Abstract
Background: ST elevation is commonly seen in young, healthy men. The exact mechanisms that cause ST height to be greater in young men are not yet completely understood. The purpose of the present study was to determine whether autonomic tone is responsible for age and gender differences in ST height.
Methods: Gender and age differences in ST height were studied at rest and after double autonomic blockade (DAB) with atropine and propranolol. Fifty healthy men and women were included (16 men, 14 women, age 23–32 years; 9 men, 11 women, age 65–79 years). Twelve‐lead ECGs were registered at rest and after DAB. Leads II and V1–V4 were chosen for analysis. ST height (in mm) was measured manually at the J‐point, and 40 ms and 80 ms after the J‐point. Values were corrected for QRS amplitude.
Results: Gender and age differences in ST height were seen in both rest and DAB data. Men had greater ST height compared to women at J‐point, 40 and 80 ms after the J‐point (P ≤ 0.0001), and younger subjects had greater ST height than older subjects at J‐point (P = 0.0140), 40 and 80 ms after the J‐point (P ≤ 0.0001). DAB did not change ST height at J‐point or at 40 ms, but increased ST height at 80 ms. Women had less of an increase in ST height following DAB than men did.
Conclusions: ST elevation in the absence of structural or electrical heart disease is mainly seen in young men. Age and gender difference persist after DAB and thus are not due to differences in autonomic tone.
Keywords: early repolarization, autonomic tone, age, gender
Upward coving ST elevation, or early repolarization, is commonly seen in young men, 1 , 2 , 3 , 4 , 5 , 6 , 7 and is characterized by an elevated concave ST segment in the precordial leads. Prior studies have not identified the cause of this syndrome with certainty. Testosterone, increased vagal tone, and slower heart rates in young men have been suggested in the past to cause ST elevation in young men. Both testosterone and estrogen receptors have been found in the heart suggesting theoretically that female and male sex hormones may be responsible for the gender differences in ST height. 4 , 8 , 9 , 10 Some data suggest that testosterone has a major effect on ST height, 4 whereas other studies of ST height during the menstrual cycle 11 suggest that fluctuations in female sex hormones may modulate ST height as well. Increased vagal tone has also been suggested to be a mechanism behind the ST elevation using analysis of heart rate variability in patients with early repolarization. 12 Other possible mechanisms on ST height may include direct effects of heart rate on cardiac ion current activity. 13 A second syndrome that shows ST elevation is the Brugada syndrome, which in contrast to early repolarization in young men, is associated with QRS prolongation and potentially fatal arrhythmias. We hypothesized that the predominance of ST elevation in young men is due in large part to intrinsic differences perhaps mediated by sex hormones rather than the effects of autonomic tone. Therefore, we analyzed ST height both at rest and after double autonomic blockade (DAB) in 50 healthy men and women in two separate age groups.
METHODS
The patient population consisted of 50 healthy subjects including 16 young men and 14 young women with ages ranging from 23 to 32 years (26.5 ± 3.5 years), and 9 older men and 11 older women with ages ranging from 65 to 79 years (70.7 ± 3.5 years). All individuals gave informed consent to participate in a protocol approved by the Northwestern University Institutional Review Board. All subjects had normal cardiac physical examinations, no history of heart disease by echo or stress test, no hypertension or left ventricular hypertrophy either on ECG or two dimensional echo, and no autonomic dysfunction or conditions such as diabetes that are known to cause autonomic dysfunction. They were all able to receive propranolol and atropine (i.e., none had asthma or type II or greater heart block). All young women had normal menstrual cycles, and none were on hormone replacement therapy. No major ECG abnormalities were seen in any of the subjects.
Twelve‐lead surface ECGs were obtained in all subjects at rest and after DAB with propranolol and atropine. 14 Intravenous propranolol was administered using an initial dose of 0.25 mg/kg at a rate of 1 mg/min followed by an infusion of 0.002 mg/kg per min for the duration of the study. Intravenous atropine was administered as a single dose of 0.04 mg/kg at a rate of 1 mg/min. The order of drug administration was randomly assigned.
ECGs were recorded at 25 or 50 mm/s with a gain of 10 mm/mV and computer scanned and magnified 7–8 times the original size. ST height was measured with an on‐screen measuring tool to the nearest 0.2 mm. Leads II, V1, V2, V3, and V4 were chosen for analysis. The isoelectric baseline was defined as the height of the midpoint of a line drawn from the end of the previous T wave to the beginning of the P wave, and ST height in relation to the baseline was measured at cut‐points of 0, 40, and 80 milliseconds (ms) after the J‐Point (Fig. 1). QRS amplitude was also measured and was defined as the difference between the highest and lowest deflection of the QRS complex, measured to the nearest 0.2 mm. Heart rates during rest and DAB were also measured. ST‐height measurements had been validated in the past in our lab by a second observer using ECG measurements of a total of 525 ST‐height measurement points and yielded an intraclass correlation coefficient of 0.83.
Figure 1.
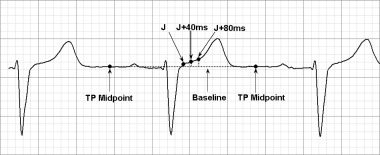
ECG measurement example. ST height (in mm) was measured at the J‐point, and at 40 ms and 80 ms after the J‐point down to the isoelectric baseline deferred as the midpoint of the TP segment.
Data Analysis
Data are expressed as mean ± standard deviation (SD). Statistical analysis was performed using commercially available software (StatView 5.0.1). Age, gender, and DAB effects were analyzed using repeated measure ANOVA with age and gender as factors. Analyses were repeated using ST‐height divided by the QRS complex amplitude data (ST/QRS). 15 A P‐value ≤0.05 was considered statistically significant for all purposes.
RESULTS
Differences among leads II, and V1–4 were evaluated at rest at J‐point, and 40 ms and 80 ms after the J‐point (Fig. 2). ST height in leads II and V1 was generally lower compared to the precordial leads V2–4. Thus, analysis of the gender and age influence both at rest and after DAB were performed on pooled data for the mid‐precordial leads V2–4, and leads V1 and lead II are reported separately.
Figure 2.
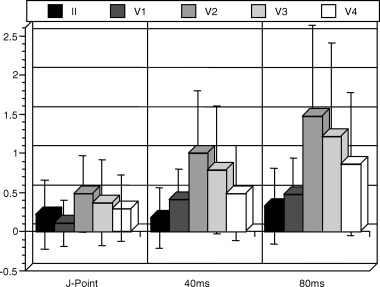
ST height (in mm) for each lead measured at the J‐point, and at 40 ms and 80 ms after the J‐point. Data shown as mean ± 1 standard deviation.
Analysis of ST height at baseline in leads V2–V4 showed that overall, men (young and old combined) had greater ST height than women measured at the J‐point (0.64 ± 0.50 mm men vs. 0.13 ± 0.32 mm women; P < 0.0001), 40 ms (1.16 ± 0.82 mm men vs. 0.36 ± 0.44 mm women, P < 0.0001) and 80 ms (1.78 ± 1.22 mm men vs. 0.59 ± 0.56 mm women; P < 0.0001). Younger subjects (male and female combined) had greater ST height than older subjects measured at the J‐point (0.49 ± 0.50 mm younger vs. 0.22 ± 0.43 mm older at rest, P = 0.0140, at 40 ms (1.00 ± 0.85 mm younger vs. 0.41 ± 0.44 mm older; P < 0.0001) and at 80 ms (1.56 ± 1.24 younger vs. 0.62 ± 0.51 mm older; P < 0.0001). Similar results were found for analysis in lead II, but no age or gender differences in ST height were seen in lead V1.
Results for separate age and gender subgroups during rest are shown in Table 1. ST height was highest in young men at all cut‐points in leads V2–V4, followed by older men, young women, and older women. Gender differences were significant in both young and older subgroups at all the three cut‐points. Age differences were significant in the male subgroup at all the three cut‐points, and in the female subgroup at J‐point. Results obtained after dividing ST height by QRS amplitude mirrored the above‐described findings. Analysis for leads II and V1 are shown in Table 1.
Table 1.
ST Height (in mm) in Leads V2–V4, V1 and II for Each Age‐Gender Subgroup at Each Cut‐point at Rest (Mean ± SD)
| Lead V2–4 | Heart Rate | J‐point | 40 ms | 80 ms |
|---|---|---|---|---|
| Young men (n = 16) | 62.3 ±10.9 | 0.73 ± 0.51a,b,c | 1.49 ± 0.81a,b,c | 2.30 ± 1.18a,b,c |
| Young women (n = 14) | 66.9 ± 9.9 | 0.21 ± 0.33b,c | 0.44 ± 0.46 | 0.73 ± 0.64 |
| Older men (n = 9) | 65.0 ± 10.8 | 0.48 ± 0.45a | 0.59 ± 0.44a | 0.87 ± 0.56a |
| Older women (n = 11) | 63.2 ± 9.8 | 0.01 ± 0.28 | 0.26 ± 0.39 | 0.42 ± 0.37 |
| Lead V1 | ||||
| Young men (n = 16) | 62.3 ± 10.9 | 0.13 ± 0.32 | 0.59 ± 0.23a | 0.69 ± 0.37 |
| Young women (n = 14) | 66.9 ± 9.9 | 0.01 ± 0.20b | 0.22 ± 0.06b | 0.34 ± 0.12 |
| Older men (n = 9) | 65.0 ± 10.8 | 0.16 ± 0.37 | 0.31 ± 0.12 | 0.33 ± 0.10 |
| Older women (n = 11) | 63.2 ± 9.8 | 0.20 ± 0.28 | 0.47 ± 0.09 | 0.47 ± 0.11 |
| Lead II | ||||
| Young men (n = 16) | 62.3 ± 10.9 | 0.34 ± 0.56 | 0.45 ± 0.17a,b,c | 0.73 ± 0.30a,b,c |
| Young women (n = 14) | 66.9 ± 9.9 | 0.13 ± 0.32 | 0.14 ± 0.08 | 0.24 ± 0.08b |
| Older men (n = 9) | 65.0 ± 10.8 | 0.31 ± 0.36 | 0.04 ± 0.10 | 0.13 ± 0.14 |
| Older women (n = 11) | 63.2 ± 9.8 | 0.09 ± 0.43 | −0.07 ± 0.08 | 0.00 ± 0.08 |
aP ≤ 0.05 versus opposite gender in same age group.
bP ≤ 0.05 versus same gender in opposite age group.
cP ≤ 0.05 versus opposite gender in opposite age group.
Following DAB, heart rate significantly increased from 64.3 ± 10.4 bpm at rest to 87.7 ± 15.6 bpm after DAB. Age and gender differences persisted after DAB and were similar to findings observed at rest (Table 2). ST height again was found to be highest in young men in leads V2–V4, followed by older men, young women, and older women. Gender differences were significant in the young subgroup at J‐point, 40 ms, and 80 ms, and in the older subgroup at J‐point and 40 ms. Age differences were significant in the male subgroup at 40 ms and 80 ms, but no age differences were seen in the female subgroup. Analysis for lead II and lead V1 are shown in Table 2.
Table 2.
ST Height (in mm) in Leads V2–V4 for Each Age‐Gender Subgroup at Each Cut‐point after DAB (Mean ± SD)
| Lead V2‐4 | Heart Rate | J‐point | 40 ms | 80 ms |
|---|---|---|---|---|
| Young men (n = 16) | 93.3 ± 14.8a,b,c | 0.70 ± 0.71a,c | 1.59 ± 1.0a,b,c | 2.77 ± 1.63a,b,c |
| Young women (n = 14) | 98.9 ± 12.6b,c | 0.11 ± 0.40c | 0.34 ± 0.47c | 0.77 ± 0.75 |
| Older men (n = 9) | 76.7 ± 6.9 | 0.56 ± 0.52a | 0.72 ± 0.51a | 1.10 ± 0.66 |
| Older women (n = 11) | 74.6 ± 9.0 | 0.0 ± 0.26 | 0.29 ± 0.38 | 0.55 ± 0.53 |
| Lead V1 | ||||
| Young men (n = 16) | 93.3 ± 14.8a,b,c | 0.11 ± 0.36 | 0.49 ± 0.50 | 0.73 ± 0.67 |
| Young women (n = 14) | 98.9 ± 12.6b,c | −0.03 ± 0.22 | 0.19 ± 0.37 | 0.34 ± 0.49 |
| Older men (n = 9) | 76.7 ± 6.9 | 0.24 ± 0.46 | 0.42 ± 0.46 | 0.53 ± 0.58 |
| Older women (n = 11) | 74.6 ± 9.0 | 0.06 ± 0.16 | 0.27 ± 0.14 | 0.35 ± 0.20 |
| Lead II | ||||
| Young men (n = 16) | 93.3 ± 14.8a,b,c | 0.29 ± 0.53 | 0.49 ± 0.58a,b,c | 0.84 ± 0.79a,b,c |
| Young women (n = 14) | 98.9 ± 12.6b,c | 0.06 ± 0.27 | 0.01 ± 0.23 | 0.20 ± 0.28 |
| Older men (n = 9) | 76.7 ± 6.9 | 0.27 ± 0.57 | 0.16 ± 0.28 | 0.27 ± 0.39 |
| Older women (n = 11) | 74.6 ± 9.0 | 0.06 ± 0.38 | −0.02 ± 0.21 | 0.09 ± 0.30 |
aP ≤ 0.05 versus opposite gender in same age group.
bP ≤ 0.05 versus same gender in opposite age group.
cP ≤ 0.05 versus opposite gender in opposite age group.
There was no effect of DAB on ST height at J‐point, and at 40 ms after the J‐point in leads V2–V4 (Fig. 3). At 80 ms after the J‐point, autonomic blockade tended to increase ST height in leads V2–V4, particularly in young men. In lead II and lead V1, autonomic blockade had no effect on ST height at any cut‐point.
Figure 3.
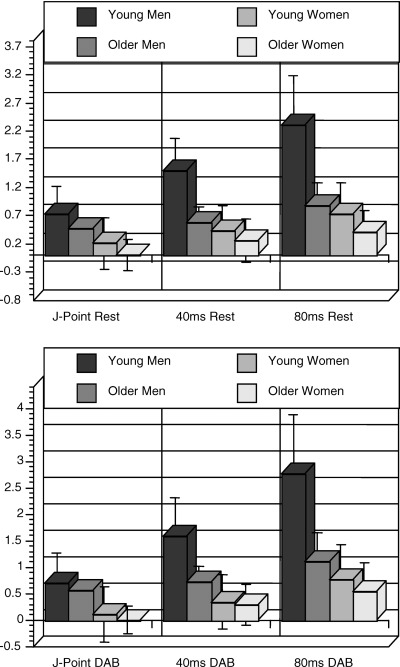
ST height (in mm) at the J‐point 40 ms and 80 ms after the J‐point and leads V2–V4 at rest and after double autonomic blockade. See text for details.
There was no interaction between autonomic blockade and sex on ST height at the J‐point. However, an interaction between sex and the effect of autonomic blockade was noted at 40 ms and at 80 ms after the J‐point. In both cases men had a slight increase in ST height, whereas women had little change in ST height. This difference was present in leads V2–V4, but not in lead V1 or in lead II. Correcting these analyses for QRS height produced similar results with the exception that sex difference in the effects of autonomic blockade was also significant in lead I at 40 ms and 80 ms after the J‐point.
DISCUSSION
The major new finding of the present study is that while resting sympathetic and parasympathetic tone may exert some effect on ST height, these effects are not responsible for the age and sex differences in ST height. ST elevation on the 12‐lead surface ECG is predominantly seen in young men. 1 , 5 , 13 Its persistence after DAB blockade suggests that intrinsic gender and age differences, perhaps due to sexual hormones, play a major role. In addition, differences in autonomic tone are not the mechanism for ST elevation in young men in that ST height was unchanged or increased in young men following autonomic blockade.
Experimental studies have shown that the increased ST height, or J Wave, as seen in early repolarization and the Brugada syndrome, may be due to regional differences in the distribution of specific ion currents in the ventricular wall that produce a transmural gradient in current flow. One of these currents is the transient outward potassium current Ito. Transmural dispersion of this current provides a voltage gradient over the ventricular wall that manifests as J‐point or ST‐height elevation on the 12‐lead surface ECG. 16 , 17 IKs, too, has been shown to be distributed differently throughout the ventricular wall with a smaller contribution of IKs in cells from the M region. 18 ST elevation in the arrhythmogenic Brugada syndrome has been shown to be due to regional differences in Ito as well. 19 , 20 Di Diego et al. 19 showed in a canine model that the Brugada phenotype is the result of a more prominent Ito in the epicardium of the right ventricle of male canines compared to female canines.
Sex hormones such as testosterone have been postulated to modulate ST height and the development of ST elevation in young men. Increases in ST height, for example, have been shown to occur predominantly in adolescent males after puberty, 21 and a decline in ST height in men occurs with increasing age. 1 , 3 , 21 , 22 The decline in ST height with age has been thought to be due to age‐dependent changes in men rather than women. 4 These observations would therefore suggest that changes in testosterone levels during puberty and a decline of testosterone level in men with increasing age may be responsible for, or related to, changes in ST height. Studies in normal women, castrated men, and women with virilization syndrome found that testosterone likely plays an important role. 4 Matsuo et al. 23 reported two cases with the Brugada syndrome in whom the Brugada ECG pattern disappeared after orchiectomy for prostate cancer, suggesting testosterone plays a major role in the Brugada phenotype of repolarization, too. The discovery of testosterone receptors in the atria and ventricles, 8 , 10 as well as androgen messenger RNA in human cardiac myocytes, 24 supports these conclusions.
Increased vagal tone in young men is another factor that has been suggested to contribute to the prominence of ST elevation in young men. Demir et al. 12 used spectral heart rate variability analysis in patients with ER to assess the activity of parts of the autonomic nervous system and suggested an increased vagal tone, rather than a decreased sympathetic tone, to be responsible for increased ST height in young men. Haydar et al. 25 suggested that slower heart rates and higher ST heights are of vagal origin in patients with early repolarization by showing increased aerobic capacity in their patients, which reflects increased vagal tone.
Sympathetic influence on ST height has also been studied. Patients with spinal cord injury, thus disruption of central sympathetic outflow, were found to have considerably elevated ST height, which suggested that sympathetic activity may modulate ST height. 26 Exogenous stimulation with isoproterenol in these patients markedly decreased ST height. Thus, central sympathetic activity, as well as exogenous sympathetic activation (isoproterenol), are thought to be able to influence ST height.
In the present study, DAB was performed to eliminate both vagal and sympathetic influences on ST height. Since the predominance of ST‐segment elevation in young men persisted after DAB, we conclude that increased ST height in young men is due to intrinsic differences between gender and age groups, rather than to autonomic influence. This does not exclude that ST height may be modulated by the autonomic system in general, but suggests that dramatic ST elevation seen in young men is not due to an alteration in autonomic tone. Intrinsic gender and age differences in ST height may be due to sexual hormonal influences or other factors.
Slower heart rates have also been thought to be responsible for ST elevation in young men. It has been suggested that heart rate possibly alters ion currents activity and therefore ST height. Lehman and Yang 13 found decreased ST height with increasing resting heart rate in their male subset of study subjects using spatial ST‐T vector variables. Heart rate changes in the form of rapid pacing have been shown to alter ion current activity, 27 repolarization, and ST height. In our study, heart rate at rest was not significantly different among the four groups and thus was not responsible for sex and age differences in ST height.
LIMITATIONS
There are several limitations to the present study. First, our study population included only a small number of subjects. Secondly, effects of heart rate on ST height were not independently studied. However, the purpose of the present study was to determine whether differences in ST height between sexes and different age groups that had been previously described were predominantly due to differences in resting autonomic tone and the results of the present study refute this hypothesis. Finally, patients with “early repolarization syndrome” were not specifically studied. Since this syndrome has been described predominantly in young men, including patients with early repolarization alone would not have allowed us to compare different age and sex subgroups.
Acknowledgments
Acknowledgment: This study was supported in part by grants from the NHLBI (R01HL075382), from the National Institute of Aging (1RO3 AG14490‐01) and from the National Center for Research Studies (MO1 RR‐00048), Baltimore, Maryland.
REFERENCES
- 1. Mehta M, Jain A. Early repolarization on scalar electrocardiogram. Am J Med Sci 1995;309:305–311. [DOI] [PubMed] [Google Scholar]
- 2. Mehta M, Jain A, Mehta A. Early repolarization. Clin Cardiol 1999;22:59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Green L, Lux R, Haws C, et al Effects of age, sex, and body habitus on QRS and ST‐T potential maps of 1100 normal subjects. Circulation 1985;71(2):244–253. [DOI] [PubMed] [Google Scholar]
- 4. Bidoggia H, Maciel J, Capalozza N, et al Sex differences on the electrocardiographic pattern of cardiac repolarization: Possible role of testosterone. Am Heart J 2000;140(4):678–683. [DOI] [PubMed] [Google Scholar]
- 5. Bidoggia H, Maciel J, Capalozza N, et al Sex‐dependent electrocardiographic pattern of cardiac repolarization. Am Heart J 2000;140(3):430–436. [DOI] [PubMed] [Google Scholar]
- 6. Yang H, Elko P, Fromm B, et al Maximal ascending and descending slopes of the T wave in men and women. J Electrocardiol 1997;30(4):267–276. [DOI] [PubMed] [Google Scholar]
- 7. Surawicz B. Brugada syndrome: Manifest, concealed, “asymptomatic,” suspected and simulated. J Am Coll Cardiol 2001;38(3):775–777. [DOI] [PubMed] [Google Scholar]
- 8. McGill HC Jr, Anselmo VC, Buchanan JM, et al The heart is a target organ for androgen. Science 1980;207(4432):775–777. [DOI] [PubMed] [Google Scholar]
- 9. Stumpf WE, Sar M, Aumuller G. The heart: A target organ for estradiol. Science 1977;196(4287):319–321. [DOI] [PubMed] [Google Scholar]
- 10. Krieg M, Smith K, Bartsch W. Demonstration of a specific androgen receptor in rat heart muscle: Relationship between binding, metabolism, and tissue levels of androgens. Endocrinology 1978;103(5):1686–1694. [DOI] [PubMed] [Google Scholar]
- 11. Endres S, Mayuga K, Cristofaro A, et al Menstrual cycle and ST height. Ann Noninvasive Electrocardiol 2004;9(2):121–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Demir A, Soylu M, Balbay Y, et al Assessment of autonomic function in subjects with early repolarization. Am J Cardiol 2002;89(8):990–992. [DOI] [PubMed] [Google Scholar]
- 13. Lehmann M, Yang H. Sexual dimorphism in the electrocardiographic dynamics of human ventricular repolarization. Characterization in true time domain. Circulation 2001;104:32–38. [DOI] [PubMed] [Google Scholar]
- 14. Jose A, Taylor R. Autonomic blockade by propranolol and atropine to study intrinsic myocardial function in man. J Clin Invest 1969;48:2019–2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Surawicz B, Orr C, Hermiller J, et al QRS changes during percutaneous transluminal coronary angioplasty and their possible mechanisms. J Am Coll Cardiol 1997;30(2):452–458. [DOI] [PubMed] [Google Scholar]
- 16. Yan G, Antzelevitch C. Cellular basis for the electrocardiographic J wave. Circulation 1996;93(2):372–379. [DOI] [PubMed] [Google Scholar]
- 17. Gussak I, Antzelevitch C. Early repolarization syndrome: clinical characteristics and possible cellular and ionic mechanisms. J Electrocardiol 2000;33(4):299–309. [DOI] [PubMed] [Google Scholar]
- 18. Lui D, Antzelevitch C. Characteristics of the delayed rectifier current (IKr and IKs) in canine ventricular epicardial, midmyocardial, and endocardial myocytes. A weaker IKs contributes to the longer action potential of the M cell. Circ research 1995;76(3):351–365. [DOI] [PubMed] [Google Scholar]
- 19. Di Diego J, Cordeiro J, Goodrow R, et al Ionic and cellular basis for the predominance of the Brugada syndrome phenotype in males. Circulation 2002;106(15):2004–2011. [DOI] [PubMed] [Google Scholar]
- 20. Antzelevitch C. The Brugada syndrome: Ionic basis and arrhythmia mechanisms. J Cardiovasc Electrophysiol 2001;12(2):268–272. [DOI] [PubMed] [Google Scholar]
- 21. Surawicz B, Parikh SR. Prevalence of male and female patterns of early ventricular repolarization in the normal ECG of males and females from childhood to old age. J Am Coll Cardiology 2002;40(10):1870–1876. [DOI] [PubMed] [Google Scholar]
- 22. Gambill CL, Wilkins ML, Haisty WK Jr, et al. T wave amplitudes in normal populations. Variation with ECG lead, sex, and age. J Electrocardiol 1995;28(3):191–197. [DOI] [PubMed] [Google Scholar]
- 23. Matsuo K, Akahoshi M, Seto S, et al Disappearance of the Brugada‐type electrocardiogram after surgical castration: A role for testosterone and an explanation for the male preponderance. Pacing Clin Electrophysiol 2003;26(7):1551–1553. [DOI] [PubMed] [Google Scholar]
- 24. Marsh J, Lehmann M, Ritchie R, et al Androgen receptors mediate hypertrophy in cardiac myocytes. Circulation 1998;98(3):256–261. [DOI] [PubMed] [Google Scholar]
- 25. Haydar Z, Brantley D, Gittings N, et al Early repolarization: An electrocardiographic predictor of enhanced aerobic fitness. Am J Cardiol 2000;85:264–266. [DOI] [PubMed] [Google Scholar]
- 26. Lehmann K, Shandling A, Yusi A, et al Altered ventricular repolarization in central sympathetic dysfunction associated with spinal cord injury. Am J Cardiol 1989;63:1498–1504. [DOI] [PubMed] [Google Scholar]
- 27. Viswanathan PC, Shaw RM, Rudy Y. Effects of IKr and IKs heterogeneity on action potential duration and its rate dependence: A simulation study. Circulation 1999;99(18):2466–2474. [DOI] [PubMed] [Google Scholar]


