictive power of Tp‐e and cTp‐e were similar in STEMI and NSTEMI.
Tail versus Tangent Tp‐e
Tp‐e measured by the tangent method was a significant multivariable predictor of mortality (RR 1.2 [1.0–1.5], P = 0.033). When Tp‐e measured according to both the tail and the tangent method were included, tail Tp‐e was significant (P = 0.006), whereas tangent Tp‐e became non‐significant (P = 0.60) (likelihood ratio 6.7 (1DF), P<0.01). This implies that tail Tp‐e adds prognostic power beyond the tangent method.
Tp‐e and Cause of Death
In multivariable analyses Tp‐e was a particularly strong predictor of fatal arrhythmia (RR 1.6, 95% CI [1.2–2.1]), besides also of death from non‐cardiac causes (RR 1.4 [1.1–1.8]). For nonarrhythmic cardiac causes there was a similar trend. Similar patterns were seen when analyzing cTp‐e (details not shown).
Troponin T
Troponin T (STEMI patients) was borderline significant in multivariable analyses (RR 1.3 [1.0–1.8], P = 0.05). Tp‐e's prognostic power was increased when troponin T was added to the multivariable model.
Exploratory Analyses
Other ECG Variables
QRS and QT duration were significant univariable predictors of mortality. QT duration was significant in multivariable analyses similar to those presented in Table 3 when HR was included (RR 1.3 [1.1–1.6]), but not when Tp‐e was included. No significant associations were found between mortality and T wave inversions, prominent U waves, LBBB, or RBBB.
Short‐ and Long‐term Mortality
Tp‐e was a strong multivariable predictor of death when observation time was restricted to ≤ 30 days (45 deaths, 80% cardiac) (RR 1.6, [1.3–2.1]). When analyzing only the patients who survived more than 30 days, Tp‐e was still significant (RR 1.3, [1.0–1.6]).
Tp‐e and Drugs
Twenty‐five patients treated with amiodarone on admission had significantly longer Tp‐e than those who were not (mean Tp‐e 133 ± 23 vs. 114 ± 20 ms, P < 0.001). Otherwise, no significant associations were found between use of drugs and Tp‐e; particularly not beta‐blockers.
Tp‐e and Acute Heart Failure
Among the 175 patients with acute heart failure (mean Tp‐e 115 ± 21), 37 (21.1%) died (nine fatal arrhythmia, 22 nonarrhythmic cardiac death). In this group, Tp‐e was the strongest predictor of mortality (RR 1.6 [1.2–2.2]).
ROC Analysis
For total mortality, the area under the ROC curve (AUC) for cTp‐e at 1 year was 0.77 [0.70–0.84]. The optimal cut‐off value as suggested by the ROC analysis was 132 ms, corresponding to a sensitivity of 0.68 and a specificity of 0.74.
DISCUSSION
Tp‐e – a measure of the terminal part of the QT interval, was a strong and independent predictor of mortality during the first year post‐AMI. Moreover, Tp‐e measured according to the tail method was a better prognostic instrument than Tp‐e measured according to the traditional tangent method. Heart rate correction appeared to improve the prognostic value of Tp‐e.
Prior Investigations
This is the first study of AMI survivors that assesses the prognostic significance of Tp‐e measured according to the tail method, whereas only two previous studies have reported the prognostic value of Tp‐e post‐AMI using the tangent method.( 14 , 15 ) In a 1992–96 study (n = 280) no significant associations between ECG variables reflecting dispersion of repolarization and subsequent death were found,( 14 ) although there was a trend towards longer Tp‐e in patients who died. In a recent study (n = 101) long Tp‐e predicted mortality in STEMI patients undergoing PCI,( 15 ) in concert with our findings. Dispersion of electrical repolarization has been reported as a major arrhythmogenic factor post‐AMI,( 26 , 27 ) and QT dispersion was introduced as a measure of electrical repolarization in 1994.( 28 ) Tp‐e may possibly be a more accurate marker of electrical dispersion than QT, not including depolarization but focusing on repolarization.
Tp‐e and Cause of Death
VentricularAarrhythmias
We found a strong association between long Tp‐e and fatal cardiac arrhythmia, suggesting that long Tp‐e may be a marker of increased ventricular arrhythmogenicity. This is supported by the close correlation between Tp‐e and maximal spatial dispersion of cardiac repolarization,( 5 ) that inhomogeneous repolarization and delayed ventricular conduction due to scarred myocardium both contribute to reentrant arrhythmias post‐AMI,( 29 ) and that increased T‐wave dispersion after myocardial infarction appears related to susceptibility to ventricular tachycardia.( 10 )
Nonarrhythmic Cardiac Causes
Long Tp‐e and cTp‐e was also associated with risk of dying from nonarrhythmic cardiac causes. Since Tp‐e was the strongest multivariable predictor of death in patients with acute heart failure, and 60% of these died from nonarrhythmic cardiac causes, combined acute heart failure and long Tp‐e may be particularly ominous.
Other Causes
Long Tp‐e was also associated with increased risk of non‐cardiac death, implying that repolarization disturbances may be a marker of other serious conditions related to vital functions. Interestingly, implantable cardioverter defibrillator (ICD) in patients with heart failure and high risk of ventricular arrhythmia post‐AMI reduced the incidence of fatal arrhythmia, but not total mortality.( 30 , 31 )
Timing of ECG Recordings
ECGs in STEMI patients were taken shortly after onset of chest pain, whereas ECGs in NSTEMI patients were recorded after varying intervals from symptom start. Despite these differences, Tp‐e had similar prognostic impact in both groups. Since repolarization abnormalities can be dynamic in the post‐AMI period, it is conceivable that an optimal “time window” for measuring Tp‐e may exist. If so, Tp‐e measured with optimal timing ought to have higher predictive power than reported in our study.
The QT interval and Tp‐e
Similar to previous studies( 1 , 2 , 3 ) our data show that the QT interval was a significant predictor of mortality. However, when both QT and Tp‐e were included, only Tp‐e remained significant. This suggests that the terminal part of the QT interval carries most of the prognostic information during the first year post‐AMI.
Significance of the T wave “tail”
The end of the QT interval is conventionally defined as the point where the steepest tangent of the terminal part of the T wave intersects with the isoelectric line. However, the T wave mostly continues beyond that point, creating a “tail” that gradually becomes horizontal when reaching the isoelectric line. We found that tail Tp‐e provided significant prognostic information beyond tangent Tp‐e. Conceivably therefore, the T wave tail may be of particular prognostic importance.
Heart Rate Correction
HR correction may be relevant since the time course of repolarization depends on the length of the RR interval. Interestingly, HR potentiated the predictive power of Tp‐e in multivariable analyses. Although we have kept main focus on the predictive value of uncorrected Tp‐e in this article, the predictive power of cTp‐e—which efficiently integrates information from both Tp‐e and HR—is higher. cTp‐e may therefore be a more useful prognostic marker in clinical practice. The best way to integrate the information from HR and Tp‐e is, however, still an open question.
Limitations
The ECGs were interpreted as typed out on paper by the ECG recorder in a typical clinical setting. Therefore, resolution was not optimal.
Determination of the end of the T wave may sometimes be difficult, e.g., in presence of flat T waves. Although we found a good interobserver agreement, a computer algorithm based on the tail method could be a helpful standardization tool.
By omitting troponin T data in NSTEMI patients, Tp‐e's predictive power in NSTEMI patients may theoretically have been overestimated. However, in STEMI patients, Tp‐e's prognostic power was slightly increased statistically when troponin was added.
LVEF is an important predictor of mortality post‐AMI, but echocardiography was performed only when clinically indicated. However, inclusion of heart failure and/or LVEF had minimal effect on Tp‐e's predictive power. Still we cannot rule out that optimally measured LVEF might have some effect on Tp‐e's predictive power.
Conclusions
Tp‐e measured with the tail method–and in particular cTp‐e, was an important predictor of mortality during the first year post‐AMI. Tp‐e may provide substantial prognostic information additional to established post‐AMI risk factors.
Grants: Inger and John Fredriksen's foundation.
REFERENCES
- 1. Schwartz PJ, Wolf S. QT interval prolongation as predictor of sudden death in patients with myocardial infarction. Circulation 1978;57:1074–1077. [DOI] [PubMed] [Google Scholar]
- 2. Gaudron P, Kugler I, Hu K, et al Time course of cardiac structural, functional, and electrical changes in asymptomatic patients after acute myocardial infarction: Their interRelation and prognostic impact. J Am Coll Cardiol 2001;38:33–40. [DOI] [PubMed] [Google Scholar]
- 3. Padmanabhan S, Silvet H, Amin J, et al Prognostic value of the QT interval and QT dispersion in patients with left ventricular systolic dysfunction: Results from a cohort of 2265 patients with an ejection fraction of ≤ 40%. Am Heart J 2003;145:132–138. [DOI] [PubMed] [Google Scholar]
- 4. Xia Y, Liang Y, Kongstad O, et al In vivo validation of the coincidence of the peak and end of the T wave with full repolarization of the epicardium and endocardium in swine. Heart Rhythm 2005;2:162–169. [DOI] [PubMed] [Google Scholar]
- 5. Opthof T, Coronel R, Wilms‐Schopman FJG, et al Dispersion of repolarization in canine ventricle and the electrocardiographic T wave: Tp‐e does not reflect transmural dispersion. Heart Rhythm 2007;4:341–348. [DOI] [PubMed] [Google Scholar]
- 6. Opthof T, Coronel R, Janse MJ. Is there a significant transmural gradient in repolarization time in the intact heart? Repolarization gradients in the Intact heart. Circ Arrythmia Electrophysiol 2009;2:89–96. [DOI] [PubMed] [Google Scholar]
- 7. Lubinski A, Leweick‐Nowak E, Kempa M, et al New insight into repolarization abnormalities in patients with congenital long QT syndrome. The increased transmural dispersion of repolarization. PACE 1998;21:172–175. [DOI] [PubMed] [Google Scholar]
- 8. Castro Hevia J, Antzelevitch C, Barzaga FT. T‐peak to T‐end and T‐peak to T‐end dispersion as risk factors for ventricular tachycardia/ventricular fibrillation in patients with the Brugada syndrome. J Am Coll Cardiol 2006;47:1828–1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shimizu M, Ino H, Okeie K, et al T‐peak to T‐end may be a better predictor of high‐risk patients with hypertrophic cardiomyopathy associated with a cardiac troponin I mutation than QT dispersion. Clin Cardiol 2002;25:335–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Oikarinen L, Viitasalo M, Korhonen P, et al Postmyocardial infarction patients susceptible to ventricular tachycardia show increased T‐wave dispersion independent of delayed ventricular conduction. J Cardiovasc Electrophysiol 2001;12:1115–1120. [DOI] [PubMed] [Google Scholar]
- 11. Perkiömäki JS, Koistinen MJ, Yli‐Mäyry S, et al Dispersion of QT interval in patients with and without suceptibility to ventricular tachyarrhythmias after previous myocardial infarction. J Am Coll Cardiol 1995;26:174–179. [DOI] [PubMed] [Google Scholar]
- 12. Zabel M, Portnoy S, Franz MR. Electrocardiographic indexes of disperion of repolarization: An isolated heart validation study. J Am Coll Cardiol 1995;25:746–752. [DOI] [PubMed] [Google Scholar]
- 13. Krahn AD, Nguyen‐Ho P, Klein GJ, et al QT dispersion: An electrocardiographic derivative of QT prolongation. Am Heart J 1999;137:104–108. [DOI] [PubMed] [Google Scholar]
- 14. Zabel M, Klingenheben T, Franz MR, et al Assessment of QT dispersion for prediction of mortality or arrhythmic events after myocardial infarction: Results of a prospective, long‐term follow‐up study. Circulation 1998;97:2543–2550. [DOI] [PubMed] [Google Scholar]
- 15. Haarmark C, Hansen PR, Vedel‐Larsen E, et al The prognostic value of the T‐peak to T‐end interval in patients undergoing primary percutaneous coronary intervention for ST‐segment elevation myocardial infarction. J Electrocardiol 2009;42:555–560. [DOI] [PubMed] [Google Scholar]
- 16. Charbit B, Semain E, Merckx P, et al QT interval measurement. Anesthesiology 2006;104:255–260. [DOI] [PubMed] [Google Scholar]
- 17. Salles GF, Cardoso CRL, Leocardio SM, et al Recent ventricular repolarization markers in resistant hypertension: Are they different from the traditional QT interval? Am J Hypertens 2008;21:47–53. [DOI] [PubMed] [Google Scholar]
- 18. The task force on the management of acute coronary syndromes of the European Society of Cardiology : Management of acute coronary syndromes in patients without persistent ST‐segment elevation. Eur Heart J 2002;23:1809–1840. [DOI] [PubMed] [Google Scholar]
- 19. The task force on the management of acute myocardial infarction of the European society of cardiology : Management of acute myocardial infarction I patients presenting with ST‐segment elevation. Eur Heart J 2003;24:28–66. [DOI] [PubMed] [Google Scholar]
- 20. Rautaharju PM, Surawicz B, Gettes LS. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram. J Am Coll Cardiol 2009;53:982–991. [DOI] [PubMed] [Google Scholar]
- 21. Malik M. The imprecision in heart rate correction may lead to artificial observations of drug induced QT interval changes. PACE 2002;25:209–216. [DOI] [PubMed] [Google Scholar]
- 22. Tanabe Y, Inagaki M, Kurita T, et al Sympathetic stimulation produces a greater increase in both transmural and spatial dispersion of repolarization in LQT1 than LQT2 forms of congenital long QT syndrome. J Am Coll Cardiol 2001;37:911–919. [DOI] [PubMed] [Google Scholar]
- 23. Bazett HC. An analysis of the time‐relations of electrocardiograms. Heart 1920;7:353–370. [Google Scholar]
- 24. Hinkle LE Jr, Thaler HT. Clinical classification of cardiac deaths. Circulation 1982;65:457–464. [DOI] [PubMed] [Google Scholar]
- 25. Stefania M, Barbabei L, De Caterina R. Receiver operating characteristic (ROC) curves and the definition treshold levels to diagnose coronary artery disease on electrocardiographic stress testing. Part II: The use of ROC curves in the choice of electrocardiographic stress test markers of ischemia. J Cardiovasc Med 2008;9:22–31. [DOI] [PubMed] [Google Scholar]
- 26. Vassallo JA, Cassidy DM, Kindwall KE, et al Nonuniform recovery of excitability in the left ventricle. Circulation 1988;78:1365–1372. [DOI] [PubMed] [Google Scholar]
- 27. Spragg DD, Akar FG, Helm RH, et al Abnormal conduction and repolarization in late‐activated myocardium of dyssynchronously contracting hearts. Cardiovasc Res 2005;67:77–86. [DOI] [PubMed] [Google Scholar]
- 28. Barr CS, Naas A, Freeman M, et al QT dispersion and sudden unexpected death in chronic heart failure. Lancet 1994;343:327–329. [DOI] [PubMed] [Google Scholar]
- 29. Michael G, Xiao L, Oi XY, et al Remodelling of cardiac repolarization: How homeostatic responses can lead to arrhythmogenesis. Cardiovasc Res 2009;81:491–499. [DOI] [PubMed] [Google Scholar]
- 30. Hohnloser SH, Kuck KH, Dorian P, et al Prophylactic use of an implantable cardioverter‐defibrillator after acute myocardial infarction. N Engl J Med 2004;351:2481–2488. [DOI] [PubMed] [Google Scholar]
- 31. Steinbeck G, Andersen D, Seidl K, et al Defibrillator implantation early after myocardial infarction. N Engl J Med 2009;361:1427–1436. [DOI] [PubMed] [Google Scholar]


