Abstract
Background: To date, prevalence and clinical significance of noninvasive arrhythmia risk predictors in apparently healthy, middle‐aged persons are largely unknown.
Methods: A total of 110 apparently healthy persons 20–75 years old were enrolled in this prospective observational monocenter study and followed up for 32 ± 15 months. Baseline investigations included symptom‐limited bicycle ergometry, echocardiography, time‐domain analysis, and spectral turbulence analysis of the signal‐averaged electrocardiogram (ECG), ventricular arrhythmias, and heart rate variability on 24‐hour Holter ECG, baroreflex sensitivity, and t‐wave alternans in all persons.
Results: The prevalence of an abnormal signal‐averaged ECG was 1% for spectral turbulence analysis and varied between 1% and 37% for time‐domain analysis depending upon the definition used for an abnormal time‐domain analysis. A reduced heart rate variability defined as a standard deviation of normal‐to‐normal intervals ≤105 ms, <100 ms and <70 ms was found in 12%, 9%, and 1% of persons. A baroreflex sensitivity <6 ms/mmHg and <3 ms/mmHg was present in 15% and 2% of persons. Microvolt t‐wave alternans was found to be positive in 5%, negative in 88%, and indeterminate in 7% of persons, respectively. During the 32 ± 15 months follow‐up, no arrhythmic events and no cardiovascular mortality were observed in this population.
Conclusions: Abnormal findings of noninvasive arrhythmia risk stratification can be found in 1–37% of healthy, middle‐aged persons when previously reported cut‐off values are used.
Keywords: signal‐averaged ECG, heart rate variability, baroreflex sensitivity, t‐wave alternans
Several noninvasive techniques are widely used for arrhythmia risk prediction in patients with structural heart disease including the signal‐averaged electrocardiogram (ECG), 1 , 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 ventricular arrhythmias and heart rate variability on 24‐hour Holter ECG, 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 baroreflex sensitivity, 25 , 26 , 27 , 28 and microvolt t‐wave alternans. 29 , 30 Most studies evaluating the prognostic significance of noninvasive arrhythmia risk predictors used previously reported cut‐off values, retrospectively optimized cut‐off values, or arbitrarily defined cut‐off values to differentiate between patients with high risk versus low risk for arrhythmic events. To date, only limited information is available about normal values for potential noninvasive arrhythmia risk predictors. Therefore, the present study was designed to determine the prevalence and clinical significance of potential noninvasive arrhythmia risk predictors in apparently healthy persons using previously published cut‐off values to define abnormal findings.
METHODS
Healthy Study Persons
During a 4‐year period from June 1996 to March 2000, a total of 110 healthy volunteers were prospectively enrolled in this study at our institution. Men and women 20–75 years old were eligible for study enrollment if the following inclusion criteria were met: (1) absence of any form of cardiovascular disease by history, physical examination, and noninvasive evaluation including 12‐lead ECG, bicycle ergometry, and echocardiography, (2) absence of noncardiovascular disorders including diabetes mellitus, arterial hypertension, kidney or liver disease, (3) absence of any medication other than hormonal contraception. This study was approved by the Institutional Ethics Committee of the Philipps‐University of Marburg. All 110 study persons were followed prospectively from the time of study enrollment until May 2001.
Signal‐Averaged ECG
All recordings were performed in the supine position using a Predictor system (Corazonix Corporation, Oklahoma City, OK, USA). After skin preparation, orthogonal bipolar X, Y, and Z leads were used to record an average of 327 ± 153 cycles in order to reach a noise level of 0.3 μV. 11 All signal‐averaged data were continuously stored on an optical disk for subsequent off‐line analysis. The recorded signal was digitized, and the resultant data underwent signal averaging using a bidirectional band‐pass filter with a range of 40–250 Hz. A high‐pass cut‐off frequency of 40 Hz was used for filtering because time‐domain results analyzed at 40 Hz showed the highest sensitivity and specificity for predicting arrhythmic events in previous studies. 3 , 12 The following time‐domain variables of the filtered signal averaged ECG were evaluated: 11 total QRS duration; root‐mean‐square voltage (RMS 40) in the last 40 ms of the QRS complex; and LAS 40, the duration of low amplitude signal (<40 μV) in the terminal QRS portion. In addition to time‐domain analysis of the signal‐averaged ECG, spectral turbulence analysis was performed using the default (automatic) mode of the software of the Del Mar Cardiac Early Warning System (model 183 CEWSTM). An abnormal spectral turbulence analysis was defined as a score of 3 or 4 out of 4. 6 , 7 , 8
Arrhythmias and Heart Rate Variability on 24‐Hour Holter ECG
Spontaneous arrhythmias and heart rate variability were determined exclusively from digital 24‐hour Holter recordings (Oxford FD2TM or FD3TM recorders, and Oxford Medilog Excel 2 system, version 7.5, Oxford Instruments, Abington, UK). At least 20 hours of artifact‐free recording time was required for a Holter ECG to be included for analysis. Otherwise, the Holter ECG was discarded, and a new Holter ECG was started. The number of ventricular premature beats during each hour was assessed and the mean value calculated. Nonsustained ventricular tachycardia was defined as ≥3 consecutive ventricular premature beats at a rate of ≥120 beats/min lasting less than 30 seconds. 18 Analysis of heart rate variability was performed as previously reported in detail. 28 For each Holter recording, the mean of all coupling intervals between normal beats (RRm) was calculated, and the following time‐domain parameters were determined: the standard deviation of all normal R‐R intervals (SDNN), the square root of the mean of the sum of squared differences between adjacent normal R‐R intervals (RMSSD), and the percentage of differences between adjacent normal R‐R intervals that are >50 ms (pNN50).
Baroreflex Sensitivity Analysis
Analysis of baroreflex sensitivity was performed by intravenous administration of phenylephrine according to the method described by Smyth and co‐workers. 25 , 28 Briefly, a bolus dose of phenylephrine (2 μg/kg) was given intravenously to evaluate the magnitude of the resulting blood pressure increase. If the systolic blood pressure did not increase as desired (15–40 mmHg), additional injections were given at intervals of 10 minutes with incremental bolus doses of phenylephrine up to a maximum dose of 10 μg/kg. Phenylephrine injections were repeated until at least two recordings were obtained using the optimal bolus dose. The heart rate and blood pressure were continuously recorded noninvasively by a finger cuff (Finapres 2300, Ohmeda, Hatfield, UK). All blood pressure data and corresponding R‐R interval data were transferred on‐line from the Finapres 2300 system to an IBM personal computer. A linear regression analysis of R‐R cycles and systolic blood pressure values was performed, including all values between the beginning and the end of the blood pressure increase. Baroreflex sensitivity was then calculated as the mean of at least two measurements of the slope of the regression line relating changes of R‐R intervals to systolic blood pressure. Similar to previous investigators, 26 , 27 exclusively regression lines with statistical significant coefficients (P < 0.05) were accepted for baroreflex sensitivity analysis.
Measurement of T‐Wave Alternans
T‐wave alternans analysis was performed noninvasively at rest and during symptom‐limited bicycle ergometry using the CH 2000 system (Cambridge Heart Inc., Bedford, MA, USA). The presence of t‐wave alternans was determined using the Cambridge Heart spectral analytic method for measurement of microvolt level t‐wave alternans (software release 1.7.1.). Newly developed, multicontact electrodes were used for noise reduction (High Resolution Electrodes, Cambridge Heart Inc.). The spectral method of t‐wave alternans and the definitions for positive, negative, and indeterminate t‐wave alternans using this system have been described previously in detail. 29 , 30 Briefly, studies were classified as positive if sustained alternans was present at rest or with an onset heart rate ≤110 beats/min. Studies were classified as negative if they did not meet the criteria for positivity, and the highest heart rate at which sustained alternans was definitely not present was ≥105 beats/min. Studies that did not meet the criteria for positivity or negativity were classified as indeterminate. Off‐line analysis and interpretation of all t‐wave alternans tests of the present study were carried out by an independent blinded experienced observer.
Statistical Analysis
Continuous data are expressed as mean ± SD. In addition, continuous data of noninvasive risk stratification are descriptively presented as medians, 2.5th, 5th, 95th and 97.5th percentiles in Tables 2 and 4. Comparisons between groups were conducted with the Mann‐Whitney U test for continuous variables allowing for deviations from normal distribution, and Fisher's exact test for categorical variables. To evaluate the effect of age and gender on potential noninvasive arrhythmia risk predictors, all results were stratified according to gender and according to the median age of 45 years. A P value of <0.05 was considered statistically significant.
Table 2.
Results of Signal‐Averaged Electrocardiography Stratified for Gender and Age
| Mean | SD | 2.5th | 5th | Percentiles | |||
|---|---|---|---|---|---|---|---|
| Median | 95th | 97.5th | |||||
| Time‐Domain Analysis (all controls) | |||||||
| QRS duration (ms) | 101 | 10 | 87 | 88 | 100 | 119 | 120 |
| RMS 40 (μV) | 32 | 23 | 6 | 9 | 28 | 64 | 96 |
| AS 40 (ms) | 34 | 9 | 19 | 21 | 33 | 49 | 54 |
| Subgroup of Men (n = 76) | |||||||
| QRS duration (ms) | 103a | 9 | 87 | 88 | 102 | 119 | 122 |
| RMS 40 (μV) | 32 | 23 | 8 | 9 | 28 | 63 | 106 |
| LAS 40 (ms) | 34 | 8 | 20 | 21 | 33 | 49 | 54 |
| Subgroup of Women (n = 34) | |||||||
| QRS duration (ms) | 97a | 9 | 78 | 86 | 94 | 119 | 119 |
| RMS 40 (μV) | 34 | 23 | 3 | 5 | 28 | 88 | 96 |
| LAS 40 (ms) | 33 | 10 | 12 | 19 | 32 | 54 | 57 |
| Subgroup of Controls ≤45 Years (n = 56) | |||||||
| QRS duration (ms) | 101 | 10 | 86 | 87 | 99 | 119 | 120 |
| RMS 40 (μV) | 38b | 27 | 9 | 11 | 34 | 96 | 106 |
| LAS 40 (ms) | 32b | 8 | 19 | 21 | 31 | 49 | 54 |
| Subgroup of Controls ≥45 Years (n = 54) | |||||||
| QRS duration (ms) | 102 | 9 | 89 | 90 | 101 | 118 | 119 |
| RMS 40 (μV) | 26b | 17 | 6 | 8 | 22 | 60 | 62 |
| LAS 40 (ms) | 36b | 9 | 20 | 22 | 35 | 50 | 54 |
| Spectral Turbulence Analysis (all controls)c | |||||||
| LSCR | 65 | 5 | 53 | 56 | 66 | 72 | 75 |
| ISCM | 94 | 1 | 92 | 92 | 94 | 96 | 96 |
| ISCSD | 84 | 21 | 49 | 54 | 81 | 118 | 137 |
| Spectral Entropy | 10 | 2 | 6 | 7 | 10 | 13 | 14 |
a P < 0.05 for women versus men. b P < 0.05 for persons aged < 45 years versus ≥ 45 years. c p = n.s for differences between age and gender. ISCM = interslice correlation mean; ISCSD = interslice correlation standard deviation; LAS 40 = the terminal low amplitude (<40 mV) signal duration; LSCR = low slice correlations ratio; RMS 40 = root‐mean‐square voltage of the terminal 40 ms of the QRS complex.
Table 4.
Baroreflex Sensitivity and Heart Rate Variability on 24‐Hour Holter ECG Stratified for Gender and Age
| Mean | SD | 2.5th | 5th | Percentiles | |||
|---|---|---|---|---|---|---|---|
| Median | 95th | 97.5th | |||||
| Baroreflex Sensitivity Analysis (ms/mmHg) | |||||||
| All controls (n = 105)a | 12 | 6 | 3 | 4 | 11 | 25 | 27 |
| Men (n = 75) | 12 | 6 | 3 | 4 | 11 | 24 | 27 |
| Women (n = 30) | 12 | 7 | 3 | 4 | 10 | 26 | 28 |
| Age < 45 years (n = 53) | 15b | 6 | 7 | 8 | 13 | 27 | 28 |
| Age ≥ 45 years (n = 52) | 10b | 5 | 3 | 3 | 9 | 22 | 24 |
| Heart Rate Variability (all controls) | |||||||
| RRm (ms) | 817 | 96 | 640 | 677 | 809 | 976 | 1055 |
| SDNN (ms) | 154 | 44 | 83 | 92 | 153 | 228 | 272 |
| RMSSD (ms) | 42 | 50 | 12 | 14 | 33 | 75 | 158 |
| PNN50 (%) | 12 | 11 | 0 | 1 | 9 | 34 | 39 |
| Subgroup of Men (n = 76) | |||||||
| RRm (ms) | 825 | 89 | 677 | 699 | 823 | 1019 | 1055 |
| SDNN (ms) | 159c | 38 | 94 | 99 | 158 | 228 | 249 |
| RMSSD (ms) | 44 | 56 | 12 | 14 | 34 | 75 | 133 |
| Pnn50 (%) | 12 | 10 | 0 | 1 | 10 | 34 | 36 |
| Subgroup of Women (n = 34) | |||||||
| RRm (ms) | 799 | 110 | 602 | 635 | 791 | 975 | 1092 |
| SDNN (ms) | 143c | 54 | 66 | 81 | 134 | 272 | 322 |
| RMSSD (ms) | 37 | 34 | 8 | 13 | 25 | 158 | 162 |
| PNN50 (%) | 11 | 15 | 0 | 1 | 5 | 45 | 70 |
| Subgroup of Controls ≤45 years (n = 56) | |||||||
| RRm (ms) | 824 | 102 | 635 | 675 | 810 | 1019 | 1055 |
| SDNN (ms) | 168b | 50 | 83 | 92 | 163 | 272 | 283 |
| RMSSD (ms) | 46b | 30 | 14 | 17 | 39 | 133 | 158 |
| PNN50 (%) | 16b | 13 | 1 | 2 | 14 | 39 | 45 |
| Subgroup of Controls ≥45 Years (n = 54) | |||||||
| RRm (ms) | 810 | 90 | 664 | 694 | 808 | 959 | 1022 |
| SDNN (ms) | 140b | 31 | 91 | 91 | 137 | 202 | 205 |
| RMSSD (ms) | 37b | 65 | 12 | 12 | 25 | 66 | 67 |
| PNN50 (%) | 7b | 8 | 0 | 1 | 5 | 23 | 24 |
a Five patients were excluded from baroreflex sensitivity analysis due to nonsignificant correlation coefficients. b P < 0.05 for persons aged <45 years versus ≥45 years. c P < 0.05 for women versus men.
RESULTS
Clinical Characteristics of Healthy Persons and Follow‐up Results
The clinical characteristics of the 110 persons in the study including the results of echocardiography, symptom‐limited bicycle ergometry, and 24‐hour Holter monitoring are summarized in Table 1. During 32 ± 15 months follow‐up, no cardiovascular deaths and no arrhythmic events occurred in any of the study persons. Two patients (2%) died during follow‐up. The cause of death was cancer in one patient and an accident during a thunderstorm in the other patient.
Table 1.
Clinical Characteristics of 110 Healthy Volunteers
| All Controls | Men | Women | |
|---|---|---|---|
| Number of Healthy Volunteers | 110 | 76 (69%) | 34 (31%) |
| Age (years) | 45 ± 12 | 45 ± 11 | 43 ± 14 |
| Range | 21–71 | 27–66 | 21–71 |
| Height (cm) | 175 ± 8 | 179 ± 6 | 167 ± 7a |
| Weight (kg) | 78 ± 14 | 81 ± 14 | 70 ± 11a |
| Echocardiographic Evaluation | |||
| LV end‐diastolic diameter (mm) | 50 ± 4 | 51 ± 4 | 47 ± 4a |
| Range | 38–58 | 38–58 | 39–54 |
| LV ejection fraction (%) | 71 ± 5 | 71 ± 5 | 70 ± 4 |
| Bicycle Ergometry | |||
| Maximum exercise (W) | 153 ± 45 | 168 ± 43 | 117 ± 22a |
| Range | 75–300 | 100–300 | 75–175 |
| Heart rate at rest (beats/min) | 76 ± 12 | 74 ± 12 | 78 ± 13 |
| Maximum heart rate (beats/min) | 157 ± 17 | 157 ± 18 | 157 ± 15 |
| 24‐Hour Holter ECG | |||
| Mean R‐R interval (ms) | 817 ± 96 | 825 ± 89 | 799 ± 110 |
| Frequent VPDs (>10/hour) | 7 (6%) | 5 (7%) | 2 (6%) |
| Couplets of VPDs | 6 (5%) | 2 (3%) | 4 (12%) |
| Nonsustained VT | 1 (1%) | 0 (0%) | 1 (3%) |
a P < 0.05 for men compared to women. LV = left ventricular; VPD = ventricular premature depolarization; VT = ventricular tachycardia.
Signal‐Averaged ECG
The results of time‐domain analysis and spectral turbulence analysis of the signal‐averaged ECG are shown in Table 2 (see also Fig. 1). In addition, Table 3 summarizes the incidence of false‐positive signal‐averaged ECG findings when various previously published criteria for an abnormal signal‐averaged ECG were applied. An abnormal spectral turbulence analysis was found only in 1% of the 110 study persons. An abnormal time‐domain analysis was present in 1–37% of the 110 study persons depending upon the definition used for an abnormal time‐domain result (Table 3). The incidence of abnormal time‐domain analyses of the signal‐averaged ECG was similar in men compared to women. Persons aged ≥45 years were found to have a higher incidence of abnormal time‐domain analyses compared to younger patients when one out of three criteria or two out of three criteria proposed by Gomes et al. 4 , 5 were used to define an abnormal time‐domain analysis (Table 3).
Figure 1.
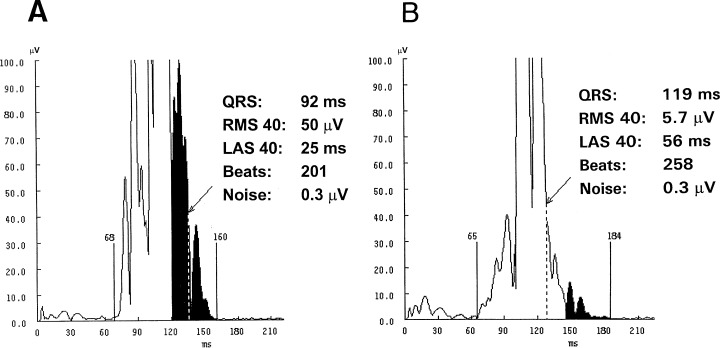
Normal (A) and abnormal time‐domain analysis (B) of the signal‐averaged electrocardiogram in an apparently healthy person without arrhythmic events during follow‐up.
Table 3.
Abnormal Signal‐Averaged ECG Results Using Previously Reported Cut‐off Values
| All Controls | Men | Women | Age ≤ 45 years | Age ≥ 45 years | |
|---|---|---|---|---|---|
| Number of Healthy Volunteers | 110 | 76 (69%) | 34 (31%) | 56 (51%) | 54 (49%) |
| Abnormal Time‐Domain Analysis (n) | |||||
| QRS duration > 114 ms (2,3) | 12 (11%) | 10 (13%) | 2 (6%) | 7 (13%) | 5 (9%) |
| RMS 40 < 20 μV (2,3) | 36 (33%) | 27 (36%) | 9 (26%) | 12 (21%) | 24 (44%)a |
| LAS 40 > 38 ms (2,3) | 29 (26%) | 21 (28%) | 8 (24%) | 11 (20%) | 18 (33%)b |
| 1 of the above (2,3,5,16) | 41 (37%) | 31 (41%) | 10 (29%) | 16 (29%) | 25 (46%)b |
| 2 of the above (6,9,12–16) | 29 (26%) | 21 (28%) | 8 (24%) | 11 (20%) | 18 (33%)b |
| 2 of the above + QRS > 114 ms (10) | 8 (7%) | 7 (9%) | 1 (3%) | 3 (5%) | 5 (9%) |
| All 3 of the above 9 (16) | 7 (6%) | 6 (8%) | 1 (3%) | 3 (5%) | 4 (7%) |
| QRS > 120 ms + RMS 40 < 20 μV (1) | 2 (2%) | 2 (3%) | 0 (0%) | 1 (2%) | 1 (2%) |
| QRS > 120 ms + RMS 40 < 20 μV + LAS 40 > 38 ms (16) | 1 (1%) | 1 (1%) | 0 (0%) | 0 (0%) | 1 (2%) |
| Abnormal Spectral Turbulence Analysis (n) | |||||
| Score 3 or 4 out of 4 (6,7,8) | 1 (1%) | 1 (1%) | 0 (0%) | 1 (2%) | 0 (0%) |
aP < 0.05 for persons aged <45 years versus ≥45 years. b P = 0.1 for persons aged <45 years versus ≥45 years. pNN50 = percent of differences between adjacent normal R‐R intervals that are >50 ms; RMSSD = square root of the mean of the sum of the squared differences between adjacent normal R‐R intervals; RRm = normal R‐R intervals; SDNN = standard deviation of all R‐R intervals; VPD = ventricular premature depolarization.
Heart Rate Variability, Baroreflex Sensitivity, and T‐Wave Alternans
The results of 24‐hour digital Holter recordings and baroreflex sensitivity analysis are summarized in Tables 4 and 5. Persons aged ≥45 years had a higher incidence of ventricular premature beats/hour, a decreased heart rate variability, and a reduced baroreflex sensitivity when compared to persons aged <45 years. In addition, women were found to have a lower standard deviation of normal‐to‐normal R‐R intervals (SDNN) compared to men. All other parameters of heart rate variability and baroreflex sensitivity were similar in men compared to women. The distribution of heart rate variability (SDNN) and baroreflex sensitivity in the 110 study persons is shown in Figure 2. Microvolt level t‐wave alternans was found to be present in 5 of the 110 study persons (5%) without significant age‐ or gender‐related differences (Table 5).
Table 5.
Abnormal Results of 24‐Hour Holter ECG, Baroreflex Sensitivity, and T‐Wave Alternans Analysis Using Previously Reported Cut‐off Values
| All Controls | Men | Women | Age < 45 years | Age ≥ 45 years | |
|---|---|---|---|---|---|
| Number of Healthy Volunteers | 110 | 76 (69%) | 34 (31%) | 56 (51%) | 54 (49%) |
| Ventricular Arrhythmias | |||||
| >10 VPDs per hour | 7 (6%) | 5 (7%) | 2 (6%) | 0 (0%) | 7 (13%)a |
| Couplets of VPDs | 6 (5%) | 2 (3%) | 4 (12%) | 4 (7%) | 2 (4%) |
| Nonsustained ventricular tachycardia | 1 (1%) | 0 (0%) | 1 (3%) | 0 (0%) | 1 (2%) |
| Abnormal Heart Rate variability (n) | |||||
| SDNN ≤ 105 ms (27) | 13 (12%) | 6 (8%) | 7 (21%) | 5 (9%) | 8 (15%) |
| SDNN < 100 ms (20,22,32) | 10 (9%) | 4 (5%) | 6 (18%) | 3 (5%) | 7 (13%) |
| SDNN < 70 ms (19,24,27) | 1 (1%) | 0 (0%) | 1 (3%) | 1 (2%) | 0 (0%) |
| SDNN < 50 ms (20,22,26) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Abnormal Baroreflex Sensitivity | |||||
| <6 ms/mmHg (27) | 16 (15%) | 11 (15%) | 5 (17%) | 0 (0%) | 16 (31%)a |
| <3 ms/mmHg (26,27) | 2 (2%) | 1 (1%) | 1 (3%) | 0 (0%) | 2 (4%) |
| T‐Wave Alternans analysis (n) | |||||
| Positive | 5 (5%) | 2 (3%) | 3 (9%) | 1 (2%) | 4 (7%) |
| Negative | 8 (89%) | 69 (91%) | 29 (85%) | 54 (96%) | 44 (81%) |
| Indeterminate | 7 (6%) | 5 (7%) | 2 (6%) | 1 (2%) | 6 (11%) |
a P < 0.05 for persons aged <45 years versus ≥45 years.
Figure 2.
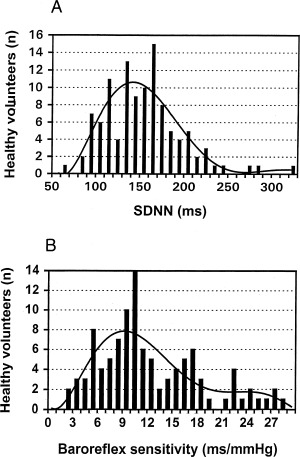
Distribution of the standard deviation of normal‐to‐normal R‐R intervals (SDNN) (A), and baroreflex sensitivity (B) in 110 healthy, middle‐aged persons.
DISCUSSION
In this study, we determined the prevalence of abnormal findings of the important potential noninvasive arrhythmia risk predictors in 110 healthy middle‐aged persons and using previously published cut‐off values to define abnormal findings. Importantly, no cardiovascular deaths and no arrhythmic events occurred in any of the study persons during a mean follow‐up of almost 3 years. Depending upon the definition used for an abnormal result, the present study showed abnormal findings of noninvasive arrhythmia risk stratification in up to 37% of persons for time‐domain analysis of the signal‐averaged ECG, in up to 12% of persons for heart rate variability (SDNN), in up to 15% of persons for baroreflex sensitivity, and in 5% of persons for t‐wave alternans analysis (see Fig. 3).
Figure 3.
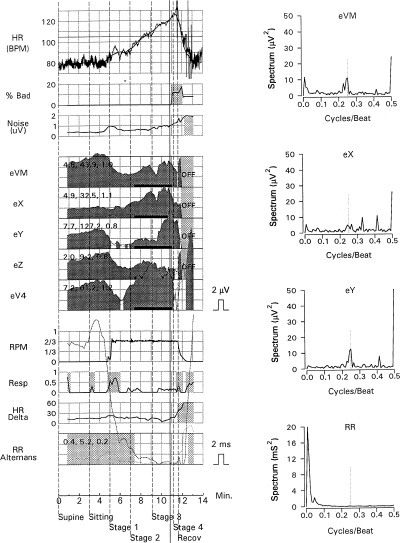
Positive t‐wave alternans analysis in an apparently healthy person without arrhythmic events during follow‐up. Alternans analysis during symptom‐limited bicycle ergometry for leads VM, X, Y, Z, and V4 is shown on the left side. The t‐wave spectrum during exercise testing with a distinct peak at a 0.5 cycle/beat frequency is shown on the right side. The dark shaded areas with black bars for each lead also indicate positive microvolt t‐wave alternans during exercise in this person. HR, heart rate trend; % bad, percentage of beats more than 10% premature; noise, mean noise in lead VM; RPM, bicycle ergometer pedaling rate; resp, respiratory frequency; HR delta, the difference between the highest and lowest instantaneous heart rates for a 128‐consecutive beat interval; R‐R alternans, the amplitude of R‐R interval alternans.
Signal‐Averaged ECG
The majority of previous studies 3 , 5 , 6 , 9 , 12 , 13 , 14 , 15 , 16 used one or two out of three criteria (QRS >114 ms, LAS > 38 ms, RMS 40 <20 μV) originally proposed by Gomes et al. 2 to define an abnormal timedomain analysis of the signal‐averaged ECG at 40 Hz filtering. Only a few of these studies, however, investigated the incidence of abnormal time‐domain analyses of the signal‐averaged ECG in healthy controls. 4 , 10 , 14 , 15 , 17 In the largest reported series of signal averaging in healthy persons, Marques‐Vidal et al. 15 investigated 487 healthy middle‐aged men from Southwestern France. Similar to the results of our study, Marques‐Vidal et al. 15 found a high incidence of 21% for abnormal time‐domain analyses of the signal‐averaged ECG using two out of the three criteria proposed by Gomes et al. 2 In current practice, time‐domain analyses of the signal‐averaged ECG are widely used to stratify risk of sustained ventricular tachyarrhythmias in postinfarct patients, because there is convincing evidence from previous studies 2 , 3 , 4 , 9 , 12 , 13 that the presence of an abnormal signal‐averaged ECG is associated with an increased risk for ventricular tachycardia and sudden death in these patients. To date, however, the CABG‐Patch trial 5 is the only reported interventional trial that used abnormalities of the signal‐averaged ECG in combination with a left ventricular ejection fraction <36% at the time of coronary artery bypass surgery as inclusion criteria for randomization to defibrillator therapy versus no defibrillator therapy. Surprisingly, no evidence of improved survival was found in the CABG‐Patch trial 5 between patients with and without defibrillator therapy. An abnormal time‐domain analysis of the signal‐averaged ECG at 40 Hz high‐pass filtering in the CABG‐Patch trial was diagnosed if at least one out of three criteria proposed by Gomes et al. 2 was positive. Using this definition, we found an incidence of 37% falsepositive time‐domain analyses in 110 healthy, middle‐aged controls in the present study.
Heart Rate Variability, Baroreflex Sensitivity, and T‐Wave Alternans
Experimental and clinical evidence suggests that decreased heart rate variability and decreased baroreflex sensitivity as markers of tonic and reflex vagal activity of the heart have independent prognostic value in postinfarct patients as well as in patients with heart failure of various etiologies. 19 , 20 , 21 , 22 , 23 , 24 , 25 Similar to previous investigations, 21 , 23 only one apparently healthy person in our study showed a markedly reduced heart rate variability with an SDNN < 70 ms whereas no person had an SDNN < 50 ms. In addition, only 2% of apparently healthy
persons in our study had a baroreflex sensitivity <3 ms/mmHg, which is frequently used as the cut‐off value for an abnormal baroreflex sensitivity analysis in patients with structural heart disease. 26 , 27 Blinded analysis of all microvolt t‐wave alternans tests by an experienced investigator revealed 5% abnormal findings in 110 apparently healthy middle‐aged persons in the present study.
Study Limitations
The number of studied healthy volunteers is too small to establish normal values for potential noninvasive arrhythmia risk predictors and to determine the prognostic significance of these tests with any certainty. This is particularly true for subgroup analyses of persons stratified for age and gender.
Clinical Implications and Conclusions
Abnormal results of noninvasive arrhythmia risk stratification can be found in 1–37% of healthy, middle‐aged persons when previously reported cut‐off values are used. It is noteworthy that no arrhythmic events and no cardiovascular death occurred in any study person during 32 ± 15 months prospective follow‐up indicating that the observed abnormal results in these healthy, middle‐aged persons are likely to be false‐positive results of noninvasive arrhythmia risk stratification. An extremely low incidence of false‐positive results of noninvasive arrhythmia risk stratification, however, should be considered as a prerequisite for risk stratification techniques in order to be used in interventional trials to prevent sudden cardiac death. This is particularly true for studies using noninvasive arrhythmia risk predictors with regard to prophylactic defibrillator implantation.
This study was supported by a grant from the German Science Foundation, Bonn, Germany (grant no. GR 1130/2‐2).
REFERENCES
- 1. Kuchar DL, Thorburn CW, Sammel NL. Prediction of serious arrhythmic events after myocardial infarction: Signal averaged electrocardiogram, Holter monitoring and radionuclide ventriculography. J Am Coll Cardiol 1987;9: 531–538. [DOI] [PubMed] [Google Scholar]
- 2. Gomes JQ, Winters SL, Stewart D, et al. A new noninvasive index to predict sustained ventricular tachycardia and sudden death in the first year after myocardial infarction: Based on signal‐averaged electrocardiogram, radionuclide ejection fraction and Holter monitoring. J Am Coll Cardiol 1987;10: 349–357. [DOI] [PubMed] [Google Scholar]
- 3. Gomes JA, Winters SL, Martinson M, et al. The prognostic significance of quantitative signal‐averaged variables relative to clinical variables, site of myocardial infarction, ejection fraction and ventricular premature beats: A prospective study. J Am Coll Cardiol 1989;13: 377–384. [DOI] [PubMed] [Google Scholar]
- 4. Breithardt G, Cain ME, El‐Sherif N, et al. Standards for analysis of ventricular late potentials using high‐resolution or signal‐averaged electrocardiography. A statement of the European Society of Cardiology, the American Heart Association, and the American College of Cardiology. J Am Coll Cardiol 1991;17: 999–1006. [DOI] [PubMed] [Google Scholar]
- 5. Bigger JT Jr, for the Coronary Artery Bypass Graft Patch Trial Investigators . Prophylactic use of implanted cardiac defibrillators in patients at high risk for ventricular arrhythmias after coronary artery bypass graft surgery. N Engl J Med 1997;337: 1569–1575. [DOI] [PubMed] [Google Scholar]
- 6. Keeling PJ, Kulakowski P, Yi G, et al. Usefulness of signal‐averaged electrocardiogram in idiopathic dilated cardiomyopathy for identifying patients with ventricular arrhythmias. Am J Cardiol 1993;72: 78–84. [DOI] [PubMed] [Google Scholar]
- 7. Turitto G, Ahuja RK, Caref EB, et al. Risk stratification for arrhythmic events in patients with nonischemic dilated cardiomyopathy and nonsustained ventricular tachycardia: Role of programmed ventricular stimulation and the signal‐averaged electrocardiogram. J Am Coll Cardiol 1994;24: 1523–28. [DOI] [PubMed] [Google Scholar]
- 8. Yi G, Keeling PJ, Goldman JH, et al. Prognostic significance of spectral turbulence analysis of the signal averaged electrocardiogram in patients with idiopathic dilated cardiomyopathy. Am J Cardiol 1995;75: 494–497. [DOI] [PubMed] [Google Scholar]
- 9. Silverman ME, Pressel MD, Brackett JC, et al. Prognostic value of the signal‐averaged electrocardiogram and a prolonged QRS in ischemic and nonischemic cardiomyopathy. Am J Cardiol 1995;75: 460–464. [DOI] [PubMed] [Google Scholar]
- 10. Grimm W, Menz V, Hoffmann J, et al. Value of time and frequency domain analysis of signal averaged electrocardiograms for arrhythmia risk prediction in idiopathic dilated cardiomyopathy. PACE 1996;19: 1923–1927. [DOI] [PubMed] [Google Scholar]
- 11. Steinberg J, Bigger JT. Importance of the endpoint of noise reduction in analysis of the signal‐averaged electrocardiogram. Am J Cardiol 1989;63: 556–562. [DOI] [PubMed] [Google Scholar]
- 12. Savard P, Rouleau JL, Ferguson J, et al. Risk stratification after myocardial infarction using signal‐averaged electrocardiographic criteria adjusted for sex, age, and myocardial infarction location. Circulation 1997;96: 202–213. [DOI] [PubMed] [Google Scholar]
- 13. Hohnloser SH, Franck P, Klingenheben T, et al. Open infarct artery, late potentials, and other prognostic factors in patients after acute myocardial infarction in the thrombolytic era. Circulation 1994;90: 1747–1756. [DOI] [PubMed] [Google Scholar]
- 14. Mercando AD, Aronow WF, Epstein S, et al. Signal‐averaged electrocardiography in elderly subjects with and without disease. PACE 1994;17: 166–171. [DOI] [PubMed] [Google Scholar]
- 15. Marques‐Vidal P, Ruidavets JB, Prouteau N, et al. Prevalence of late potentials in a sample of 487 healthy, middle‐aged men from southwestern France. PACE 2000;23: 888–890. [DOI] [PubMed] [Google Scholar]
- 16. Steinberg JS, Prystowsky E, Freedman RA, et al. Use of the signal‐averaged electrocardiogram for predicting inducible ventricular tachycardia in patients with unexplained syncope: Relation to clinical variables in a multivariable analysis. J Am Coll Cardiol 1994;23: 99–106. [DOI] [PubMed] [Google Scholar]
- 17. Raineri AA, Traina M, Rotolo A, et al. Quantitative analysis of ventricular late potentials in healthy subjects. Am J Cardiol 1990;66: 1359–1362. [DOI] [PubMed] [Google Scholar]
- 18. Hohnloser SH, Klingenheben T, Zabel M, et al. Prevalence, characteristics and prognostic value during long‐term follow‐up of nonsustained ventricular tachycardia after myocardial infarction in the thrombolytic era. J Am Coll Cardiol 1999;33: 1895–1902. [DOI] [PubMed] [Google Scholar]
- 19. Farrell TG, Bashir Y, Cripps TR, et al. Risk stratification for arrhythmic events in postinfarction patients based on heart rate variability, ambulatory electrocardiographic variables and the signal‐averaged electrocardiogram. J Am Coll Cardiol 1991;18: 687–697. [DOI] [PubMed] [Google Scholar]
- 20. Kleiger RE, Miller JP, Bigger JT, et al. Decreased heart rate variability and its association with increased mortality after acute myocardial infarction. Am J Cardiol 1987;59: 256–262. [DOI] [PubMed] [Google Scholar]
- 21. Bigger JT Jr, Fleiss JL, Steinman RC, et al. RR variability in healthy, middle‐aged persons compared with patients with chronic coronary heart disease or recent acute myocardial infarction. Circulation 1995;91: 1936–1943. [DOI] [PubMed] [Google Scholar]
- 22. Nolan J, Batin PD, Andrews R, et al. Prospective study of heart rate variability and mortality in chronic heart failure. Circulation 1998;98: 1510–1516. [DOI] [PubMed] [Google Scholar]
- 23. Umetani K, Singer DH, McCraty R, et al. Twenty‐four hour time domain heart rate variability and heart rate. Relations to age and gender over nine decades. J Am Coll Cardiol 1998;31: 593–601. [DOI] [PubMed] [Google Scholar]
- 24. Thomsen PEB, Huikuri H, Kober L, et al. Lessons from the Nordic ICD pilot study. Lancet 1999;353: 2130. [DOI] [PubMed] [Google Scholar]
- 25. Smyth HS, Sleight P, Pickering GW. Reflex regulation of arterial pressure during sleep in man: A quantitative method of assessing baroreflex sensitivity. Circ Res 1969;24: 109–121. [DOI] [PubMed] [Google Scholar]
- 26. Farrell TG, Paul V, Cripps TR, et al. Baroreflex sensitivity and electrophysiological correlates in patients after acute myocardial infarction. Circulation 1991;83: 945–952. [DOI] [PubMed] [Google Scholar]
- 27. LaRovere MT, Bigger JT, Marcus FI, et al. for the ATRAMI Investigators . Baroreflex sensitivity and heart‐rate variability in prediction of total cardiac mortality after myocardial infarction. Lancet 1998;351: 478–484. [DOI] [PubMed] [Google Scholar]
- 28. Hoffmann J, Grimm W, Menz V, et al. Heart rate variability and baroreflex sensitivity in idiopathic dilated cardiomyopathy. Heart 2000;83: 531–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rosenbaum DS, Jackson LE, Smith JM, et al. Electrical alternans and vulnerability to ventricular arrhythmia. N Engl J Med 1994;330: 235–241. [DOI] [PubMed] [Google Scholar]
- 30. Ikeda T, Sakata T, Takami M, et al. Combined assessment of T wave alternans and late potentials used to predict arrhythmic events after myocardial infarction. J Am Coll Cardiol 2000;35: 722–730. [DOI] [PubMed] [Google Scholar]


