Abstract
Background: Subjects with microvolt‐level T‐wave alternans (TWA) in association with structural heart disease have an increased risk for sudden cardiac death. The presence of diabetes (DM) is associated with an increased risk of sudden death but there is limited data on the impact of DM and previous myocardial infarction (MI) on TWA prevalence.
Methods: We performed a case‐control cross‐sectional study in 140 patients referred for routine exercise testing within a large multispecialty clinic. All patients with a history of DM and MI status within the past year were eligible: group 1 (no DM or MI), group 2 (DM only), group 3 (MI only), group 4 (DM and MI). Patients performed a symptom‐limited Bruce protocol exercise test with assessment of TWA by the spectral method using commercially available equipment. We used published criteria for the blinded interpretation of TWA; all tests not unequivocally negative were considered abnormal.
Results: Age and gender were similar in all groups. The prevalence of abnormal TWA in groups 1–4 was 24%, 20%, 48%, and 62%, respectively (between group P = 0.002). Logistic regression analysis in all patients showed that abnormal TWA was related to prior MI [OR (95% CI): 4.0 (1.8–8.9), P < 0.001] but not to prevalent DM [0.9 (0.4–1.8), P = 0.72]. In patients with DM, the prevalence of abnormal TWA was related to reduced ejection fraction (P = 0.034) but not to BMI, DM duration, glycemic control, insulin use, or the presence of microvascular complications.
Conclusion: The presence of DM alone does not increase risk of abnormal TWA. Prospective studies are required to establish the prognostic value of TWA in patients with DM.
Keywords: T‐wave alternans, diabetes, myocardial infarction, exercise testing
Sudden cardiac death accounts for approximately 250,000 deaths annually in the United States and is the mode of death in one in seven of the general population. The presence of diabetes (DM) is associated with an increased risk of sudden cardiac death. 1 , 2 , 3 , 4 , 5 Moreover, prognosis in patients with DM is especially poor following myocardial infarction (MI). 6 Screening for cardiac disease may be especially useful in patients with DM because coronary disease is more often asymptomatic in patients with diabetes compared to those without DM.
Recent advances in the noninvasive evaluation of patients potentially at risk for life‐threatening arrhythmias include the development of a technique to detect microvolt T‐wave alternans (TWA). When compared with other currently available noninvasive modalities, assessment of TWA appears to be both sensitive and specific for risk stratification of patients with cardiovascular disease and associated left ventricular dysfunction who may be at risk for sudden cardiac death. 7 Recent studies of patients following MI have found that abnormal TWA can predict arrhythmic mortality in patients with well‐preserved ventricular function. 8 Although diabetic patients without overt heart disease have been reported to have approximately 25% prevalence of abnormal TWA, there has been only one controlled study of TWA prevalence in patients with DM, 10 and no studies reporting the additional influence of previous MI on TWA prevalence. 9 , 10
Since TWA has only been evaluated in limited patient populations, the present study sought to evaluate its potential for risk assessment in patients with and without DM and/or previous MI. The aim of this study was to assess the prevalence of abnormal TWA in patients with and without DM and/or previous MI; our secondary aim was to determine the clinical correlates of abnormal TWA in these patients.
METHODS
We enrolled unselected patients referred by primary care and cardiovascular physicians to the Lahey Clinic for exercise testing to screen for coronary disease in asymptomatic subjects, or to evaluate symptoms suggestive of cardiac disease. Exclusion criteria were: high frequency of ectopic beats or atrial fibrillation at the time of the test, bundle branch block, unstable angina, severe hypertension, inability to exercise, and severe aortic stenosis. Patients were also excluded if testing was performed in the first 60 days after MI since abnormal TWA may not be present early after MI. Assessment of DM and MI status using standard diagnostic criteria 11 , 12 was based on questionnaire responses at enrollment and review of medical case‐note data within 1 year of study entry. Patients were classified into one of four groups based on the presence of DM and/or previous MI: group 1 (no DM or MI), group 2 (DM only), group 3 (MI only) group 4 (DM and MI). Hypertension was defined by patient's report and medical record review of physician diagnosis. Retinopathy, neuropathy, and nephropathy were defined by standard criteria based upon questionnaire responses and review of medical record.
Patients performed a symptom‐limited treadmill exercise test with assessment of TWA by the spectral method using commercially available equipment (Cambridge Heart Inc., Bedford, MA, USA). The exercise was performed according to either standard or modified Bruce protocols as determined by patient factors with the intent of achieving a stable heart rate >105 bpm for at least 3 minutes. Use of beta‐blocker and other medications at the time of testing was determined by the clinician ordering the test. A single author (DTM) used published criteria for the blinded interpretation of TWA; all tests not unequivocally negative were considered abnormal. 13 , 14 All patients gave informed consent as approved by the Lahey Clinic Institutional Review Board.
Statistical Analysis
Categorical variables were assessed using the chi‐square or Fisher's exact test. Continuous variables were compared using the Student's t‐test or Mann‐Whitney rank‐sum test depending on data distribution. Logistic regression analysis with TWA as the outcome variable included age, gender, body mass index (BMI), DM (categorical), previous MI (categorical), and ejection fraction as predictor variables. In a separate analysis in patients with DM, the logistic model included duration of DM, glycosylated hemoglobin concentration, presence of microvascular complications, resting heart rate, and use of insulin as predictor variables. Sample size calculations indicated that 46 patients in groups 1 and 2 were required to show a significant between‐group difference in the prevalence of TWA positivity (power 80%, 2‐tailed P < 0.05) assuming that the prevalence of abnormal TWA would be 3% in group 1 and 25% in group 2. Analysis was performed using SigmaStat for Windows (Systat Software Inc., San Jose, CA). Two‐tailed P < 0.05 was considered statistically significant.
RESULTS
Table 1 shows the clinical and exercise test characteristics of the four study groups. A total of 140 patients were enrolled in this study and of these 135 completed the exercise test and provided analyzable data. Age, gender, and the prevalence of ST segment depression during exercise were similar between groups. Patients with DM (groups 2 and 4) had higher BMI (P = 0.03); patients with history of MI (groups 3 and 4) exhibited blunted heart rates at peak exercise (P < 0.001) compared with those without a history of MI (groups 1 and 2).
Table 1.
Clinical and Exercise Characteristics of the Study Groups
| Clinical | Group 1 No MI, no DM (N = 54) | Group 2 DM, no MI (N = 47) | Group 3 MI, no DM (N = 26) | Group 4 DM and MI (N = 13) |
|---|---|---|---|---|
| Age | 53 ± 13 | 60 ± 10 | 58 ± 9 | 60 ± 8 |
| Gender: female [no. (%)] | 10 (19) | 12 (26) | 4 (15) | 1 (8) |
| BMI | 27 ± 4 | 29 ± 6 | 27 ± 4 | 31 ± 5 |
| Insulin use [no. (%)] | NA | 11 (23) | NA | 4 (31) |
| HbA1c (%) | NA | 7.2 ± 1.4 | NA | 7.7 ± 1.5 |
| Microvascular complications [no. (%)] | NA | 9 (19) | NA | 3 (23) |
| LV Ejection fraction (%) | 61 ± 6 | 63 ± 7 | 53 ± 16 | 44 ± 11 |
| Total cholesterol (mg/dL) | 209 ± 45 | 175 ± 36 | 155 ± 32 | 167 ± 36 |
| Triglycerides (mg/dL) | 132 ± 77 | 121 ± 66 | 131 ± 70 | 140 ± 65 |
| High density lipoprotein (mg/dL) | 57 ± 16 | 45 ± 11 | 44 ± 9 | 40 ± 7 |
| Current smoker [no. (%)] | 4 (7) | 5 (11) | 1 (4) | 1 (8) |
| Hypertension [no. (%)] | 17 (31) | 30 (64) | 11 (42) | 11 (85) |
| Beta‐blocker use [no. (%)] | 12 (22) | 7 (15) | 21 (81) | 12 (92) |
| Statin use [no. (%)] | 11 (20) | 17 (36) | 23 (89) | 7 (54) |
| Exercise test data | ||||
| Duration (seconds) | 760 ± 169 | 652 ± 178 | 659 ± 201 | 582 ± 148 |
| Resting HR (bpm) | 75 ± 12 | 80 ± 13 | 65 ± 11 | 71 ± 13 |
| Peak HR (bpm) | 155 ± 23 | 141 ± 21 | 128 ± 21 | 128 ± 25 |
| HR 1 m post (bpm) | 126 ± 23 | 122 ± 19 | 104 ± 20 | 111 ± 21 |
| Resting sBP (mmHg) | 127 ± 17 | 126 ± 17 | 121 ± 19 | 137 ± 21 |
| Resting dBP (mmHg) | 79 ± 11 | 76 ± 10 | 73 ± 8 | 79 ± 8 |
| Peak sBP (mmHg) | 171 ± 23 | 175 ± 26 | 159 ± 20 | 180 ± 28 |
| Peak dBP (mmHg) | 80 ± 11 | 76 ± 9 | 75 ± 7 | 84 ± 15 |
| STD [no. (%)] | 5 (9) | 6 (13) | 3 (12) | 3 (23) |
Data are number (%), or mean ± SD unless stated.
Microvascular complications—includes any combination of neuropathy, nephropathy, and/or retinopathy.
BMI = body mass index (wt in Kg/square of height in meters); HR = heart rate; LV = left ventricle; sBP = systolic blood pressure; dBP = diastolic blood pressure; STD = ischemic ST segment depression during exercise.
There were 94 negative tests for TWA and 41 abnormal tests. The indeterminacy rate was 32/135 (24%) with almost all indeterminate tests related to patient factors such as inadequately low heart rate or frequent ventricular ectopy during exercise. The definitively positive tests were distributed as follows: 1 in group 1, 2 in group 2, and 3 each in groups 3 and 4. The proportions of abnormal tests between the four groups were statistically different (ANOVA, P = 0.002). Between‐group differences related more to the presence of prior MI than the presence of known DM (Fig. 1). The proportion of abnormal tests was not significantly different between groups 1 and 2 or between groups 3 and 4 (both P > 0.5).
Figure 1.
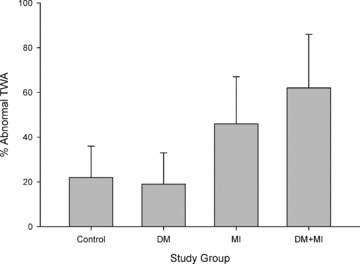
Distribution of abnormal T‐wave alternans in the four study groups.
Assessment of left ventricular ejection fraction was available for 92 (64%) of patients. Median (IQR and range) values are shown in Figure 2. Logistic regression in all patients showed that abnormal TWA was associated with history of MI and with left ventricular dysfunction but not with known DM. In patients with DM (groups 2 and 4), there was no relationship between abnormal TWA and duration of diabetes, glycemic control (HbA1c level), presence of microvascular complications, resting heart rate, or use of insulin (Table 2).
Figure 2.

Box plot showing distribution of left ventricular ejection fraction in the four study groups. One way RM ANOVA on ranks: P < 0.001. Pairwise comparisons: P < 0.05 for group 1 versus 3, 1 versus 4, and 2 versus 4.
Table 2.
Univariate Predictors of Abnormal TWA by Logistic Regression
| Odds Ratio | Confidence 95% Intervals | P | |
|---|---|---|---|
| All Patients | |||
| History of MI | 4.0 | 1.8–8.9 | 0.001 |
| Ejection fraction | 3.5 | 1.2–10.1 | 0.022 |
| Diabetes | 0.9 | 0.4–1.8 | 0.72 |
| BMI | 1.0 | 0.9–1.1 | 0.99 |
| Patients with diabetes | |||
| Duration of diabetes | 1.0 | 0.9–1.1 | 0.77 |
| Glycemic control (HbA1c) | 0.9 | 0.6–1.4 | 0.79 |
| Use of insulin | 0.5 | 0.1–1.9 | 0.30 |
| Resting heart rate | 1.0 | 0.9–1.0 | 0.08 |
| Microvascular complications | 2.1 | 0.6–7.8 | 0.28 |
DISCUSSION
The main finding of this study was that in out‐patients referred for treadmill testing a history of DM was not associated with an increased likelihood of exercise‐induced abnormal TWA in the absence of prior MI. Although there is evidence that DM is associated both with increased cardiovascular and sudden death risk, the similar prevalence of abnormal TWA in patients with and without DM in this study provides little support for the notion that clinically useful risk stratification regarding sudden death in patients with DM can be achieved with such exercise testing.
There is a paucity of data on life‐threatening ventricular arrhythmic risk stratification in patients with DM; however, recent studies by Molon et al. have shown that in patients with DM and no known cardiovascular disease, abnormal TWA prevalence was similar to that found in the present study (∼20%). 9 , 10 We extend this observation by reporting the prevalence of abnormal TWA in a larger sample stratified by history of DM and prior MI. Molon showed that abnormal TWA was related to the presence of DM and to HbA1c level but we were unable to confirm these associations. Although HbA1c levels were similar in both studies, we demonstrated no association between abnormal TWA and HbA1c, duration of diabetes, or the presence of microvascular complications.
There was a high prevalence (22%) of abnormal TWA in our control subjects (group 1: no DM, no MI). Although abnormal TWA has been found in approximately 2–12% of healthy individuals, 15 , 16 our control group comprised patients referred for exercise testing who, as a group, are likely to have a higher prevalence of cardiovascular disease and diabetes than volunteers. In patients with hypertension, abnormal TWA is found in ∼8% of individuals and in ∼33% of patients with both hypertension and left ventricular hypertrophy (LVH). 17 Although we did not perform coronary angiography, oral glucose tolerance testing, or assess echocardiographic LVH in all subjects we speculate that, in addition to between‐study differences in abnormal TWA definitions, occult coronary disease, DM, or LVH in our subjects could partly explain the high prevalence of TWA in our controls.
The use of noninvasive testing to stratify patients with heart disease who may be at risk for sudden cardiac death, is well established, and since the introduction of the implantable defibrillator, this practice has gained additional importance with regard to refining the application of such expensive therapy. TWA is the most recently introduced of such tests and shares with other tests such as signal averaging of the ECG, and heart rate variability, a powerful negative predictive value for arrhythmic events in patients with coronary artery disease, dilated cardiomyopathy, and implanted defibrillators. 18 , 19 , 20 The relationship of inducible TWA in our study with previous MI and reduced left ventricular ejection fraction is consistent with many previous reports.
Our study evaluates TWA in 60 patients with DM—the largest number of such patients studied to date. The patients enrolled are representative of the typical population referred for out‐patient cardiac management, and we expect that these data are relevant to clinical practice. A particular strength of this study is that control subjects without a history of DM or previous MI are included and that data on left ventricular function are available for many patients.
This study is limited by its relatively small sample size and lack of oral glucose tolerance testing; since we relied upon medical records and patient reporting of medical history, we cannot be confident that all subjects without DM were correctly classified. The prevalence of abnormal TWA in our patients with heart disease or diabetes is consistent with previous reports but the prevalence in our control group (group 1) was higher than expected—a finding that could be explained by our use of hospital‐based controls.
In conclusion, we have shown that in this hospital cohort the presence of TWA was related to a history of MI and impaired LV function, but was not strongly related to presence of DM, diabetic complications, or glycemic control. Future studies should enroll large numbers of well‐characterized patients with DM and determine the prognostic role of TWA for sudden and coronary death.
Acknowledgments
Acknowledgment: The authors wish to gratefully acknowledge the support of Jeanne MacDonald and the technical staff of the ECG department at Lahey Clinic.
Funding: Eleanor Dana Research Foundation, New York, NY.
Presented in part at the 43rd Annual Meeting of The European Society for Study of Diabetes, September 17–21, 2007, Amsterdam, The Netherlands (abstract no. 1205).
Conflict of Interest: None declared.
REFERENCES
- 1. El‐Atat FA, McFarlane SI, Sowers JR, et al Sudden cardiac death in patients with diabetes. Curr Diab Rep 2004;4:187–193. [DOI] [PubMed] [Google Scholar]
- 2. Kannel WB, McGee DL. Diabetes and glucose tolerance as risk factors for cardiovascular disease: The Framingham study. Diabetes Care 1979;2:120–126. [DOI] [PubMed] [Google Scholar]
- 3. Kannel WB, Wilson PW, D’Agostino RB, et al Sudden coronary death in women. Am Heart J 1998;136:205–212. [DOI] [PubMed] [Google Scholar]
- 4. Curb JD, Rodriguez BL, Burchfiel CM, et al Sudden death, impaired glucose tolerance, and diabetes in Japanese American men. Circulation 1995;91:2591–2595. [DOI] [PubMed] [Google Scholar]
- 5. Jouven X, Desnos M, Guerot C, et al Predicting sudden death in the population: The Paris Prospective Study I. Circulation 1999;99:1978–1983. [DOI] [PubMed] [Google Scholar]
- 6. Miettinen H, Lehto S, Salomaa V, et al Impact of diabetes on mortality after the first myocardial infarction. The FINMONICA Myocardial Infarction Register Study Group. Diabetes Care 1998;21:69–75. [DOI] [PubMed] [Google Scholar]
- 7. Narayan SM. T‐wave alternans and the susceptibility to ventricular arrhythmias. J Am Coll Cardiol 2006;47:269–281. [DOI] [PubMed] [Google Scholar]
- 8. Ikeda T, Yoshino H, Sugi K, et al Predictive value of microvolt T‐wave alternans for sudden cardiac death in patients with preserved cardiac function after acute myocardial infarction: Results of a collaborative cohort study. J Am Coll Cardiol 2006;48:2268–2274. [DOI] [PubMed] [Google Scholar]
- 9. Molon G, Targher G, Costa A, et al Measurement of microvolt T‐wave alternans, a new arrhythmic risk stratification test, in type 2 diabetic patients without clinical cardiovascular disease. Diabet Med 2006;23:207–210. [DOI] [PubMed] [Google Scholar]
- 10. Molon G, Costa A, Bertolini L, et al Relationship between abnormal microvolt T‐wave alternans and poor glycemic control in type 2 diabetic patients. Pacing Clin Electrophysiol 2007;30:1267–1272. [DOI] [PubMed] [Google Scholar]
- 11. Alpert JS, Thygesen K, Antman E, Bassand JP. Myocardial infarction redefined–a consensus document of The Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction. J Am Coll Cardiol 2000;36:959–969. [DOI] [PubMed] [Google Scholar]
- 12. Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: Diagnosis and Classification of Diabetes Mellitus Provisional Report of a WHO consultation. Diabet Med 1998;15:539–553. [DOI] [PubMed] [Google Scholar]
- 13. Kaufman ES, Bloomfield DM, Steinman RC, et al “Indeterminate” microvolt T‐wave alternans tests predict high risk of death or sustained ventricular arrhythmias in patients with left ventricular dysfunction. J Am Coll Cardiol 2006;48:1399–1404. [DOI] [PubMed] [Google Scholar]
- 14. Bloomfield DM, Hohnloser SH, Cohen RJ. Interpretation and classification of microvolt T wave alternans tests. J Cardiovasc Electrophysiol 2002;13:502–512. [DOI] [PubMed] [Google Scholar]
- 15. Grimm W, Liedtke J, Muller HH. Prevalence of potential noninvasive arrhythmia risk predictors in healthy, middle‐aged persons. Ann Noninvasive Electrocardiol 2003;8:37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Weber S, Tillmanns H, Waldecker B. Prevalence of T wave alternans in healthy subjects. Pacing Clin Electrophysiol 2003;26:49–52. [DOI] [PubMed] [Google Scholar]
- 17. Hennersdorf MG, Niebch V, Perings C, et al T wave alternans and ventricular arrhythmias in arterial hypertension. Hypertension 2001;37:199–203. [DOI] [PubMed] [Google Scholar]
- 18. Bloomfield DM, Steinman RC, Namerow PB, et al Microvolt T‐wave alternans distinguishes between patients likely and patients not likely to benefit from implanted cardiac defibrillator therapy: A solution to the Multicenter Automatic Defibrillator Implantation Trial (MADIT) II conundrum. Circulation 2004;110:1885–1889. [DOI] [PubMed] [Google Scholar]
- 19. Gold MR, Bloomfield DM, Anderson KP, et al A comparison of T‐wave alternans, signal averaged electrocardiography and programmed ventricular stimulation for arrhythmia risk stratification. J Am Coll Cardiol 2000;36:2247–2253. [DOI] [PubMed] [Google Scholar]
- 20. Hohnloser SH, Ikeda T, Bloomfield DM, et al T‐wave alternans negative coronary patients with low ejection and benefit from defibrillator implantation. Lancet 2003;362:125–126. [DOI] [PubMed] [Google Scholar]


