Abstract
Repolarization dynamics and variability are of increasing interest as Holter‐derived parameters reflecting changes in myocardial vulnerability and contributing to increased risk of arrhythmic events and sudden death. Repolarization dynamics is usually defined as phenomenon describing and quantifying QT adaptation to changing heart rate. The analysis of QT–R‐R slopes in long ECG recordings is one of the ways to evaluate repolarization dynamics. Increased QT–R‐R slopes are frequently observed in patients at risk for cardiac death and arrhythmic events: postinfarction patients, long QT syndrome patients, patients with nonischemic cardiomyopathy as well as in patients taking drugs affecting repolarization. QT variability reflects beat‐to‐beat changes in repolarization duration and morphology and such changes can be quantified using a number of algorithms currently in various phases of development and validation. Increased QT variability is observed in several conditions with increased risk of arrhythmias. Recent data from MADIT II indicate that increased QT variability is a powerful predictor of arrhythmic events in postinfarction patients with left ventricular dysfunction. More studies are needed to determine further the potential clinical usefulness for diagnosing patients and for risk stratification purposes using both QT dynamics and QT variability methods, and compare these methods with exercise‐induced T wave alternans.
Keywords: QT dynamics, QT variability, repolarization, risk stratification
The QT interval measured in the ECG is a representation of global repolarization duration in the ventricular myocardium. Repolarization is a complex electrical process governed by a multitude of transmembrane ion currents having different timing, distinct kinetics, and operating in different layers of myocardium. Last decade witnessed major breakthrough in understanding of electrophysiological background of repolarization process, mainly due to identification of genes for the long QT syndrome. 1 Various genetic forms of LQTS revealed involvement of the ion currents in repolarization process: IKs potassium current and min‐K component of this current, IKr potassium current and MiRP1 component of this current, and SCN5A sodium current. Function of these channels is heart rate dependent and therefore, repolarization as a whole process is also heart rate dependent.
Heart rate dependency of repolarization process represents a form of repolarization dynamics appreciated already in 1920s by several researchers including Bazett 2 and Fridericia 3 who described the QT–R‐R relationship and established heart rate correction formulae that are in use to date. Dynamic behavior of repolarization might also be manifested by beat‐to‐beat changes in repolarization duration and morphology. T wave alternans, described for the first time nearly a 100 years ago, 4 is the prime example of repolarization dynamics and in its microvolt form is being increasingly used as a risk marker of arrhythmic events. 5 , 6 , 7 The 2:1 behavior of repolarization changes, typical for T wave alternans, is rarely observed at heart rates below 90–100 bpm. However, non‐2:1 changes in repolarization morphology and duration are observed more often and this so‐called repolarization variability (also known as QT variability or T wave lability), is considered as yet another emerging marker of cardiac events. 8 , 9 , 10
QT–R‐R relationship and QT variability are described in this review together, because they seem to represent somewhat related phenomena reflecting increased vulnerability of myocardium. Increased vulnerability of myocardium together with altered myocardial substrate and changes in autonomic control of the heart constitute conditions contributing to increased risk of cardiac death. 11 Each person shows his/her own individual QT–R‐R relationship, which could be described by the QT–R‐R slope. There is physiologic range of QT variability (Fig. 1) that could be altered by excessive changes in RR interval (tachycardia or bradycardia), by changes in QT duration (ischemia, drugs), or by changes in both parameters simultaneously. 12 Above certain level of the QT–R‐R relationship, there is a likelihood of developing T wave alternans, a form of repolarization variability showing a 2:1 pattern of changes. Both decreased and increased QT duration might predispose to cardiac arrhythmias (in the mechanism of reentry) and therefore, tracking overall QT–R‐R relationship and instantaneous beat‐to‐beat changes in QT interval might help identifying subjects prone to develop cardiac arrhythmias.
Figure 1.
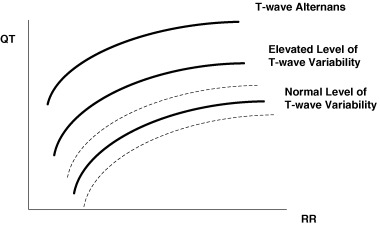
The conceptual link between ECG measures of myocardial vulnerability: the QT–R‐R relationship, T wave (or QT) variability, and T wave alternans (reproduced with permission from Zareba 12 ).
QT–R‐R RELATIONSHIP
The relationship between QT and RR is frequently simplified to a curvilinear relationship represented by Bazett 2 or Fridericia 3 QTc correction formulae. However, Holter recordings demonstrate varying nature of QT–R‐R relationship, frequently described by linear formula as well as by other more complicated equations. The analysis of the slope of linear correlation between QT and RR intervals showed higher values during day than night, steeper slope in females than males. 13 As demonstrated by Malik et al., 14 the QT–R‐R slope is highly individual. This subject‐specific uniqueness is most likely determined by genotypic variations governing heart rate, autonomic response, as well as structure and function of repolarizing currents in myocardium.
Adjustment of QT to changing heart rate is a dynamic phenomenon consisting of fast adaptation phase and slow adaptation phase. 15 Franz et al., 15 showed that after rapid change in heart rate, fast adaptation phase of repolarization usually lasts 30–60 seconds followed by a 2‐minute slow adaptation. This adjustment also seems highly individual and among other factors is dependent on the nature of heart rate changes. Repolarization increases faster to increasing heart rate than it does to decreasing heart rate. This differential response is known as repolarization hysteresis. 16 Measures of repolarization hysteresis are proposed for diagnosing long QT syndrome patients who usually show larger difference in QT duration between exercise and recovery than control subjects. 17
Analyzing just RR interval preceding measured QT is not sufficient to determine dynamics of QT interval. Several beats, usually minutes of RR cycles, influence QT interval duration of given beat. Therefore, to adjust for this time lag, QT–R‐R relationship is increasingly analyzed using RR interval averaged from at least 30–60 seconds preceding tested beat. 18
It is important to realize that QT–R‐R relationship is to major extent governed by changes in autonomic nervous system. Increased vagal tone prolongs repolarization what is observed when monitoring QT during night hours. 19 Autonomic blockade decreases QT–R‐R slope whereas no changes are observed after propranolol given alone, therefore, indicating significant vagal modulation of repolarization. 20
PROGNOSTIC SIGNIFICANCE OF QT–R‐R SLOPE
The association between QT–R‐R slope and cardiac (and arrhythmic) events was evaluated in few studies mostly using retrospective case control design. 21 , 22 The largest one by Chevalier et al. 23 demonstrated that the slope of QT–R‐R relationship, evaluated 9–14 days after index myocardial infarction, has prognostic significance for predicting both total mortality and sudden death in 265 postinfarction patients during a mean 7‐year follow‐up. Steeper slope (>0.18) was associated with a two‐fold increased risk of mortality, indicating that excessive shortening of QT with fast heart rate and/or excessive lengthening of QT with slow heart rates might contribute to arrhythmic events (Fig. 2). The concept of excessive QT shortening with fast heart rates might be supported by the observations that most episodes of tachyarrhythmias leading to sudden death are preceded by sinus tachycardia, 24 and also by the observation that both short‐ and long‐QT intervals found in Holter monitoring are related to sudden death. 25 Facilitation of reentry by shortening of the refractory period might be considered as potential mechanism behind this phenomenon. QT–R‐R slope was tested simultaneously in these patients with clinical variables including ejection fraction, late potentials, and heart rate variability. In univariate analyses, 7‐year all‐cause mortality was predicted by all the above factors. When tested simultaneously in the multivariate Cox model, the slope of QT–R‐R relationship was the most powerful independent predictor of both total and sudden mortality. This observation emphasizes the importance of myocardial vulnerability independently of myocardial dysfunction in the risk stratification process.
Figure 2.
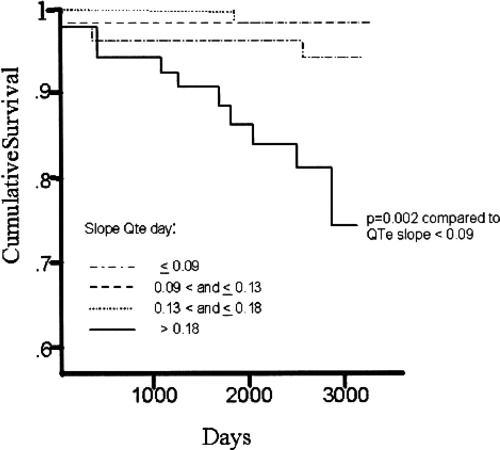
Event‐free curves of sudden death according to daytime slope of QTc/RR plotted using the Kaplan–Meier method (reproduced with permission from Chevalier et al. 23 ).
These observations indicate that QT–R‐R slope might be considered as useful Holter‐derived parameter for identifying high‐risk patients after myocardial infarction. The prognostic significance of QT–R‐R slope in patients with other conditions remains to be determined as well as there is no data regarding whether the analysis of QT–R‐R slope will be redundant with T wave alternans and T wave variability.
Misadaptation of QT to rapid changes in heart rate is most likely the reason for increased number of beats showing substantial QT prolongation in patients with arrhythmic events when compared to controls. Homs et al. 26 showed that number of cardiac beats with QTc exceeding 500 ms in 24‐hour Holter recordings and patients that present these peaks was significantly higher in group of postmyocardial infarction patients with ventricular tachyarrhythmias than in control group (Table 1).
Table 1.
Automatic Corrected QT (QTc) Interval Analysis in Postinfarction Patients with and without Ventricular Tachyarrhythmias (Reproduced with Permission from Homs et al. 26 )
| VT Group (n = 14) | No VT Group (n = 28) | P value | |
|---|---|---|---|
| Total of beats automatically analyzed | 682,960 | 1,276,498 | – |
| Mean QTc interval (ms) | 425 ± 20 | 405 ± 17 | <0.01 |
| Mean QT interval (ms) | 376 ± 33 | 373 ± 30 | NS |
| Mean RR interval (ms) | 780 ± 80 | 830 ± 114 | NS |
| Total number of peaks of QTc >500 ms | 11,112 (1.62%) | 823 (0.06%) | <0.005 |
| Patients with peaks of QTc >500 ms | 7 (50%) | 2 (7%) | <0.005 |
| Patients with grouped peaks (clusters) of QTc >500 ms | 4 (28%) | None | <0.02 |
QT VARIABILITY
Repolarization process is closely correlated with heart rate and therefore, similarly to heart rate remains under a strong influence of the autonomic nervous system. Heart rate variability is a Holter‐based parameter, which provides an insight into the autonomic regulation of the heart. Changes in heart rate and heart rate variability influence changes in QT interval, although QT variability cannot be entirely explained by changes in autonomic nervous system. 9 It is likely that beat‐to‐beat changes in action potential duration are dependent on instantaneous changes in ion channel activity or number of channels involved in repolarization process. Even with stable RR interval there is a possibility of repolarization changes due to variations in number of channels involved. 27 Myocardial conditions (ischemia, fibrosis) might alter number of channels involved in repolarization process and changes in cycle length (RR interval) might further potentate beat‐to‐beat variability of repolarization. T wave alternans is a prime example of such beat‐to‐beat changes.
The association between microvolt T wave alternans and cardiac death and arrhythmic events is well documented in postinfarction patients and patients with nonischemic cardiomyopathy. 5 , 6 , 7 This association illustrates the importance of myocardial vulnerability in arrhythmogenic conditions. However, T wave alternans is mostly evaluated in exercise‐induced conditions since elevated heart rate is needed to induced T wave alternans. Holter recordings are also amenable to detect T wave alternans, although experience with such approach is limited. 28 , 29 As classical 2:1 T wave alternans might not be frequent in standard Holter recordings, there is an increasing interest in the analysis of QT variability in Holter recordings. Several methods quantifying beat‐to‐beat repolarization variability have been developed.
Berger et al. 10 developed a time‐stretching algorithm to quantify changes in repolarization duration and morphology. They found that congestive heart failure have increased variability of repolarization when compared to healthy controls. Three different methods were developed and applied by our Rochester group to quantify repolarization. Burattini and Zareba 30 described repolarization variability method based on cross‐correlation function, also accounting for T wave morphology since unique QT interval changes might not sufficiently illustrate dynamics of repolarization. Increased levels of repolarization variability were observed in coronary patients and long QT syndrome patients in comparison to healthy controls. The wavelet‐based method to measure beat‐to‐beat variability of repolarization amplitude and duration was developed by Couderc et al. 31 who found higher levels of repolarization variability in LQT3 carriers than noncarriers. In the study by Perkiomaki et al. 32 we evaluated repolarization variability quantified by standard deviation of QT and T wave complexity in long QT syndrome patients compared to their unaffected family members. QT variability and T wave complexity variability were increased in the LQTS patients compared with unaffected family members (QT‐SD: 38 ± 20 ms vs 19 ± 7 ms, P = 0.0001; TWC‐SD: 6.7 ± 2.8% vs 4.6 ± 1.8%, P = 0.003, respectively). At the same time, the measures of heart rate variability were similar between the affected and unaffected LQTS subjects.
There is growing number of studies documenting increased repolarization variability in patients with acute myocardial ischemia, 33 left ventricular hypertrophy, 34 hypertrophic cardiomyopathy, 35 left ventricular dysfunction, 10 long QT syndrome, 32 renal failure, 36 , and in patients with anxiety, depression, and panic disorders. 37 , 38 All these conditions predispose to arrhythmic events and increased QT variability might reflect increased myocardial vulnerability to arrhythmias.
PROGNOSTIC SIGNIFICANCE OF QT VARIABILITY
Increased beat‐to‐beat changes in repolarization duration and morphology predisposes to electrical instability of myocardium. Beat‐to‐beat changes in repolarization, increasing heterogeneity of repolarization throughout myocardium, might favor the initiation and maintenance of reentry arrhythmias.
Atiga et al. 39 were first to demonstrate the association between increased levels of QT variability and arrhythmic events. They measured QT variability index (QTVI, adjusting QT variability for heart rate variability) in 95 patients presenting for electrophysiologic study. Sudden cardiac death or sustained monomorphic ventricular tachycardia on presentation and during follow‐up of 23.7 ± 14.3 months was considered as the primary endpoint. Fourteen patients had arrhythmic events during follow‐up. The QTVI was higher in patients with SCD than in other patients with heart disease (0.0 ± 0.6 vs −0.8 ± 0.5, P < 0.05). The QTVI was the only clinical variable that identified patients who presented with SCD in multivariate regression model. In a Kaplan–Meier analysis of arrhythmic events, QTVI greater than or equal to 0.1 was a discriminator for higher risk of arrhythmic events (P < 0.05). It is important to emphasize that QTVI outperformed as predictor the following parameters tested simultaneously in studied patients: QT dispersion, T wave alternans ratio during atrial pacing, VT inducibility, signal‐averaged EGG, heart rate variability, and ejection fraction.
We studied 47 patients with decreased left ventricular function and ICDs who had high‐resolution 10‐minute ECG recordings and were followed for 781 ± 258 days (mean ± SD) on average. 40 The interval from the R peak to the T wave peak with maximum amplitude (RTmax) and from the R peak to the T wave offset (RToff) were determined automatically on a beat‐to‐beat basis. Standard deviation of RTmax and RToff were considered as standard measures of beat‐to‐beat repolarization variability. Nonlinear dynamics measures of variability and complexity included approximate entropy (ApEn) and the short‐term scaling exponent (α1). Eight (17%) patients died and 16 (34%) patients experienced death/appropriate ICD shock during follow‐up. RTmax‐ApEn was significantly higher in patients who died compared with patients who survived (1.24 ± 0.13 vs 1.01 ± 0.21, respectively, P = 0.008). Approximate entropy remained an independent predictor of mortality in ICD patients, however, none of the repolarization parameters was significantly associated with combined endpoint of ICD therapy or death.
Recently, Haigney et al. 41 reported the association between QT variability and arrhythmic events (VT or VF) documented by ICD interrogation in 817 MADIT II patients. In this study, we found that QT variability (unadjusted for heart rate variability) was significantly higher in patients with VT/VF than those without (median QT variability was 0.179 and 0.125, respectively; P = 0.001). Two‐year risk of VT/VF from Kaplan–Meier curves was 40% in highest quartile versus 21% in lower three quartiles for QTVN (Fig. 3). In multivariate Cox regression models adjusting for clinical covariates (race, New York Heart Association functional class, time after myocardial infarction), top‐quartile was independently associated with VT/VF (hazard ratio for QTVN = 2.18; P = 0.002).
Figure 3.
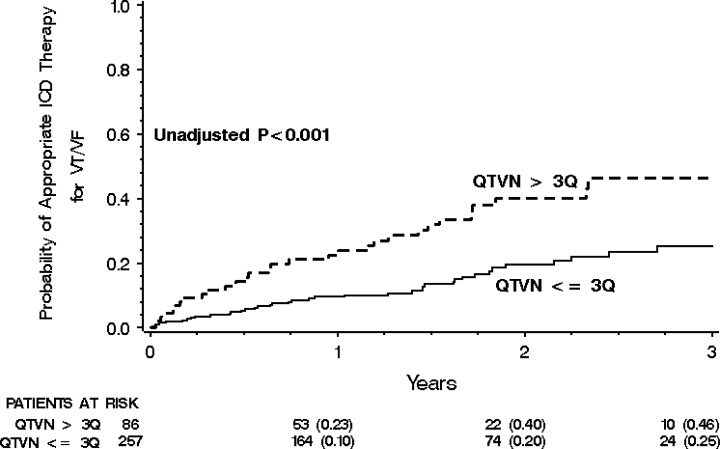
Cumulative probability of first appropriate defibrillator therapy for ventricular tachycardia (VT) or ventricular fibrillation (VF) in patients without bundle branch block with QT variability (QTVN) in the highest quartile versus lower three quartiles (reproduced with permission from Haigney et al. 41 ).
These studies provide evidence for QT variability emerging as important Holter‐derived risk stratifier helping to identify patients at high risk of arrhythmic events. Most likely increased variability of repolarization representing myocardial vulnerability should be analyzed together with clinical and ECG markers describing abnormalities in myocardial substrate to optimize risk stratification. 42 Low ejection fraction, presence of gene mutations causing long QT syndrome or hypertrophic cardiomyopathy, wide QRS complex are important predictors of outcome in respective clinical conditions, and QT variability will refine this risk stratification.
QT–R‐R SLOPE AND QT VARIABILITY IN DRUG STUDIES
All drugs recently introduced to medicine need to undergo scrutiny regarding their potential QT prolonging effect and potential for drug‐induced torsade de pointes. Heart rate correction formulae used for analyzing drug‐induced changes in QT duration might lead to both under‐ and overestimates of real changes, especially if drug changes heart rate. Therefore, there is increasing interest in using QT–R‐R slopes to determine whether there is a significant change in QT duration across different heart rates. 43 , 44 Individual subject‐specific slopes are being derived during drug‐free conditions and serve as subject‐specific reference for evaluating drug testing. 44 Also, there is interest in using QT variability to identify early signs of drug action on repolarization. However, this field requires further studies.
SUMMARY
Dynamic features of repolarization expressed as QT adaptation to changing heart rate and QT variability are coming of age being more and more frequently used to identify patients with increased vulnerability of myocardium. Modern Holter technology supports digital signal processing of repolarization signal with high‐precision enabling dynamic tracking of repolarization duration and morphology. Increased QT–R‐R slope and increased QT variability should remain in focus of clinical research to determine further their potential clinical usefulness for both diagnosing patients and for risk stratification purposes.
REFERENCES
- 1. Kass RS, Moss AJ. Long QT syndrome: Novel insights into the mechanisms of cardiac arrhythmias. J Clin Invest 2003;112: 810–815.DOI: 10.1172/JCI200319844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bazett HC. An analysis of time relations of electrocardiograms. Heart 1920;7: 353–367. [Google Scholar]
- 3. Fridericia LS. Duration of systole in electrocardiogram. Acta Med Scand 1920;53: 469. [Google Scholar]
- 4. Mines GR. On functional analysis by the action of electrolytes. J Physiol 1913;46: 188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rosenbaum DS, Jackson LE, Smith JM, et al Electrical alternans and vulnerability to ventricular arrhythmias. N Engl J Med 1994;330: 235–241.DOI: 10.1056/NEJM199401273300402 [DOI] [PubMed] [Google Scholar]
- 6. Gold MR, Bloomfield DM, Anderson KP, et al A comparison of T‐wave alternans, signal averaged electrocardiography and programmed ventricular stimulation for arrhythmia risk stratification. J Am Coll Cardiol 2000;36: 2247–2253.DOI: 10.1016/S0735-1097(00)01017-2 [DOI] [PubMed] [Google Scholar]
- 7. Nearing BD, Huang AH, Verrier RL. Dynamic tracking of cardiac vulnerability by complex demodulation of the T wave. Science 1991;252: 437–440. [DOI] [PubMed] [Google Scholar]
- 8. Merri M, Moss AJ, Benhorin J, et al Relation between ventricular repolarization duration and cardiac cycle length during 24‐hour Holter recordings: Findings in normal subjects and patients with long QT syndrome. Circulation 1992;85: 1816–1821. [DOI] [PubMed] [Google Scholar]
- 9. Merri M, Alberti M, Moss AJ. Dynamic analysis of ventricular repolarization duration from 24‐hour Holter recordings. IEEE Trans Biomed Eng 1993;40: 1219–1225.DOI: 10.1109/10.250577 [DOI] [PubMed] [Google Scholar]
- 10. Berger RD, Kasper EK, Baughman KL, et al Beat‐to‐beat QT interval variability. Novel evidence for repolarization lability in ischemic and nonischemic dilated cardiomyopthy. Circulation 1997;96: 1557–1565. [DOI] [PubMed] [Google Scholar]
- 11. Bayes‐de‐Luna A, Vinolas X, Guindo J, et al Bayes. Triangle risk stratification after myocardial infarction: Role of electrical instability, ischemia, and left ventricular function. Cardiovasc Drugs Ther 1994;8: 335–343.DOI: 10.1007/BF00877318 [DOI] [PubMed] [Google Scholar]
- 12. Zareba W. QT–RR slope: Dynamics of repolarization in the risk stratification. J Cardiovasc Electrophysiol 2003;14: 234–235. [PubMed] [Google Scholar]
- 13. Extramiana F, Maison‐Blanche P, Badilini F, et al Circadian modulation of QT rate dependence in healthy volunteers: Gender and age differences. J Electrocardiol 1999;32: 33–43.DOI: 10.1016/S0022-0736(99)90019-5 [DOI] [PubMed] [Google Scholar]
- 14. Malik M, Farbom P, Batchvarov V, et al Relation between QT and RR intervals is highly individual among healthy subjects: Implications for heart rate correction of the QT interval. Heart 2002;87: 220–228.DOI: 10.1136/heart.87.3.220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Franz MR, Swerdlow CD, Liem LB, et al Cycle length dependence of human action potential in vivo. Effects of single extrastimuli sudden sustained rate acceleration and deceleration, and different steady state frequencies. J Clin Invest 1988;82: 972–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lau CP, Freedman AR, Fleming S, et al Lau 1988 hysteresis of the ventricular paced QT interval in response to abrupt changes in pacing rate. Cardiovasc Res 1988;22: 67–72. [DOI] [PubMed] [Google Scholar]
- 17. Krahn AD, Klein GJ, Yee R. Hysteresis of the RT interval with exercise: A new marker for the long‐QT syndrome? Circulation 1997;96: 1551–1556. [DOI] [PubMed] [Google Scholar]
- 18. Badilini F, Maison‐Blanche P, Childers R, et al QT interval analysis on ambulatory electrocardiogram recordings: A selective beat averaging approach. Med Biol Eng Comput 1999;37: 71–79. [DOI] [PubMed] [Google Scholar]
- 19. Browne KF, Zipes DP, Heger JJ, et al Prolongation of QT interval in man during sleep. Am J Cardiol 1983;52: 55–59. [DOI] [PubMed] [Google Scholar]
- 20. Cappato R, Alboni P, Codeca L, et al Direct and autonomically mediated effects of oral quinidine on RR/QT relation after an abrupt increase in heart rate. J Am Coll Cardiol 1993;22: 99–105. [DOI] [PubMed] [Google Scholar]
- 21. Brüggemann T, Eisenreich S, Behrens S, et al Continuous QT interval measurements from 24‐hour electrocardiography and risk after myocardial infarction. Ann Noninvasive Electrocardiol 1997;2: 264–273. [Google Scholar]
- 22. Extramiana F, Neyroud N, Huikuri HV, et al QT interval and arrhythmic risk assessment after myocardial infarction. Am J Cardiol 1999;83: 266–269.DOI: 10.1016/S0002-9149(98)00835-2 [DOI] [PubMed] [Google Scholar]
- 23. Chevalier P, Burri H, Adeleine P, et al QT dynamicity and sudden death after myocardial infarction: Results of a long‐term follow‐up study. J Cardiovasc Electrophysiol 2003;14: 227–233. [DOI] [PubMed] [Google Scholar]
- 24. Bayes de Luna, Coumel P, Leclercq JF. Ambulatory sudden death: Mechanisms of production of fatal arrhythmia on the basis of data from 157 cases. Am Heart J 1989;117: 151–159.DOI: 10.1016/0002-8703(89)90670-4 [DOI] [PubMed] [Google Scholar]
- 25. Algra A, Tijssen JGP, Roelandt JRTC, et al QT interval variables from 24 hour electrocardiography and the two year risk of sudden death. Br Heart J 1993;70: 43–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Homs E, Marti V, Offndo J, et al Automatic measurement of corrected QT interval in Holter recordings: Comparison of its dynamic behavior in patients after myocardial infarction with and without life‐threatening arrhythmias. Am Heart J 1997;134: 181–187. [DOI] [PubMed] [Google Scholar]
- 27. Zareba W, Badilini F, Moss AJ. Automatic detection of heterogenous repolarization. J Electrocardiol 1994;27: 65–71. [DOI] [PubMed] [Google Scholar]
- 28. Burattini L, Zareba W, Moss AJ. Correlation method for detection of transient T‐wave alternans in digital Holter ECG recordings. Ann Noninvasive Electrocardiol 1999;4: 416–424. [Google Scholar]
- 29. Verrier RL, Nearing BD, La Rovere MT, et al Ambulatory electrocardiogram‐based tracking of T wave alternans in postmyocardial infarction patients to assess risk of cardiac arrest or arrhythmic death. J Cardiovasc Electrophysiol 2003;14: 705–711. [DOI] [PubMed] [Google Scholar]
- 30. Burattini L, Zareba W. Time‐domain analysis of beat‐to‐beat variability of repolarization morphology in patients with ischemic cardiomyopathy. J Electrocardiol 1999;32(Suppl.):166–172. [DOI] [PubMed] [Google Scholar]
- 31. Couderc JP, Zareba W, Burattini L, et al Beat‐to‐beat repolarization variability in LQTS patients with the SCN5A sodium channel gene mutation. PACE 1999;22: 1581–1592. [DOI] [PubMed] [Google Scholar]
- 32. Perkiomaki JS, Zareba W, Nomura A, et al Repolarization dynamics in patients with long QT syndrome. J Cardiovasc Electrophysiol 2002;14: 651–656.DOI: 10.1046/j.1540-8167.2002.00651.x [DOI] [PubMed] [Google Scholar]
- 33. Bonnemeier H, Hartmann F, Wiegand UK, et al Course and prognostic implications of QT interval and QT interval variability after primary coronary angioplasty in acute myocardial infarction. J Am Coll Cardiol 2001;37: 44–50. [DOI] [PubMed] [Google Scholar]
- 34. Piccirillo G, Germano G, Quaglione R, et al QT‐interval variability and autonomic control in hypertensive subjects with left ventricular hypertrophy. Clin Sci (Lond) 2002;102: 363–371. [PubMed] [Google Scholar]
- 35. Cuomo S, Marciano F, Migaux ML, et al Abnormal QT interval variability in patients with hypertrophic cardiomyopathy: Can syncope be predicted? J Electrocardiol 2004;37: 113–119. [DOI] [PubMed] [Google Scholar]
- 36. Johansson M, Gao SA, Friberg P, et al Elevated temporal QT variability index in patients with chronic renal failure. Clin Sci (Lond) 2004;107: 583–588. [DOI] [PubMed] [Google Scholar]
- 37. Piccirillo G, Cacciafesta M, Lionetti M, et al Influence of age, the autonomic nervous system and anxiety on QT‐interval variability. Clin Sci (Lond) 2001;101: 429–438. [PubMed] [Google Scholar]
- 38. Carney RM, Freedland KE, Stein PK, et al Effects of depression on QT interval variability after myocardial infarction. Psychosom Med 2003;65: 177–180. [DOI] [PubMed] [Google Scholar]
- 39. Atiga WL, Calkins H, Lawrence JH, et al Beat‐to‐beat repolarization lability identifies patients at risk for sudden cardiac death. J Cardiovasc Electrophysiol 1998;9: 899–908. [DOI] [PubMed] [Google Scholar]
- 40. Perkiomaki JS, Couderc JP, Daubert JP, et al Temporal complexity of repolarization and mortality in patients with implantable cardioverter defibrillators. PACE 2003;26: 1931–1936. [DOI] [PubMed] [Google Scholar]
- 41. Haigney MC, Zareba W, Gentlesk PJ, et al QT interval variability and spontaneous ventricular tachycardia or fibrillation in MADIT II patients. J Am Coll Cardiol 2004;44: 1481–1487.DOI: 10.1016/j.jacc.2004.06.063 [DOI] [PubMed] [Google Scholar]
- 42. Kudaiberdieva G, Gorenek B, Goktekin O, et al Combination of QT variability and signal‐averaged electrocardiography in association with ventricular tachycardia in postinfarction patients. J Electrocardiol 2003;36: 17–24.DOI: 10.1054/jelc.2003.50003 [DOI] [PubMed] [Google Scholar]
- 43. Zareba W, Moss AJ, Rosero SZ, et al Electrocardiographic findings in patients with diphenhydramine overdose. Am J Cardiol 1997;80: 1168–1173.DOI: 10.1016/S0002-9149(97)00634-6 [DOI] [PubMed] [Google Scholar]
- 44. Malik M, Camm AJ. Evaluation of drug‐induced QT interval prolongation: Implications for drug approval and labelling. Drug Saf 2001;24: 323–351. [DOI] [PubMed] [Google Scholar]


