Abstract
Background: The electrocardiogram (ECG) of the athlete displays particular characteristics as a consequence of both electrophysiological and autonomic remodeling of the heart that follows continued physical training. However, doubts persist on how these changes directly interact during ventricular activation and repolarization ultimately affecting surface ECG waveforms in athletes.
Objective: This article considers an in deep rationale for the electrocardiographic pattern known as early repolarization based on both electrophysiological mechanisms at cellular level and the vectorial theory of the cardiac activation.
Methods: The mechanism by which the autonomic remodeling influences the cardiac electrical activation is reviewed and an insight model of the ventricular repolarization based on ionic models and the vectorial theory of the cardiac activation is proposed.
Results: Considering the underlying processes related to ventricular electrical remodeling, we propose that, in athletes' heart: 1) vagal modulation increases regional electrophysiological differences in action potential phases 1 and 2 amplitudes, thus enhancing a voltage gradient between epicardial and endocardial fibers; 2) this gradient affects depolarization and repolarization timing sequences; 3) repolarization wave front starts earlier on ventricular wall and partially overcomes the end of depolarization causing an upward displacement of the J‐point, ST segment elevation, and inscription of magnified T‐waves amplitudes leading to characteristic surface ECG waveform patterns.
Conclusions: In athletes, the association between epicardial to endocardial electrophysiological differences and early repolarization ECG pattern can be demonstrated by the vectorial theory of the ventricular activation and repolarization.
Keywords: computer modeling/simulations, noninvasive techniques—electrocardiography
The electrocardiogram (ECG) of the athlete often mimics repolarization alterations found in diverse cardiopathies, as those observed in hypertrophic cardiomyopathy, arrhythmogenic right ventricular dysplasia, ischemic cardiopathy, and Brugada syndrome. However, the electrocardiographic pattern characterized by both J‐point and ST‐segment elevation and known as early repolarization is highly prevalent among athletes. Furthermore, considering the scenario where the above pathologic conditions have been ruled out, cohort studies have demonstrated that those electrocardiographic patterns in athletes are benign and not associated with adverse cardiovascular events in the long term. 1 Thus, it is clinically evident that electrophysiological mechanisms underlying typical ST‐T segment changes in healthy athletes are diverse from those observed in individuals with cardiopathies. This article focuses on the mechanisms of exercise‐induced autonomic remodeling of the heart and considers explanation for the electrocardiographic pattern known as early repolarization based on electrophysiological mechanisms and the vectorial theory of the cardiac activation.
The Remodeling of the Autonomic Modulation of the Heart in Athletes
Continued high‐performance physical training produces significant modifications in cardiovascular and autonomic nervous systems, known as conditioning or fitness. The physical conditioning has been traditionally assessed by the determination of the chronotropic response to exercise. 2 The reduction of the heart rate at rest and during exercise has been interpreted as a combined effect of sympathetic and parasympathetic systems modifications reported to occur after continued regular exercises. 3 , 4 Studies that analyzed heart rate variability in the frequency domain demonstrated that either low‐frequency components, which reflect contributions of both parasympathetic and sympathetic modulation, or high‐frequency components, which reflect predominantly vagal activity, are both positively influenced by physical training. 5 , 6 , 7 , 8 Among trained individuals, heart rate variability under controlled ventilation shows an increased spectral power in the high‐frequency range when compared to untrained ones, suggesting an increase of the vagal activity. 7 The remodeling of the autonomic tonus of the heart secondary to training, which includes the reduction of the sympathetic and increase of the parasympathetic activities, has been associated with the following mechanisms:
-
1
Desensitization of the cardiac beta‐adrenergic receptors or reduction of their density consequent to the frequent and sustained stimulation that occurs with the physical training. The receptors density reduction can be spread 9 , 10 or situated, specially, in the inferior wall of the left ventricle, as demonstrated by studies that evaluated the integrity of the sympathetic nervous system with 123I‐metaiodobenzylguanidine (MIBG) scintilography; 11
-
2
The increased inotropism of the trained heart generates a stronger baroreflex stimulation including the carotid bulb receptors and the left ventricular pressoceptors with consequent vagal afferent stimulation and increment of parasympathetic tonus; 4 , 12 , 13
-
3
The increase in vagal tonus contributes for reduction of sympathetic tonus through the so‐called accentuated antagonism phenomenon. 14 The faster release of acetylcholine (ACh) in the terminal synaptic antagonizes the adrenergic effects through two mechanisms: prejunctional and postjunctional. The first one is secondary to the simultaneous release of ACh and neuropeptide Y. Neuropeptide Y has the function of inhibiting the release of norepinephrine in the synaptic terminals. The second is due to a competitive action between the ACh and norepinephrine on the enzyme adenylate‐cyclase, the former inhibiting and the later activating this enzyme. These mechanisms are responsible for the characteristic autonomic balance observed in athletes; and
-
4
Intrinsic electrophysiological changes in the cardiac cell caused by stretching and myocardial hypertrophy that come along with continuous physical training. These changes induce sinus bradycardia and slowing of atrioventricular conduction in athletes, even when autonomic nervous system is pharmacologically blocked. 15 These mechanisms result in athletes' characteristic autonomic balance.
The Ionic Bases of the Electric Activation of the Heart in Athletes
The autonomic modulation of the heart rate is exerted primordially through sympathetic and parasympathetic influences on sinus node automaticity. 14 Under normal conditions, sinus node cells have a faster frequency of depolarization than other automatic cells throughout the heart and therefore play the key role of dominant pacemaker. The autorhythmicity of pacemaker cells is a consequence of the presence of a higher density of a type of ionic channel responsible for voltage‐dependent inward current, composed by potassium and sodium ions, and activated when voltage reaches a threshold of −40 mV. 16 This so‐called pacemaker current (If) promotes spontaneous diastolic depolarization until it reaches the potential threshold of the T‐type Ca2+ channels, which completes depolarization. Increases in If channel conductance result in more stimuli per minute and consequently higher heart rates. Adrenergic stimulation—through cardiac beta‐receptors—as well as parasympathetic stimulation—through type II muscarinic receptors—modifies some ionic currents. 14 , 17 The coupling of norepinephrine with beta‐1 receptors activates Gs ‐protein and release alpha subunit in the cellular membrane. As a result, activated adenylate‐cyclase catalyzes the formation of adenosine cyclic 5′‐monophosfate (cAMP) from adenosine triphosfate. The increase of the intracellular concentration of cAMP promotes selective phosphorylation of some proteins including the Kinase A, which, in turn, phosphorylates regulatory proteins of ionic channels leading to the increase of conductance. This increment in conductance includes three types of currents: (1) the L‐type Ca2+ current, causing an increase in inotropism, (2) the delayed rectifier potassium current, leading to shortening of both repolarization and refractory period, and (3) the If current with consequent increase of heart rate. On the contrary, to adrenergic stimulation, vagal tonus, through release of Ach, acts by reducing the heart rate. The binding of ACh to M2 muscarinic receptors releases Gi‐protein alpha subunit from subunits gamma and beta, thus, promoting inhibition of adenylate‐cyclase, reducing intracellular cAMP concentration, and decreasing If channel conductance. Evidence suggest that Gi‐ protein activated by muscarinic receptors can act as a second messenger and, thus, directly interact with potassium channels, promoting efflux of this ion and reducing heart rate. In fact, sinus bradycardia is a common feature in the athlete ECG.
Electrophysiology of Ventricles
Epicardial and endocardial fibers in the ventricular wall demonstrate different ionic content and properties, accounting for particular action potential waveform characteristics. Regional differences of the electrophysiological properties of cardiac fibers are associated with a prominent phase 1 in epicardial fibers action potential. 18 Phase 1 constitutes an early repolarization drift of transitory outward potassium current (Ito) activated by voltage and calcium. 19 , 20 , 21 , 22 Besides being more prominent, the magnitude of phase 1 in epicardial fibers is more dependent of the heart rate than in the endocardial fibers. Beta‐adrenergic stimulation diminishes heart rate‐dependent variation in amplitude of action potential phase 1 of epicardial fibers. 23 The early repolarization pattern is also rate dependent. The transient slow component of outward K+ current (Ito1) shows slow kinetics of recovery from inactivation. Thereafter, at faster heart rate (usually above 600 ms cycle length), a decrease in the population of prompt reactivation channels is observed with further attenuation of the magnitude of phase 1 (or notch) and a much less pronounced spike‐and‐dome morphology in epicardium. The morphology of the endocardial action potential shows far less rate dependence. This regional difference between EPI and endocardium action potential waveforms is consistent with a greater density of Ito1 in the former. Both notch attenuation and restoration of the dome in epicardium observed under heart rate increment, usually abolish early repolarization ECG pattern entirely. 23
Although parasympathetic stimulation has a discrete direct effect on the ventricles, it does promote through the phenomenon of accentuated antagonism a reversion of the effect of sympathetic tonus. The net result is a reduction of the heart rate and an increment in the variation of the amplitude of the phase 1 potential in the epicardium as compared to the endocardium. Thus, a voltage gradient between epicardial and endocardial fibers during phase 1 and phase 2 of the action potential is elicited, and there are strong electrophysiological evidences connecting transmural voltage gradient to the rise in the J point and ST segment on the surface ECG. 23 , 24 (Fig. 1). The relation between autonomic shifts, characterized by increased vagal tonus and decreased sympathetic activity, and early repolarization on ECG have already been observed in healthy athletes and nonathletes. In Haydar et al. study, 25 the presence or absence of early repolarization on the electrocardiogram at rest was correlated with aerobic exercise capacity in healthy volunteers. Individuals with early repolarization had both longer treadmill exercise duration and higher peak oxygen consumption than age‐ and gender‐matched control subjects. Marcus et al. 26 examining the prevalence of early repolarization in patients with spinal cord injury observed a higher prevalence of this electrocardiographic pattern in individuals with levels of injury that can disrupt central sympathetic command of the heart. According to the authors, 26 it appears that either enhanced vagal tonus or loss of sympathetic tonus, or both were responsible for ST elevation.
Figure 1.
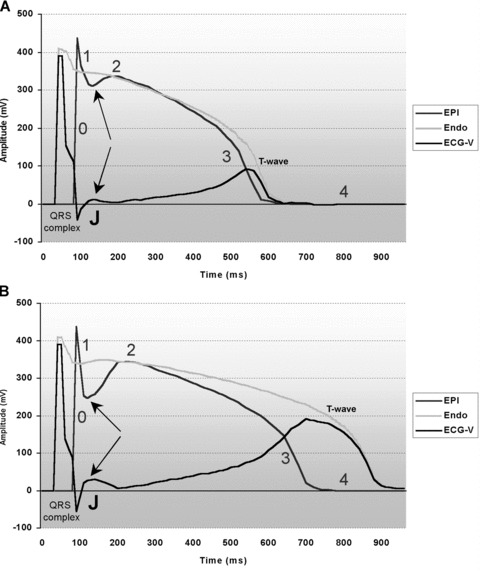
Computational surface electrocardiogram waveform (ECG‐V) reconstruction as proposed by Antzelevich et al. 23 and based on vectorial difference between epicardial (EPI) and endocardial (ENDO) regional action potentials. EPI and ENDO action potentials were computationally reconstructed from actual and respective isolated canine heart action potentials. Numbers 0, 1 2, 3, and 4 in (A) and (B) represents respective action potential phases. (A) EPI and ENDO action potentials at 100 bpm, and receptive computational ECG‐V. Upward arrows points to post‐spike phase 1 dip. On ECG‐V, J point (J) is indicated by downward arrow. (B) EPI and ENDO action potentials at 60 bpm, and receptive computational ECG‐V. EPI shows pronounced “phase 1” magnitude (spike and dome shape, upward arrow points to post‐spike phase 1 dip). ENDO action potential shows less pronounced phase 1 magnitude, virtually unchanged as compared to 100 bpm pacing rate. On ECG‐V, note a pronounced notch (J wave, downward arrow) arising at the junction between reconstructed QRS complex and following ST segment, caused by marked regional EPI to ENDO phase 1 repolarization differences.
Vectorial Basis of Athlete's Electrocardiogram
The association between regional electrophysiological differences of action potential phase 1 amplitude and athlete T wave can be demonstrated by the vectorial theory of the ventricular depolarization and repolarization (Fig. 2). The QRS complex on surface ECG results from depolarizing currents that begin in the endocardium and travels toward the epicardium. In normal hearts, after the end of depolarization, there is a time gap in phase 2 of the action potential corresponding to ST segment on ECG, before the beginning of the repolarization. The latter, represented by T wave on ECG, initiates in the epicardium and goes toward the endocardium. In contrast to the depolarization, repolarization is a negative‐charge current expressed on surface ECG as a wave of same polarity as that of depolarization. Therefore, in leads where QRS complex is predominantly positive, T wave also tends to be positive. In the case of a prominent phase 1 in the epicardial cells, this current can be detected by the surface ECG as early repolarization, causing shortening of the ST segment. In the athlete's heart, on the other hand, in leads where R waves are predominant, as left precordial leads, an early and also positive repolarizing wave interrupts the descending phase of R wave causing an upward shift of both the J point and the ST segment. In this setting, an increase in the number of repolarizing cells in the epicardium‐to‐endocardium direction eventually leads to even more precocious beginning of the repolarization in the epicardium, thus generating taller T waves. This theory is supported by our observations of vectocardiographic loops in vagotonic subjects (Figs. 2 and 3). In such a manner, on the horizontal plane of Frank's vectorcardiogram, the rollback of the loop of the QRS complex in point‐zero potential is prematurely interrupted by a precocious beginning of the T‐wave loop.
Figure 2.
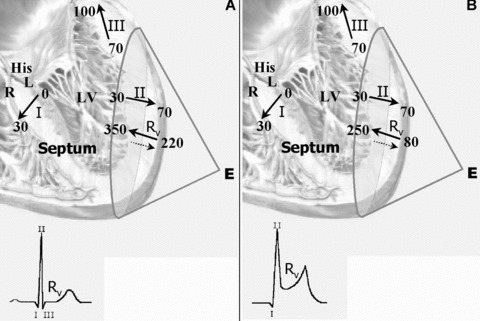
(A) Vectorial representation of transmural electrical activation registered at a unipolar exploring electrode (E) in normal hearts. Symbols I, II, and III represent instantaneous moments (vectors) in ventricular depolarization sequence (respectively, septal wall, free lateral wall, and posterior‐basal wall). Rv represents ventricular repolarizing vector. In each segment, arrows represent, respectively, the onset (head) and the offset (tail) of regional activation times (in milliseconds). Full arrow next to Rv represents the actual direction (epicardial‐to‐endocardial), whereas broken arrow represents the direction of repolarizing vector (endocardial‐to‐epicardial) observed on electrode E. Note that between the end of depolarization (III) and the repolarization onset (Rv) there is a time gap of 120 ms, corresponding to the ST segment on surface electrocardiogram in normal heart (ECG inset). (B) Vectorial representation of transmural electrical activation registered at a unipolar exploring electrode (E) Note that, in athlete's heart, the onset of the ventricular repolarization overlaps in 20ms the offset the ventricular activation causing both J –point and ST‐T‐segment to move upward (ST‐T elevation) on regular surface ECG (III). Once Rv goes in opposite direction to vector III, a ST elevation with upside concavity is registered, causing interruption of the descending limb of the R wave on surface ECG, characterizing early repolarization (ECG inset).
Figure 3.
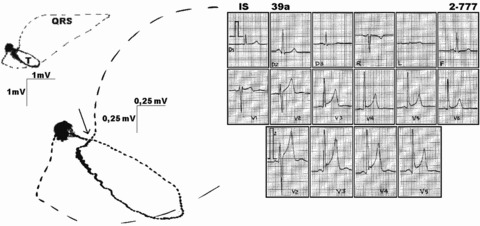
Typical electrocardiogram and respective vectorcardiogram in a healthy marathon runner. Note concave ST segment elevation on electrocardiogram, corresponding on vectorcardiogram to a take off of the zero potential at the QRS complex terminal loop (in detail—full arrow). Open droplets in vectorcardiogram loops indicate counterclockwise QRS complex and T‐wave loops orientations. Potential scales are, respectively, 1 mV and 0.25 mV on vectorcardiogram.
In conclusion, considering the fact that vagal modulation of Ito current leads to more pronounced phases 1 and 2 in epicardial action potentials, we in addition, postulate that, in athletes' heart: (1) increased vagal activity affects depolarization and repolarization timings sequence, (2) repolarization wave front starts earlier on ventricular wall and partially superposes the end of depolarization causing upward displacement in point J, ST segment elevation, and inscription of magnified amplitude T waves leading to characteristics surface ECG waveform patterns, known as early repolarization or vagotonic heart.
Authors Eduardo C. Barbosa, Alfredo de S. Bomfim, and Paulo R. Benchimol‐Barbosa contributed equally to this article.
REFERENCES
- 1. Pelliccia A, Maron BJ, Culasso F, et al Clinical significance of abnormal electrocardiographic patterns in trained athletes. Circulation 2000;102:278–284. [DOI] [PubMed] [Google Scholar]
- 2. Scheuer J, Tipton CM. Cardiovascular adaptations to physical training. Ann Rev Physiol 1977;39:221–227. [DOI] [PubMed] [Google Scholar]
- 3. Bedford GB, Tipton CM. Exercise training and arterial baroreflex. J Appl Physiol 1987;63:19–26. [DOI] [PubMed] [Google Scholar]
- 4. Pagani M, Somers V, Furlan R, et al Changes in autonomic regulation induced by physical training in mild hypertension. Hypertension 1988;12:600–607. [DOI] [PubMed] [Google Scholar]
- 5. Goldsmith RL, Bigger JT, Steinman RC, et al Comparison of 24‐hour parasympathetic activity in endurance‐trained and untrained young men. J Am Coll Cardiol 1992;20:552–558. [DOI] [PubMed] [Google Scholar]
- 6. La Rovere MT, Mortara A, Sandrone G, et al Autonomic nervous system adaptations to short‐term exercise training. Chest 1992;101(5 Suppl.):299s–303s. [DOI] [PubMed] [Google Scholar]
- 7. Dixon EM, Kamath MV, McCartney N, et al Neural regulation of heart rate variability in endurance athletes and sedentary controls. Cardiovasc Res 1992;26:713–719. [DOI] [PubMed] [Google Scholar]
- 8. Strano S, Lino S, Calcagnini G, et al Respiratory sinus arrhythmia and cardiovascular neural regulation in athletes. Med Sci Sports Exerc 1998;30:215–219. [DOI] [PubMed] [Google Scholar]
- 9. Sylvestre‐Gervais L, Nadeau A, Nguyen MH, et al Effects of physical training on b‐adrenergic receptors in rat myocardial tissue. Cardiovasc Res 1982;16:530–537. [DOI] [PubMed] [Google Scholar]
- 10. Matsuo S, Nakamura Y, Takahashi M, et al Cardiac Sympathetic Dysfunction in an Athlete's Heart Detected by 123 I‐Metaiodobenzylguanidine Scintigraphy. Jpn Circ J 2001;65:371–374. [DOI] [PubMed] [Google Scholar]
- 11. Estorch M, Serra‐Grima R, Florats A, et al Myocardial sympathetic innervation in the athlete's sinus bradycardia: Is there selective inferior myocardial wall denervation? J Nucl Cardiol 2000;7:354–358. [DOI] [PubMed] [Google Scholar]
- 12. Verani MS, Hartung GH, Hoepfel‐Harris J, et al Effects of exercise training on left ventricular performance and myocardial perfusion in patients with coronary artery disease. Am J Cardiol 1981;47:797–891. [DOI] [PubMed] [Google Scholar]
- 13. Billman GE, Schwartz PJ, Stone HL. The effects of daily exercise on susceptibility to sudden cardiac death. Circulation 1984;69:1182–1189. [DOI] [PubMed] [Google Scholar]
- 14. Matthew NL: Time dependency of the autonomic interactions that regulate heart rate and rhythm In: Zipes DP. and Jalife J. (eds.): Cardiac Electrophysiology. From Cell to Bedside. Philadelphia , W.B. Saunders Company, 1995, pp. 454–459. [Google Scholar]
- 15. Stein R, Medeiros CM, Guido AR, et al Intrinsic sinus and atrioventricular node electrophysiologic adaptations in endurance athletes. J Am Coll Cardiol 2002;39:1033–1038. [DOI] [PubMed] [Google Scholar]
- 16. DiFrancesco D, Mangoni M, Maccaferri G. The pacemaker current in cardiac cells In: Zipes DP. and Jalife J. (eds.): Cardiac Electrophysiology. From Cell to Bedside, Philadelphia , W.B. Saunders Company, 1995, pp. 96–103. [Google Scholar]
- 17. Stiles GL. Structure and function of cardiovascular membranes channels and receptors In: Schlant RC. and Alexander RE. (eds.): Hurst's The Heart: Arteries and Veins. New York , Mc Graw‐Hill, 1994. pp. 47–58. [Google Scholar]
- 18. Litovsky SH, Antzelevitch C. Transient outward current prominent in canine ventricular epicardium but not endocardium. Circ Res 1988;62:116–126. [DOI] [PubMed] [Google Scholar]
- 19. Giles W, Van Ginneken A. A transient outward current in isolated cells from the crista terminalis of rabbit heart. J Physiol 1988;405:123–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Clark RB, Giles WR, Imaizumi Y. Properties of the transient outward current in rabbit atrial cells. J Physiol 1988;405:147–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Apkon M, Nerbonne JM. Characterization of two distinct depolarization‐activated K+ currents in isolated rat ventricular myocytes. J Gen Physiol 1991;97:973–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nabauer M, Beuckelmann D, Erdmann E. Characteristics of transient outward current in human ventricular myocytes from patients with terminal heart failure. Circ Res 1993;73:386–394. [DOI] [PubMed] [Google Scholar]
- 23. Antzelevitch C, Sicouri S, Lukas A, et al Regional differences in the electrophysiology of ventricular cells: Physiological and clinical implications In: Zipes DP. and Jalife J. (eds.): Cardiac Electrophysiology. From Cell to Bedside, Philadelphia , W.B. Saunders Company, 1995. pp. 228–245. [Google Scholar]
- 24. Di Diego JM, Antzelevitch C. Cardiac innervation/conduction: High (Ca sup 2+) sub O‐induced electrical heterogeneity and extrasystolic activity in isolated canine ventricular epicardium: Phase 2 reentry. Circulation 1994;89:1839–1850. [DOI] [PubMed] [Google Scholar]
- 25. Haydar ZR, Brantley DA, Gittings NS, et al Early repolarization: An electrocardiographic predictor of enhanced aerobic fitness. Am J Cardiol 2000;85:264–266. [DOI] [PubMed] [Google Scholar]
- 26. Marcus RR, Kalisetti D, Raxwal V, et al Early repolarization in patients with spinal cord injury: prevalence and clinical significance. J Spinal Cord Med 2002;25:33–38. [DOI] [PubMed] [Google Scholar]


