Abstract
Background: Abnormalities in the electromagnetic signal of the atria during sinus rhythm could serve as markers of triggering foci or substrate for atrial fibrillation (AF). We examined atrial electrophysiologic properties noninvasively by using magnetocardiographic mapping (MCG) in patients with paroxysmal lone AF to find whether any difference exists between those who have frequent triggers of AF and who don't.
Methods: MCG was recorded over anterior chest during sinus rhythm in 80 patients with paroxysmal lone AF (44 ± 12 years, 61 males) and 80 matched controls. Atrial wave duration (Pd) and root mean square amplitudes of the last 40 ms (RMS40) of the averaged filtered atrial complex were determined automatically. Patients expressing atrial arrhythmias triggering AF episodes were classified as focal AF.
Results: The Pd was 109 ms in patients and 104 ms in controls (P = 0.007). In focal AF (72%) the Pd was slightly prolonged and its proportion of the PR interval was larger, but RMS40 was normal compared to controls. In other patients, the Pd was close to controls, but the RMS40 was reduced (59 ± 17 vs74 ± 36 fT, P = 0.006). Pd and atrial RMS amplitudes were unrelated to duration of AF history or frequency of recurrences.
Conclusion: Clinical subclasses of lone AF seem to possess distinct signal profiles of atrial depolarization. Differences in electrophysiological properties between these subclasses may reflect pathogenetic variation and could have implications on diagnostics and therapy.
Keywords: atrial fibrillation, signal analysis, magnetocardiography
Atrial fibrillation (AF), although commonly associated with cardiovascular disorders, occurs also without any evidence of heart disease. This condition, called lone AF, is frequent among patients with onset of AF before middle age 1 and it presents usually as paroxysmal. 2 AF episodes are often initiated by fast repeating premature atrial complexes (PACs) originating from the junction between the left atrium and the pulmonary veins. 3 Sustained trigger activity can maintain AF, but altered atrial substrates like areas of disorganized activation, can also be crucial for perpetuation of AF. 3 , 4
Abnormalities in atrial electromagnetic signals could serve as markers of triggering foci or the substrate facilitating the initiation of AF. Signal‐averaged electrocardiography (SAECG) can detect abnormalities in atrial signal in patients prone to AF. These include prolongation of atrial depolarization, spatial difference in signal duration, and abnormal frequency content of the atrial signal. 5 , 6 , 7 High‐resolution magnetocardiography (MCG) is a noninvasive method complementary to electrocardiography to study cardiac electromagnetic activity and it has been accurate in detecting ventricular arrhythmia substrate. 8 , 9 , 10
We aimed to explore atrial electrophysiologic properties noninvasively by using MCG mapping in patients with paroxysmal lone AF, particularly to find whether any difference exists between those who have frequent triggers of AF and who don't.
METHODS
Study Population
The study population consisted of patients with paroxysmal AF and of age‐ and gender‐matched controls. Patients were screened from those referred to a tertiary hospital due to symptomatic paroxysmal AF. Included were 80 consecutive patients aged below 65 years and without structural or other heart diseases in clinical, ECG, and cardiac ultrasound examination. Borderline arterial hypertension without left ventricular hypertrophy was allowed. Clinical arrhythmia history was collected from patients' interview and medical records.
Measurements were carried out during sinus rhythm. Any class I and III antiarrhythmic medication was discontinued at least five drug half‐lives before examination, but in severely symptomatic patients use of β‐adrenergic antagonists was allowed. None of the patients had been on amiodarone.
All patients underwent ambulatory electrocardiographic monitoring for 24 or 48 hours. The number of PACs/hour and presence of early (P‐on‐T) PACs and atrial bigeminy were analyzed. AF was defined focally triggered if it was initiated by frequent early PACs or rapid atrial tachycardia at a rate of >200 beats/minute. In the absence of AF in the recording, AF was considered also focally triggered, if there were repeated episodes of fast atrial tachycardia or uncommon flutter.
Data regarding arrhythmia history were collected from patient interview and medical records. To describe the frequency of AF recurrences the average number of arrhythmia episodes over the past year was assessed from patients' reports and medical records.
Echocardiographic examination was performed using standard views and techniques according to the guidelines of the American Society of Echocardiography. The left ventricular end diastolic diameter (LVEDD), ejection fraction (EF) and left atrial anteroposterior diameter (LAD) were determined in the parasternal long‐axis view from two‐dimensional targeted M mode tracings.
The study was approved by the Ethical Review Board of the institute and a written informed consent was obtained from all participants.
MCG and Signal‐Averaged ECG
High‐resolution MCG signals were recorded for 7 minutes over anterior chest using a 33 triple sensor unit array as described previously. 11 Signals with a 1000 Hz sampling frequency were band‐pass filtered to 0.03–300 Hz and atrial P wave and the QRS‐T complex were averaged separately using maximum cross‐correlation with the user‐selected templates. 8 , 11
The onset and end of atrial and QRS complexes were determined automatically after 40 Hz high‐pass filtering. 8 , 11 The duration of atrial activation (Pd), QRS complex duration (QRSd), and the PR interval were calculated using the medians of onset and end times in all averaged channels as described earlier. 8 , 11 The ratios Pd/PR and Pd/QRSd were calculated. 12 , 13 In addition, the root mean square (RMS) amplitudes of the magnetic field strength over the whole atrial complex and last 40 ms of atrial depolarization were automatically determined. The mean amplitudes in all included channels were used in further analysis. 8 , 11 Reproducibility of the measures has previously been assessed and found similar in AF patients and controls and somewhat better by MCG than by ECG. 11 By MCG the difference between two measurements of atrial signal duration was 3.5 ms on average and the coefficient of variation was 3.3%.
The end and the apex of the T wave were automatically determined from nonfiltered data. 9 The QT interval (from QRS onset to T‐wave end) and the time from T‐wave peak to T‐wave end (T apex–T end interval) were calculated and the QT interval was corrected for heart rate by Bazett's equation. The heart rate was obtained as a mean of the R‐R intervals preceding the averaged complexes. The fragmentation of the atrial complex was examined by computing the number of polarity changes in binomial filtered atrial complex and expressed as index M and score S by the method previously applied for QRS complex. 10
A 3‐lead orthogonal ECG was registered simultaneously with MCG. 11 Disposable Ag/AgCl electrodes were used. The ECG data were analyzed using XYZ magnitude complex and methods analogous to those applied in MCG. 8 , 11
Statistical Analysis
Continuous data are expressed as mean ± SD and categorical variables as numbers and proportion of positive cases in groups. Differences between the groups were examined using Student's t‐test for continuous and the chi‐square test for discrete variables. Pearson's correlation coefficient was used to study the relationship of continuous variables. A two‐tailed P value < 0.05 was considered statistically significant.
RESULTS
Clinical Characteristics
One hundred sixty subjects were enrolled, 80 patients and 80 age‐ and gender‐matched controls. There were 61 men and 19 women in both groups. The age, height, weight, body mass index, and presence of arterial hypertension did not differ between the groups.
Men were younger than women and also had onset of symptoms and confirmed AF diagnosis at earlier age (Table 1). The duration of AF history was similar in both genders. In all AF had occurred as recurrent, mostly self‐terminating episodes. Majority had AF episodes at least once a week. An episode had lasted >24 hours in 21% and >1 week in 10% of the patients. Electrical cardioversion had been performed in 34% of patients.
Table 1.
Clinical Characteristics of the Paroxysmal Lone AF Patient Cohort and Subgroups According to Gender
| All Patients | Female | Male | P Value | |
|---|---|---|---|---|
| Number | 80 | 19 (24) | 61 (76) | |
| Age at study (years) | 44 ± 12 | 53 ± 8 | 41 ± 12 | <0.001 |
| Height (cm) | 179 ± 8 | 169 ± 7 | 182 ± 6 | <0.001 |
| Weight (kg) | 80 ± 13 | 68 ± 13 | 84 ± 11 | <0.001 |
| BMI | 25 ± 3 | 24 ± 4 | 25 ± 3 | n.s. |
| LVEDD (mm) | 50 ± 5 | 47 ± 5 | 51 ± 4 | 0.004 |
| LVEF (%) | 64 ± 6 | 65 ± 7 | 64 ± 6 | n.s. |
| Left atrial diameter (mm) | 36 ± 5 | 36 ± 3 | 36 ± 5 | n.s. |
| Hypertension | 8 (10) | 3 (16) | 5 (8) | n.s. |
| Beta‐blocker in use | 19 (24) | 4 (21) | 15 (25) | n.s. |
| Age at first confirmed AF episode | 40 ± 12 | 50 ± 8 | 37 ± 11 | <0.001 |
| Age at onset of AF symptoms | 35 ± 13 | 43 ± 13 | 32 ± 12 | 0.006 |
| AF episode frequency | ||||
| >1/week | 44 (55) | 10 (53) | 34 (56) | n.s. |
| 1 /week–1/month | 24 (30) | 7 (37) | 17 (28) | n.s |
| <1/month | 12 (15) | 2 (10) | 10 (16) | n.s. |
| AF episodes lasting >24 hour | 17 (21) | 3 (16) | 14 (23) | n.s. |
| Frequent AF triggers | 58 (72) | 15 (79) | 43 (70) | n.s. |
The values represent the number (percentage in parentheses) of study subjects or mean ± SD. Student's t‐test and chi‐square test were used for statistics.
AF = atrial fibrillation; BMI = weight (kg) divided by height (m)2; HR = heart rate; LVEDD = left ventricular end diastolic diameter; LVEF = left ventricular ejection fraction.
In 72% of the patients AF was classified as focally triggered by observations in ambulatory ECG or telemetry monitoring (Table 1). The number of PACs was 46 ± 85 complexes/ hour in them, whereas the number of PACs was 1 ± 1 complexes/hour in other AF patients. Of the patients 74% has used a β‐adrenergic blocker, 44% a class I agent, 16% sotalol, and 4% a calcium channel blocker. Only one patient had used amiodarone, which was discontinued 1 year before study.
MCG Measurements
Duration of Atrial Depolarization
The results of MCG analysis are shown in Table 2. The duration of the filtered atrial wave (Pd) was slightly longer in patients than in controls. In women the difference was obvious, 108 ± 11 versus 98 ± 6 ms (P < 0.001), but a trend to longer Pd, 110 ± 12 versus 106 ± 12 ms, was not significant in men. The proportion of the atrial signal from the PR interval (Pd/PR ratio) as well as to QRS duration (Pd/QRSd ratio) was larger in patients (Table 2).
Table 2.
MCG Signal Measures in Patients with Paroxysmal Lone AF and in Controls
| AF Patients n = 80 | Controls n = 80 | P Value | |
|---|---|---|---|
| Heart rate (beats/minute) | 60 ± 10 | 61 ± 8 | n.s. |
| PR interval (ms) | 156 ± 23 | 159 ± 20 | n.s. |
| QRS duration (ms) | 100 ± 10 | 103 ± 10 | n.s. |
| QRS RMS (fT) | 1100 ± 400 | 1300 ± 590 | n.s. |
| QTc (ms) | 401 ± 23 | 396 ± 20 | n.s. |
| T apex – T end interval (ms) | 79 ± 10 | 76 ± 10 | n.s. |
| P duration (ms) | 109 ± 11 | 104 ± 12 | 0.007 |
| Pd/PR ratio | 0.71 ± 0.08 | 0.66 ± 0.09 | <0.001 |
| Pd/QRSd ratio | 1.10 ± 0.15 | 1.01 ± 0.12 | <0.001 |
| P RMS (fT) | 93 ± 35 | 91 ± 36 | n.s. |
| P RMS40 (fT) | 75 ± 29 | 74 ± 36 | n.s. |
| Fragmentation index M | 9.5 ± 1.7 | 9.5 ± 1.7 | n.s. |
| Fragmentation score S | 73 ± 21 | 72 ± 19 | n.s. |
The values represent the group mean ± SD. Student's t‐test was used.
AF = atrial fibrillation; fT = 10−15 Tesla; Pd = duration of filtered atrial signal; P RMS = RMS over whole atrial complex; P RMS40 = RMS over last 40 ms of atrial complex; QTc = QT value corrected by Bazett's formula; RMS = root mean square amplitude.
Atrial RMS Amplitudes and Fragmentation Analysis
The late field RMS amplitudes of 40 Hz high‐pass filtered atrial complex did not differ between patients and controls, shown in Table 2. The Pd was longer and RMS amplitudes were larger in men compared to women in controls (Pd 106 ± 12 vs 98 ± 6 ms, P < 0.001, PRMS 96 ± 36 vs 73 ± 31 fT, P = 0.009). In AF patients the variables did not differ between genders (110 ± 12 vs 108 ± 11 ms, 96 ± 34 vs 83 ± 36 fT, P = n.s.). There was no difference in fragmentation analysis between patients and controls.
PR Interval, QRS Complex, and QT Interval
The PR interval was similar in patients and controls (Table 2), but tended to be longer in men than in women. The PR interval was >200 ms in three patients (range 216–236 ms) and in one control (209 ms). The QRS duration and the QT interval variables were similar in patients and controls (Table 2). The heart‐rate corrected QT interval exceeded 450 ms in three patients and two controls (P = n.s.). The T apex – T end interval was longer in patients than in controls in women, 77 ± 9 versus 70 ± 5 ms (P = 0.01) on average, but not in men.
SAECG Measurements
In SAECG there were no differences in the atrial signal or QRSd between patients and controls (Pd 115 ± 11 vs 113 ± 14 ms and QRSd 100 ± 11 vs 103 ± 10, P = n.s.). Parallel to MCG findings, the Pd/PR and Pd/QRSd ratios were larger in patients (0.73 ± 0.08 vs 0.69 ± 0.09, P = 0.02 and 1.15 ± 0.15 vs 1.10 ± 0.15, P = 0.02).
Relation of MCG Measures to Echocardiography and AF History
The Pd did not correlate with LA diameter (r = 0.14, P = n.s.). The QRS duration and LVEDD showed a positive correlation (r = 0.34, P < 0.01) and their magnitudes were related to body size and were more pronounced in men. In patients Pd did not correlate with height or weight (r = 0.18 and r = 0.12, respectively, n.s.). The length of AF history did not correlate with P‐wave duration, LA diameter (Fig. 1), or atrial RMS amplitudes. None of these parameters differed between the patients who had AF episodes at least once a week and those with less frequent episodes.
Figure 1.
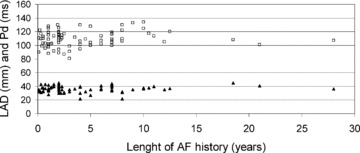
Relation of length of documented AF history to diameter of left atrium (LAD) and to P‐wave duration (Pd). □= Pd; ▴= LAD. Pearson's correlation coefficient was used for statistics; Pd versus length of AF history r = 0.14, P = n.s., LAD versus length of AF history r = 0.19, P = n.s.
Focal AF versus Nonfocal AF
Characteristics and signal measures in patient subgroups divided to focal and nonfocal AF are shown in Table 3. Compared to controls, patients with focal AF had longer Pd, larger Pd/PR and Pd/QRSd ratios and longer T apex – T end interval. Patients also had normal atrial RMS amplitudes. In nonfocal type of AF, Pd did not differ from that of controls. Their late atrial RMS amplitudes were lower than those in patients with focal AF or in controls (Fig. 2). The onset of AF was at an earlier age in the nonfocal subset (Table 3).
Table 3.
Clinical Characteristics and MCG Signal Measures in Patients with Focal AF and Nonfocal AF
| Focal AF n = 58 (72) | Nonfocal AF n = 22 (28) | P Value | |
|---|---|---|---|
| Males | 43 (74) | 18 (82) | n.s. |
| Age at time of AF diagnosis | 42 ± 10 | 33 ± 13 | 0.005 |
| AF episode frequency | |||
| > once a week | 43 (73) | 1 (4.5) | <0.001 |
| 1/week–1/month | 14 (24) | 10 (45) | |
| Heart rate (beats/min) | 58 ± 10 | 64 ± 7 | 0.004 |
| PR interval (ms) | 155 ± 23 | 158 ± 20 | n.s. |
| QRS duration (ms) | 99 ± 11* | 102 ± 10 | n.s. |
| QTc (ms) | 401 ± 25 | 399 ± 17 | n.s. |
| T apex – T end interval (ms) | 80 ± 10* | 76 ± 11 | n.s. |
| P duration (ms) | 110 ± 12** | 108 ± 9 | n.s. |
| Pd/PR ratio | 0.71 ± 0.08*** | 0.69 ± 0.07 | n.s. |
| Pd/QRSd ratio | 1.12 ± 0.15*** | 1.07 ± 0.13 | n.s. |
| P RMS (fT) | 98 ± 37 | 78 ± 21* | 0.003 |
| P RMS first 40 (fT) | 71 ± 27 | 72 ± 22 | n.s. |
| P RMS40 (fT) | 81 ± 31 | 59 ± 17** | <0.001 |
The values represent the number (percentage in parentheses) of study subjects or mean ± SD. Student's t‐test and chi‐square test were used for statistics.
*= P < 0.05, **= P < 0.01, ***= P < 0.001 comparing patient group to control group. Abbreviations as in Table 2.
Figure 2.
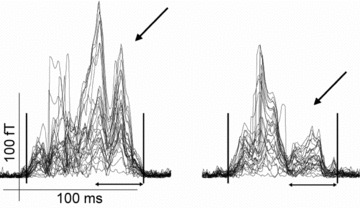
The 40 Hz high‐pass filtered atrial complex in a patient with focal AF (left) and in a patient with nonfocal AF (right). In focal AF the atrial signal strength is normal also during late phase of atrial complex when the left atrium is depolarized, but in nonfocal AF late phase amplitudes are reduced. Data are expressed as superimposed display of 33 magnetometer channels. The automatically determined onset and end of atrial complex are indicated with vertical bars.
DISCUSSION
Main Findings
In this cohort of patients with lone paroxysmal AF the duration of atrial depolarization complex was marginally prolonged. The difference was remarkable in women, but not significant in men. In focal type of AF the atrial RMS amplitudes were normal, but in AF without demonstrable triggers the late atrial RMS amplitudes were reduced. This study demonstrates also that in paroxysmal lone AF the atrial characteristics tend to remain similar, not showing obvious disease progression, even several years from the first AF episodes.
Clinical Characteristics
A majority of our patients (76%) were men, which seems to be explained by earlier presentation of AF in men than in women. In general population the incidence of AF has been reported similar in men and women, 14 but lone AF and AF appearing at early age have been more common in men. 2 , 15 A male predominance is also reflected in patient cohorts undergoing catheter ablation therapy of paroxysmal AF. 16 , 17 Our observation suggests that AF without demonstrable focal triggers starts earlier than focal type of AF.
Signal Patterns
In this study the duration of atrial depolarization was found only slightly prolonged in paroxysmal lone AF. The duration of atrial depolarization, on average 109 ms, is consistent with invasively measured duration of atrial depolarization in patients with lone paroxysmal AF. 18 The commonly reported remarkable prolongation of atrial wave in SAECG 6 , 7 seems to reflect heterogeneous etiologies of AF in the cohorts studied.
In contrast to some earlier SAECG studies, 6 , 7 we found the atrial filtered signal amplitudes in lone AF patients mostly normal. Yet in patients without demonstrable focal triggers the signal amplitudes of late atrial complex, when the left atrium is depolarizing, were reduced. This conforms to invasive measurements in patients undergoing catheter ablation of AF in which only a minority shows reduced signal amplitudes in the left atrium. 19
Our study could not show any common, noninvasively measured abnormality in atrioventricular conduction or in ventricular depolarization or repolarization in patients with paroxysmal lone AF. This supports that the abnormal conduction is confined to the atria only and is not a generalized feature of the cardiac tissues. However, there was a trend toward a longer T apex – T end interval in patients, particularly women. Prolongation of T apex – T end interval has been related to ventricular arrhythmias in patients with cardiomyopathy and genetic sarcolemmal ion channel abnormalities, some of which are also involved in pathogenesis of AF. 20 , 21 , 22 , 23
In this study, as well as some earlier AF studies, 11 , 24 there was a trend toward similar signal characteristics by the ECG and MCG techniques, but the differences between AF patients and controls were more obvious by MCG. The MCG has been accurate also in detecting ventricular arrhythmia substrate even when SAECG findings have been normal. 8 , 9 , 10 The advantages of MCG include sensitivity to currents tangential to chest, like most currents in the atrial walls are, and adequate signal to noise ratio. 25 Thus MCG may reveal abnormalities not detectable by ECG techniques explored thus far.
Relation to Characteristics of AF
This study showed that in women with paroxysmal lone AF the atrial depolarization signal is prolonged compared to healthy women, but such difference is not seen in men. This may be related to gender differences in presentation of AF, for example, normal female atria may be less vulnerable to AF unless an additional pathologic process develops.
In this study, a majority of the patients were classified as having focally triggered paroxysmal lone AF in line with earlier reports. 3 , 26 There is no common definition to focal AF and, indeed, PACs may serve as initiators in any case of paroxysmal AF. However, we aimed to separate patients who have frequent occurrence of triggering arrhythmias from those who have not. In them more triggers are required to initiate AF whereas in other patients the atria are more vulnerable and less triggers are sufficient for initiation of AF, as speculated also earlier. 4
Characteristic to focal AF seems to be frequent AF episodes, only marginal if any prolongation in atrial depolarization time and preserved atrial signal amplitudes. Yet, in nonfocal AF the left atrial depolarization signal is reduced. This may reflect altered, arrhythmogenic substrate in left atrium. That might be due to inflammation, cardiomyopathy or fibrosis, which have been observed histologically in lone AF. 27 , 28 The results raise a possibility that the atrial signal patterns could be used to assess the presence or absence of active focal triggers.
Our patients with most frequent AF episodes or longest AF history did not show more atrial signal modification. Selection bias may have excluded patients who had progression to permanent arrhythmia, but the results nevertheless indicate that paroxysmal AF does not necessarily lead to electrical or mechanical dysfunction of the atria. This is in line with prospective observation that progression from paroxysmal to permanent form is less common in lone AF compared to AF in heart disease. 14
LIMITATIONS
Since there was age difference between women and men, a contribution of age to cardiac signal may have confounded the results. However, the duration of arrhythmia history was similar in both genders, and also the controls were matched for age and gender. The observations may not be applicable to more general lone AF patient cohorts due to selection of highly symptomatic patients with frequent episodes referred to a tertiary care center. The measures of length of AF history and frequency of symptomatic AF paroxysms are only approximations due to the necessity to rely on retrospective data. The definition of focally triggered AF was based on 1–2 day's ECG recording that may not be reproducible to allow firm characterization of an individual patient. Relationship of atrial signal characteristics to the success of ablation therapy could not be assessed since only part of the patients underwent catheter ablation.
CONCLUSIONS
In general, in paroxysmal lone AF, active focal triggers are common, atrial depolarization is slightly prolonged and the depolarization amplitude is normal. However, a significant minority of patients lacks frequent focal triggers and in them the late atrial signal amplitude is reduced, signifying possibly a wider degenerative process in the left atrium. In the latter patients, the atria may be more vulnerable and fibrillate with less provocation.
Conflict of interest: none declared
Acknowledgment: This study was supported by a grant from the Finnish Cardiac Research Foundation; Helsinki, Finland.
REFERENCES
- 1. Levý S, Marek M, Coumel P, et al Characterization of different subsets of atrial fibrillation in general practice in France. The ALFA study. Circulation 1999;99:3028–3035. [DOI] [PubMed] [Google Scholar]
- 2. Patton KK, Zacks ES, Chang JY, et al Clinical subtypes of lone atrial fibrillation. Pacing Clin Electrophysiol 2005;28:630–638. [DOI] [PubMed] [Google Scholar]
- 3. Haissaguerre M, Jais P, Shah DC, et al Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med 1998;339:659–666. [DOI] [PubMed] [Google Scholar]
- 4. Pachon MJ, Pachon ME, Pachon MJ, et al A new treatment for atrial fibrillation based on spectral analysis to guide the catheter RF‐ablation. Europace 2004;6:590–601. [DOI] [PubMed] [Google Scholar]
- 5. Dilaveris PE, Gialafos JE. P‐wave dispersion: A novel predictor of paroxysmal atrial fibrillation. Ann Noninvasive Electrocardiol 2001;6:159–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fukunami M, Yamada T, Ohmori M, et al Detection of patients at risk for paroxysmal atrial fibrillation during sinus rhythm by P wave‐triggered signal‐averaged electrocardiogram. Circulation 1991;83:162–169. [DOI] [PubMed] [Google Scholar]
- 7. Abe Y, Fukunami M, Yamada T, et al Prediction of transition to chronic atrial fibrillation in patients with paroxysmal atrial fibrillation by signal‐averaged electrocardiography: A prospective study. Circulation 1997;96:2612–2616. [DOI] [PubMed] [Google Scholar]
- 8. Korhonen P, Montonen J, Mäkijärvi M, et al Late fields of the magnetocardiographic QRS complex as indicators of propensity to sustained ventricular tachycardia after myocardial infarction. J Cardiovasc Electrophysiol 2000;11:413–420. [DOI] [PubMed] [Google Scholar]
- 9. Oikarinen L, Paavola M, Montonen J, et al Magnetocardiographic QT interval dispersion in postmyocardial infarction patients with sustained ventricular tachycardia: Validation of automated QT measurements. Pacing Clin Electrophysiol 1998;21:1934–1942. [DOI] [PubMed] [Google Scholar]
- 10. Korhonen P, Montonen J, Endt P, et al Magnetocardiographic intra‐QRS fragmentation analysis in the identification of patients with sustained ventricular tachycardia after myocardial infarction. Pacing Clin Electrophysiol 2001;24:1179–1186. [DOI] [PubMed] [Google Scholar]
- 11. Koskinen R, Lehto M, Väänänen H, et al Measurement and reproducibility of magnetocardiographic filtered atrial signal in patients with lone atrial fibrillation and in healthy subjects. J Electrocardiol 2005;38:330–336. [DOI] [PubMed] [Google Scholar]
- 12. Chirife R, Feitosa GS, Frankl WS. Electrocardiographic detection of left atrial enlargement. Correlation of P wave with left atrial dimension by echocardiography. Br Heart J 1975;37:1281–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Scott C, Leier V, Kilman W, et al The effect of left atrial histology and dimension on P wave morphology. J Electrocardiol 1983;16:363–366. [DOI] [PubMed] [Google Scholar]
- 14. Ruigomez A, Johansson S, Wallander MA. Garcia Rodriguez LA. Predictors and prognosis of paroxysmal atrial fibrillation in general practice in the UK. BMC Cardiovasc Disord 2005;5:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kato T, Yamashita T, Sagara K, et al Progressive nature of paroxysmal atrial fibrillation. Observations from a 14‐year follow‐up study. Circ J 2004;68:568–572. [DOI] [PubMed] [Google Scholar]
- 16. Oral H, Chugh A, Scharf C, et al Pulmonary vein isolation for vagotonic, adrenergic, and random episodes of paroxysmal atrial fibrillation. J Cardiovasc Electrophysiol 2004;15:402–406. [DOI] [PubMed] [Google Scholar]
- 17. Pappone C, Oreto G, Rosanio S, et al Atrial electroanatomic remodeling after circumferential radiofrequency pulmonary vein ablation: Efficacy of an anatomic approach in a large cohort of patients with atrial fibrillation. Circulation 2001;104:2539–2544. [DOI] [PubMed] [Google Scholar]
- 18. Lemery R, Soucie L, Martin B, et al Human study of biatrial electrical coupling: Determinants of endocardial septal activation and conduction over interatrial connections. Circulation 2004;110:2083–2089. [DOI] [PubMed] [Google Scholar]
- 19. Verma A, Wazni OM, Marrouche NF, et al Pre‐existent left atrial scarring in patients undergoing pulmonary vein antrum isolation: An independent predictor of procedural failure. J Am Coll Cardiol 2005;45:285–292. [DOI] [PubMed] [Google Scholar]
- 20. Korhonen P, Väänänen H, Mäkijärvi M, et al Repolarization abnormalities detected by magnetocardiography in patients with dilated cardiomyopathy and ventricular arrhytmias. J Cardiovasc Electrophysiol 2001;12:772–777. [DOI] [PubMed] [Google Scholar]
- 21. Fatkin D, MacRae C, Sasaki T, et al Missense mutations in the rod domain of the lamin A/C gene as causes of dilated cardiomyopathy and conduction system disease. N Engl J Med 1999;341:1715–1724. [DOI] [PubMed] [Google Scholar]
- 22. Schwartz PJ, Priori SG, Spazzolini C, et al Genotype‐phenotype correlation in the long QT syndrome: Gene specific triggers for life‐threatening arrhythmias. Circulation 2001;103:89–95. [DOI] [PubMed] [Google Scholar]
- 23. Chen YH, Xu SJ, Bendahhou S, et al KCNQ1 gain‐of‐function mutation in familial atrial fibrillation. Science 2003;299:251–254. [DOI] [PubMed] [Google Scholar]
- 24. Winklmaier M, Pohle C, Achenbach S, et al P‐wave analysis in MCG and ECG after conversion of atrial fibrillation. Biomed Tech (Berl) 1998;43(Suppl):250–251. [DOI] [PubMed] [Google Scholar]
- 25. Baule M, McFee R. The magnetic heart vector. Am Heart J 1970;79:223. [DOI] [PubMed] [Google Scholar]
- 26. Todd M, Fynn P, Hobbs J, et al Prevalence and significance of focal sources of atrial arrhythmia in patients undergoing cardioversion of persistent atrial fibrillation. J Cardiovasc Electrophysiol 2000;11:616–622. [DOI] [PubMed] [Google Scholar]
- 27. Frustaci A, Chimenti, Bellocci F, et al Histological substrate of atrial biopsies in patients with lone atrial fibrillation. Circulation 1997;96:1180–1184. [DOI] [PubMed] [Google Scholar]
- 28. Boldt A, Wetzel U, Lauschke J, et al Fibrosis in left atrial tissue of patients with atrial fibrillation with and without underlying mitral valve disease. Heart 2004;90:400–405. [DOI] [PMC free article] [PubMed] [Google Scholar]


