Abstract
Background
The QT variability index, calculated between Q‐ and the T‐wave end (QTendVI), is an index of temporal myocardial repolarization lability associated with sudden cardiac death (SCD) in chronic heart failure (CHF). Little is known about temporal variability in the other two temporal myocardial repolarization descriptors obtained from Q–Tpeak and Tpeak–Tend intervals. We therefore investigated differences between these indexes in patients with CHF who died suddenly and in those who survived with a left ventricular ejection fraction (LVEF) ≤35% or >35%.
Methods and Results
We selected 127 ECG and systolic blood pressure (SPB) recordings from outpatients with CHF all of whom had been followed up for 30 months. We calculated RR and SPB variability by power spectral analysis and QTendVI, QTpeakVI, TpeakTendVI. We then subdivided data patients into three groups SCD, LVEF ≤ 35%, and LVEF > 35%. The LVEF was higher in the SCD than in the LVEF ≤ 35% group, whereas no difference was found between the SCD and LVEF > 35% groups. QTendVI, QTpeakVI, and TpeakTendVI were higher in the SCD and LVEF ≤ 35% groups than in the LVEF > 35% group. Multivariate analysis detected a negative relationship between all repolarization variability indexes, low frequency obtained from RR intervals and LVEF.
Conclusions
Our data show that variability in the first (QTpeakVI) and second halves of the QT interval (Tpeak–TendVI) significantly contributes to the QTendVI in patients with CHF. Further studies should investigate whether these indexes might help stratify the risk of SCD in patients with a moderately depressed LVEF.
Keywords: chronic heart failure, QT variability, heart rate variability, autonomic nervous system, sudden cardiac death
Myocardial repolarization lability may predispose patients with chronic heart failure (CHF) to sudden cardiac death (SCD) from ventricular arrhythmia.1, 2 A marker currently used to assess temporal myocardial repolarization lability is the QT variability index (QTendVI).3, 4, 5 In subjects with severe or moderate left ventricular dysfunction, an increased QTendVI is strongly associated with the SCD event.6, 7, 8, 9, 10, 11 Myocardial repolarization is nevertheless a highly complex electrophysiological phenomenon that directly implicates ventricular myocardial ion channel function and, indirectly, reflects autonomic nervous system control.1, 2 Thus, spatial12, 13 and temporal14, 15 myocardial repolarization dispersion are both influenced by several structural changes in the ventricular myocardium, as well as by autonomic cardiovascular control and many other factors including age,15, 16 and medications.17, 18, 19 Even in healthy subjects, the duration of myocardial repolarization, is inherently nonhomogeneous given that the action potential has a shorter duration in epicardial than in M‐cell layers.20, 21 Some investigators suggest that the Q–Tpeak interval on the surface electrocardiogram (ECG) (Fig. 1), measured as the distance between the Q‐wave and the T‐wave peak, mainly reflects the termination of epicardial repolarization, whereas the Tpeak–Tend interval (Fig. 1), calculated from the peak when the T‐wave ends, reflects the termination of M‐cell layer repolarization and, accordingly, could be a noninvasive marker of transmural repolarization dispersion.20, 21 In patients with CHF, whether these two repolarization variables, whose sum forms the entire Q–T interval duration (QTend) (Fig. 1), exhibit temporal nonhomogeneity as does the classically assessed Q–Tend interval, remains unclear.
Figure 1.
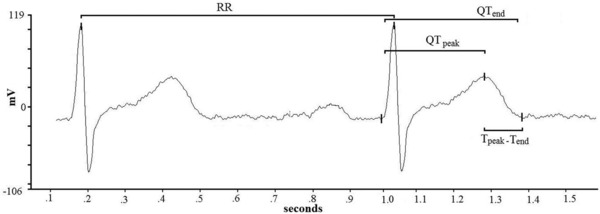
Representative example of RR, Q–Tend, Q–Tpeak, and Tpeak–Tend interval measurements from a single lead ECG.
We therefore designed this retrospective study to investigate whether one of the two temporal myocardial repolarization dispersion descriptors, QTpeakVI and TpeakTendVI, undergoes greater changes in patients with CHF and hence is stronger in predicting SCD than the classic QTendVI. To accomplish this aim, we calculated all these three indexes from a single 5‐minute surface ECG recording, and compared the data for subjects with CHF who had died of SCD and outpatients who survived whose systolic function was severely depressed (left ventricular ejection fraction, LVEF, ≤35%) or moderately depressed (LVEF > 35%).
METHODS
Study Subjects
For this study we retrospectively selected 127 short‐term (5 minutes) ECG and systolic blood pressure (SBP) recordings from clinically stable outpatients with CHF secondary to dilated postischemic cardiomyopathy, all of whom had been followed up for 30 months. We defined clinically stable patients as those who had not been hospitalized or had their therapy adjusted or had experienced any other acute coronary artery or noncoronary event during the past 3 months. All participants had undergone revascularization either cutaneously or by aorto‐coronary artery bypass at least 3 months before the study. None of the patients had malignancy, primary valve disease, atrial fibrillation, extrasystoles (one extrasystole per minute was permitted), or other arrhythmias likely to interfere with heart rate and QT analysis. None of the patients was in New York Heart Association (NYHA) class IV. Before the study none of the subjects had a documented history of cardiac arrest, ventricular tachycardia, or fibrillation. All patients were regularly contacted by phone to acquire information on their clinical conditions. Sudden (presumably arrhythmic) death was defined as natural death taking place within 1 hour after the onset of acute symptoms or death during sleep. SCD was confirmed in each patient by telephone interview with surviving relatives.
To accomplish the aim of the study, we grouped participants’ data into three categories: data from subjects who died of SCD during follow‐up, those from survivors with a severely depressed LVEF (≤35%), and those for survivors with a moderately depressed EF (>35%).
Study Protocol and Offline Data Analysis
After a 15‐minute rest lying down, each subject underwent a 5‐minute, single ECG lead, and a noninvasive beat‐to‐beat SBP recording during controlled breathing (15 breaths per minute, 0.25 Hz). All digitized signal recordings were analyzed by a single physician (G.P.) blinded to subjects’ circumstances.
We measured the following intervals from the respective time series in ECG recordings: RR, Q–Tend (from the Q‐wave to the T‐wave end), Q–Tpeak (from the Q‐wave to the T‐wave peak), and Tpeak–Tend (difference between QTend and QTpeak) (Fig. 1). We therefore calculated mean and variance values for each of these intervals and then we used the original formula proposed by Berger et al.3 to calculate three different QT variability indexes:
From the same 5‐minute ECG segments we also determined the total power of RR intervals and SBP (TPRR, TPSBP), and their total spectral density.22 For RR and SBP we calculated the following spectral components: a high‐frequency (HFRR, HFSBP) component (from 0.15 to 0.40 Hz Eq), a low‐frequency (LFRR, LFSBP) component (from 0.04 to 0.15 Hz Eq), and a very low‐frequency (VLFRR, VLFSBP) component (below 0.04 Hz Eq). We also measured LF and HF central frequencies.
The α index was calculated by dividing the square root of the spectral density for heart rate by the square root of the corresponding spectral density for SPB, as described by Robbe et al.23 and later by other investigators24, 25:
The same ECG intervals, together with beat‐to‐beat SBP recording, were also used to determine power spectral analysis with an autoregressive algorithm also for QTend, QTpeak, and Tpeak–Tend intervals (Fig. 2).22 Cross‐spectral analysis was then used to evaluate the reciprocal influence (coherence function) between RR, QTend, QTpeak, and Tpeak–Tend.3 Coherence expresses the fraction of power at a given frequency in either time series and provides an index of a linear relationship between the input and output signals. The coherence function γ[f] was then computed according to the formula described elsewhere:3, 14
where f is frequency, Pxx [f] is the RR interval spectrum, Pyy[f] is the QT interval spectrum, and Pxy[f] is the cross spectrum. The coherence function measures the degree of linear interaction between RR and QT interval oscillations as a function of their frequency. The value of the coherence function ranges between zero and one. Mean coherences were measured by averaging γ[f] over the frequency bands: from 0 to 0.50 Hz.
Figure 2.
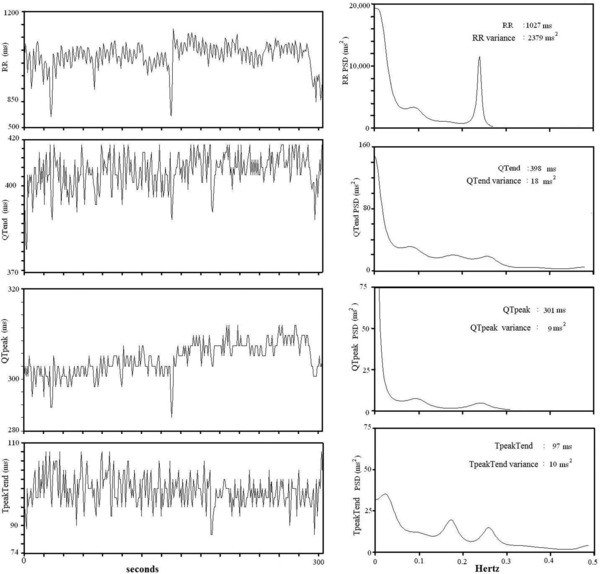
Representative example of a 5‐minute ECG recording (left panels) and related power spectral analysis (right panels) RR, Q–Tend, Q–Tpeak, Tpeak–Tend intervals in a subject with a left ventricular ejection fraction >35%.
Software for data acquisition and storage and for spectral analysis were designed and produced by our research group and are described in detail elsewhere.4, 5, 12, 13, 25, 26, 27
Last, from the same 5‐minute ECG segment, the corrected Q–Tend, Q–Tpeak, and Tpeak–Tend intervals were obtained according to the formulas proposed by Bazett (QTend/RR0.5; QTpeak/RR0.5; Tpeak–Tend/RR0.5), Friedericia (QTend/RR0.33; QTpeak/RR0.33; Tpeak–Tend/RR0.33), Lilly (QTend/RR0.4; QTpeak/RR0.4; Tpeak–Tend/RR0.4], and Framingham (QTend + [0.154 × {1000 − RR}]; QTpeak + [0.154 × {1000 – RR}]; Tpeak – Tend + [0.154 × {1000 − RR}]).
Statistical Analysis
Unless otherwise indicated all data are expressed as means ± SD. Data with skewed distribution are given as median and interquartile range [75th percentile – 25th percentile]. Categorical variables were analyzed with the chi‐square test. One‐way analysis of variance (ANOVA) and1 Bonferroni test were used to compare data for the normally distributed variables. Kruskal‐Wallis and Mann‐Whitney test were used to compare nonnormally distributed variables (as evaluated by Kolgomorov‐Smirnov test). Stepwise multiple regression analysis was used to determine possible relationships between the three indexes (QTendVI, QTpeakVI, and TpeakTendVI) and the other clinical and spectral data. P values less than or equal to 0.05 were considered statistically significant. All data were evaluated with the database SPSS‐PC+ (SPSS‐PC+ Inc, Chicago, IL, USA).
Table 1.
General Characteristics in the Three Study Groups, Patients with Chronic Heart Failure (CHF) Who Died of Sudden Cardiac Death (SCD) during Follow‐Up, and Survivors Who Had a Left Ventricular Ejection Fraction (LVEF) ≤35% or >35%
| SCD Group | LVEF ≤ 35% | LVEF > 35% | P‐Values | |
|---|---|---|---|---|
| N = 12 | Group N = 41 | Group N = 31 | ||
| Variables | Subjects | Subjects | Subjects | [ANOVA] |
| Age (years) | 67 ± 11 | 62 ± 12 | 59 ± 10 | NS |
| M/F | 10/2 | 36/5 | 29/3 | NS |
| BMI (kg/m2) | 25 ± 5 | 27 ± 4 | 27 ± 4 | NS |
| HR (beats/min) | 72 ± 11 | 68 ± 9 | 64 ± 10 | NS |
| SBP (mm Hg) | 122 ± 27 | 114 ± 21 | 118 ± 22 | NS |
| DBP (mm Hg) | 65 ± 14 | 61 ± 14 | 64 ± 11 | NS |
| QTend Bazett (ms) | 401 ± 75c | 390 ± 44 | 371 ± 30 | 0.007 |
| QTend Fridericia (ms) | 413 ± 73c | 382 ± 46 | 368 ± 33 | 0.021 |
| QTend Lilly (ms) | 416 ± 74c | 385 ± 45 | 369 ± 31 | 0.013 |
| QTend Framingham (ms) | 412 ± 74c | 384 ± 45 | 369 ± 31 | 0.023 |
| Qpeak Bazett (ms) | 305 ± 28 | 311 ± 42 | 291 ± 27 | NS |
| QTpeak Fridericia (ms) | 300 ± 28 | 305 ±43 | 289 ± 28 | NS |
| QTpeak Lilly (ms) | 302 ± 28 | 308 ± 43 | 290 ± 27 | NS |
| QTpeak Framingham (ms) | 302 ± 28 | 310 ± 41 | 291 ± 26 | NS |
| Tpeak ‐Tend Bazett (ms) | 116 ± 63a,c | 78 ± 30 | 79 ± 17 | 0.002 |
| Tpeak ‐Tend Fridericia (ms) | 113 ± 62a,c | 76 ± 30 | 79 ± 17 | 0.003 |
| Tpeak ‐Tend Lilly (ms) | 114 ± 62a,c | 77 ± 30 | 79 ± 17 | 0.003 |
| Tpeak‐Tend Framingham (ms) | 121 ± 70a,c | 89 ± 34 | 83 ± 23 | 0.015 |
| Coronary disease | ||||
| 1 Vessel | 2 | 1 | 2 | NS |
| 2 Vessels | 4 | 17 | 14 | NS |
| 3 Vessels | 6 | 23 | 15 | NS |
| LVEF (%) | 42 ± 8b | 30 ± 5d | 45 ± 7 | 0.0001 |
| NYHA class (I/II/III) | 1/8/3 | 3/19/19 | 6/19/7 | NS |
| Serum K+ | 4.1 ± 0.1 | 4.1 ± 0.2 | 4.2 ± 0.3 | NS |
| β‐blockers | 8 | 31 | 24 | NS |
| Furosemide | 4 | 26 | 8 | NS |
| ACEi/Sartans | 9 | 29 | 30 | NS |
| Spironolactone | 3 | 16 | 8 | NS |
| Digoxin | 2 | 10e | 0 | 0.033 |
| Amiodarone | 2 | 2 | 1 | NS |
Data are expressed as mean ± SD.
P < 0.05 SCD vs LVEF ≤ 35% group. bP < 0.001 SCD vs LVEF ≤ 35% group. cP < 0.05 SCD vs LVEF > 35% group. dP < 0.001 LVEF ≤ 35% vs LVEF > 35% group. eP < 0.05 LVEF ≤ 35% vs LVEF > 35% group.
M/F = male/female; BMI = body mass index; HR = heart rate; LVEF = left ventricular ejection fraction; NYHA = New York Heart Association.
RESULTS
Of the 127 participants with CHF initially enrolled, 43 subjects were excluded for various reasons and a total 84 subjects therefore completed the study. Of the 43 subjects who were excluded, 11 died of causes other than SCD, and 23 subjects refused to collaborate. No patient had an implantable cardioverter defibrillator placed for primary SCD prophylaxis either because they underwent assessment before the Multicenter Automatic Defibrillator Trial (MADIT II) was published or because they voluntarily refused ICD implantation.
Neither age, body mass index (BMI), gender distribution, heart rate, systemic arterial pressures, NYHA class, coronary disease, or drug therapy differed significantly between the three study groups, whereas the ECG recording showed a significantly longer corrected Q–Tend and Tpeak–Tend intervals in the SCD group than in the LVEF >35% group. The group with an LVEF ≤35% had a lower LVEF than the other two groups and used digoxin more frequently (Table 1).
The temporal repolarization dispersion index QTendVI was significantly higher in the LVEF ≤35% than in the LVEF >35% group. Conversely, QTpeakVI was significantly higher in the LVEF ≤35% and SCD groups than in the LVEF >35% group (Table 2). Similarly, TpeakTendVI were significantly higher in the LVEF ≤35% and SCD groups than in the LVEF >35% group (Table 2).
Table 2.
QT Data Obtained in the Three Study Groups, Patients with Chronic Heart Failure (CHF) Who Died of Sudden Cardiac Death (SCD) during Follow‐Up, and Survivors Who Had a Left Ventricular Ejection Fraction (LVEF) ≤ 35% or > 35%
| SCD Group | LVEF ≤ 35% | LVEF > 35% | ||
|---|---|---|---|---|
| N = 12 | Group N = 41 | Group N = 31 | P‐Values | |
| Variables | Subjects | Subjects | Subjects | [ANOVA] |
| QTend (ms) | 401 ± 80 | 368 ± 53 | 364 ± 44 | NS |
| QTendvariance (ms2) | 66[192] | 52[60] | 20[39] | NS |
| RR (ms) | 926 ± 221 | 895 ± 128 | 966 ± 31 | NS |
| RR variance | 324[1177] | 669[1078] | 805[1399] | NS |
| QTendVI | −0.28[1.29] | −0.22[0.83]d | −0.72[0.56] | 0.004 |
| RR→QTend, coherence | 0.265 ± 0.093 | 0.243 ± 0.061 | 0.263±0.060 | NS |
| QTpeak (ms) | 291 ± 39 | 294 ± 49 | 285 ± 35 | NS |
| QTpeakvariance (ms2) | 27[66] | 19[20]d | 8[15] | 0.044 |
| QTpeakVI | −0.36[1.54]b | −0.54[0.87]c | −0.98[0.57] | 0.001 |
| RR→QTapex, coherence | 0.277 ± 0.075 | 0.271 ± 0.068 | 0.301 ± 0.098 | NS |
| Tpeak –Tend (ms) | 109 ± 63a,b | 73 ± 29 | 78 ± 18 | 0.004 |
| Tpeak –Tendvariance (ms) | 100[195]b | 46[74]d | 22[33] | 0.044 |
| Tpeak –TendVI | 1.26[1.48]b | 1.15[1.20]d | 0.47[0.93] | 0.001 |
| RR→Tpeak –Tend, coherence | 0.217 ± 0.045 | 0.220 ± 0.056 | 0.241 ± 0.058 | NS |
Values are expressed as mean ± SD or median [interquartile range 75th percentile–25th percentile].
P < 0.05 SCD vs LVEF ≤ 35% group. bP < 0.05 SCD vs LVEF > 35% group. cP < 0.001 LVEF ≤ 35% vs LVEF > 35% group. dP < 0.05 LVEF ≤ 35% vs LVEF > 35% group.
No significant differences were found in power spectral analysis of RR intervals, SBP and the α index in the three groups, except for TP, VLF, and LF obtained from RR variability. Specifically, TPRR and VLFRR were significantly lower in the SCD and LVEF ≤35% groups than in LVEF >35% group (Table 3). LFRR had significantly lower values in the LVEF ≤35% than in the LVEF >35% group (Table 3).
Table 3.
RR and SPB Short‐Term Power Spectral Data Obtained in the Three Groups, Patients with Chronic Heart Failure (CHF) Who Died of Sudden Cardiac Death (SCD) during Follow‐Up, and Survivors Who Had a Left Ventricular Ejection Fraction (LVEF) ≤35% or >35%
| SCD Group | LVEF ≤ 35% Group | LVEF > 35% Group | ||
|---|---|---|---|---|
| N = 12 | N = 41 | N = 31 | P‐Values | |
| Variables | Subjects | Subjects | Subjects | [ANOVA] |
| TPRR (ms2) | 323 [1175]a | 667 [1012]b | 910 [1348] | 0.043 |
| VLFRR (ms2) | 139 [721]a | 304 [792]b | 552 [825] | 0.040 |
| LFRR (ms2) | 62 [274] | 62 [237]b | 180 [247] | 0.049 |
| HFRR (ms2) | 28 [196] | 49 [96] | 96 [125] | NS |
| LF/HF | 1.4 [1.9] | 1.6 [1.9] | 1.8 [2.3] | NS |
| TPSBP (mm Hg2) | 26 [50] | 20 [20] | 22 [20] | NS |
| VLFSBP (mm Hg2) | 18 [43] | 12 [14] | 18 [19] | NS |
| LFSBP (mm Hg2) | 4 [3] | 2 [3] | 3 [3] | NS |
| HFSBP (mm Hg2) | 2 [5] | 2 [3] | 1 [2] | NS |
| αLF (ms/mm Hg) | 6 [8] | 6 [4] | 8 [5] | NS |
| αHF (ms/mm Hg) | 7 [9] | 7 [8] | 8 [13] | NS |
Values are expressed as median [interquartile range 75th percentile–25th percentile].
P < 0.05 SCD vs LVEF > 35% group. bP < 0.05, patients with LVEF ≤ 35% vs LVEF > 35% group.
TP = total power; VLF = very low frequency; LF = low frequency; HF = high frequency; RR = RR interval; SBP = systolic blood pressure.
The stepwise multiple regression analysis testing QTendVI as dependent variables detected significant negative relationships with LFRR (β = –0.37; standard error = 0.04; P = 0.0001), LVEF (β = –0.33; standard error = 0.00; P = 0.0001), and BMI (β = –0.26; standard error = 0.02; P = 0.008) (Table 4, Fig. 3). Conversely, the multiple regression analysis testing QTpeakVI as dependent variables, disclosed a significant relationship with LFRR (β = –0.59; standard error = 0.04; P = 0.0001) and LVEF (β = –0.28; standard error = 0.00; P = 0.0001) but not with BMI (Table 4, Fig. 3). The regression analysis run with Tpeak–TendVI as dependent variable yielded similar results (LFRR: β = –0.42; standard error = 0.07; P = 0.0001; LVEF: β = –0.26; standard error = 0.01; P = 0.014) (Table 4).
Table 4.
Stepwise Multiple Regression Analysis between QTendVI, or QTpeakVI, or TpeakTend (Dependent Variables) and other Clinical and Spectral Data (Independent Variables)
| Ln LFRR | LVEF | BMI | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| β | SE | P | β | SE | P | β | SE | P | R2 | P | |
| QTendVI | −0.37 | 0.04 | 0.0001 | −0.33 | 0.00 | 0.001 | −0.26 | 0.02 | 0.008 | 0.366 | 0.0001 |
| QTpeakVI | −0.59 | 0.04 | 0.0001 | −0.28 | 0.00 | 0.0001 | – | – | NS | 0.492 | 0.0001 |
| TpeakTendVI | −0.42 | 0.07 | 0.0001 | −0.26 | 0.01 | 0.014 | – | – | NS | 0.284 | 0.0001 |
R2 value (a goodness‐of‐fit index) with its related P value refers to the fraction of variance explained by each multivariate model (last two columns).
β = standardized regression coefficient value; SE = standard error value.
Figure 3.
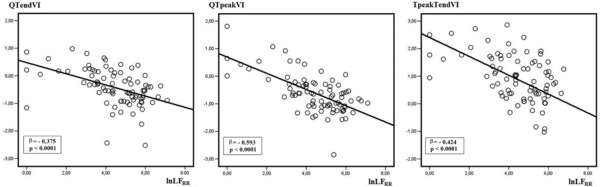
Linear regression analysis between QTendVI, QTpeakVI, and Tpeak–TendVI, natural logarithm of low frequency obtained from RR interval power spectral analysis (lnLFRR).
DISCUSSION
The major original finding in this retrospective study is that patients who died of SCD during follow‐up, notwithstanding an only moderately depressed LVEF, showed a larger QTpeakVI and TpeakTendVI than patients with CHF who survived. Accordingly, these two temporal myocardial repolarization dispersion indexes seem able to predict subjects at risk of SCD among patients who the current ACC‐AHA‐ESC guidelines consider ineligible for ICD implantation for primary SCD prophylaxis. Our second finding is that the three temporal myocardial repolarization dispersion indexes we investigated, namely QTendVI, QTpeakVI, and the TpeakTendVI, correlate inversely with LFRR. This somewhat expected finding is of clinical interest because LFRR is depressed in patients with CHF who died suddenly,28, 29, 30 improves along with the clinical and hemodynamic improvement induced by β‐blocker therapy26 or biventricular stimulation30 and is thought to mirror sinus dysfunction or low baroreceptor sensitivity or both in patients with CHF.31
The three ECG intervals we measured, Q–Tend, Q–Tpeak, and the Tpeak–Tend intervals, differ in electrophysiological meaning. Despite remaining controversial,32, 33, 34 some investigators consider that the Q–Tpeak interval depends on the action potential duration only in the epicardial layer.20, 21 Conversely, the Tpeak–Tend interval predominantly measures myocardial repolarization in the M‐cell layer and also in the layers in which depolarization lasts longer. For this reason the Tpeak–Tend interval probably reflects the maximum difference in repolarization between the myocardial layers and hence may be a noninvasive marker of transmural dispersion repolarization, reported to be increased in subjects at high risk for SCD.20, 21 Hence the derived Tpeak–TendVI, being influenced mainly by the terminal part of the action potential, namely from the rapidly (IKr) and slowly activating (IKs) components from the delayed rectifier current and the inward rectifier current (IK1), could be an exact marker of temporal myocardial repolarization dispersion. Conversely the QTpeakVI could reasonably depend mainly on oscillations in the first part of action potential (phase 0, 1, and 2) and therefore on inward Na (INa) and Ca (ICa L) currents and on transient K outward currents (Ito). Given that CHF induces profound changes in the entire repolarization phase and does so by downregulating K currents, increasing late INa currents and deregulating intracellular Ca,35 the combined change in QTpeakVI and TpeakTendVI we observed, rather than being an unexpected finding, strengthens the hypothesis that several mechanisms concur in causing the arrhythmogenesis in these patients. Most important, temporal variability in Q–Tpeak and Tpeak–Tend intervals could result in delayed after depolarizations (DADs) or early after depolarizations (EADs) and might indicate the presence of tissue areas containing nonhomogeneous refractory periods that, under favorable circumstances (i.e., ischemia, neurohumoral activation), could set up re‐entry circuits thereby triggering malignant ventricular arrhythmias.20, 21
Last, the inverse correlation we found between all the QT variability indexes and LFRR and the LVEF indicates how closely these variables are interconnected in neurohumoral activation and repolarization.36 Even though its precise pathophysiological meaning during CHF remains controversial, the LFRR diminishes during CHF,22, 31 correlates with SCD risk28, 29 and increases as treatment induces hemodynamic improvement.26, 30 Of the three multiple regression analyses we ran in this study, the one achieving major significance was that between QTpeakVI, LVEF, and LFRR. Current knowledge leaves unanswered the question whether this index correlates with LFRR because both variables are negatively influenced by the LVEF or whether an unknown shared factor causes these two variables to change according to LVEF. This possibility notwithstanding, given that both are risk factors for SCD, we conjecture that both are linked by a single causal factor. Another possible arithmetical explanation might be found in the QTVI formula itself, given that LFRR accounts for RR variance.22, 37 Nevertheless LFRR component represents just one of the spectral components of heart rate variability and no significant relationship was found between QT variability indexes and HFRR and VLFRR.
This study also helps to reinforce other findings of clinical importance already reported in earlier studies. For example, power spectral analysis investigating RR intervals of heart rate variability but not of SBP or baroreceptor sensitivity, seems able to select persons at high risk of SCD. In our study, TPRR and VLFRR, were strongly depressed in subjects who died of SCD. This finding is of clinical importance even though some investigators doubt whether VLFRR values assessed from short‐term (5 minutes) rather than from 24‐hour recordings provide reliable results.22, 38 Last, our study failed to document the previously reported reduced LFRR associated with SCD,38 probably because we had too few subjects in the SCD group.
LIMITATIONS
Although the small study sample analyzed, particularly in the SCD group, as well as our patients’ the single ischemic etiology provide homogenous data for, we acknowledged them also as limitations because they prevent us from extrapolating our findings to other patients with CHF.
CONCLUSIONS
This study provides further evidence supporting greater temporal repolarization lability and lower heart rate variability in subjects with CHF who died of SCD than in those who survived. Even though our data show that QTpeakVI and Tpeak–TendVI both contribute significantly to QTendVI in patients with CHF, further studies need to investigate their possible role in identifying patients with a moderately depressed LVEF who are at substantial risk of SCD though not yet considered eligible for ICD implantation.
Conflict of interest: none.
REFERENCES
- 1. Bayes de Luna A, Coumel P, Leclercq JF. Ambulatory sudden cardiac death: Mechanisms of production of fatal arrhythmia on the basis of data from 157 cases. Am Heart J 1989;117:151–159. [DOI] [PubMed] [Google Scholar]
- 2. Pueyo E, Martínez JP,Laguna P. Cardiac repolarization analysis using the surface electrocardiogram. Phil Trans R Soc 2009;367:213–233. [DOI] [PubMed] [Google Scholar]
- 3. Berger RD, Kasper EK, Baughman KL, et al. Beat‐to‐beat QT interval variability. Novel evidence for repolarization lability in ischemic and nonischemic dilated cardiomyopathy. Circulation 1997;96:1557–1565. [DOI] [PubMed] [Google Scholar]
- 4. Magrì D, Piccirillo G, Bucci E, et al. Increased temporal dispersion of myocardial repolarization in myotonic dystrophy type 1. Beyond the cardiac conduction system. Int J Cardiol 2012;156:259–264. [DOI] [PubMed] [Google Scholar]
- 5. Magrì D, Sciomer S, Fedele F, et al. Increased QT variability in young asymptomatic patients with β‐thalassemia major. Eur J Haematol 2007;79:322–329. [DOI] [PubMed] [Google Scholar]
- 6. Atiga WL, Calkins H, Lawrence JH, et al. Beat‐to‐beat repolarization lability identifies patients at risk for sudden cardiac death. J Cardiovasc Electrophysiol 1998;9:899–908. [DOI] [PubMed] [Google Scholar]
- 7. Haigney MC, Zareba W, Gentlesk PJ, et al. QT interval variability and spontaneous ventricular tachycardia or fibrillation in the Multicenter Automatic Defibrillator Implantation Trial [MADIT] II. J Am Coll Cardiol 2004;44:1481–1487. [DOI] [PubMed] [Google Scholar]
- 8. Piccirillo G, Magrì D, Matera S, et al. QT variability strongly predicts sudden cardiac death in asymptomatic subjects with mild or moderate left ventricular systolic dysfunction: A prospective study. Eur Heart J 2007;28:1344–1350. [DOI] [PubMed] [Google Scholar]
- 9. Dobson CP, La Rovere MT, Olsen C, et al. on behalf of the GISSI‐HF Investigators. 24‐Hour QT variability in heart failure. J Electrocardiol 2009;42:500–504. [DOI] [PubMed] [Google Scholar]
- 10. Dobson CP, La Rovere MT, Pinna GP, et al. QT variability index on 24‐Hour Holter independently predicts mortality in patients with heart failure: Analysis of GISSI‐HF trial data. Heart Rhythm 2011;8:1237–1242. [DOI] [PubMed] [Google Scholar]
- 11. Haigney MC, Zareba W, Nasir JM, et al. Gender differences and risk of ventricular tachycardia or ventricular fibrillation. Heart Rhythm 2009;6:180–186. [DOI] [PubMed] [Google Scholar]
- 12. Piccirillo G, Viola E, Bucca C, et al. QT interval dispersion and autonomic modulation in subjects with anxiety. J Lab Clin Med 1999;133:461–468. [DOI] [PubMed] [Google Scholar]
- 13. Piccirillo G, Viola E, Nocco M, et al. Autonomic modulation and QT interval dispersion in hypertensive subjects with anxiety. Hypertension 1999;34:244–246. [DOI] [PubMed] [Google Scholar]
- 14. Piccirillo G, Magrì D, Ogawa M, et al. Autonomic nervous system activity measured directly and QT‐interval variability in normal and pacing‐induced tachycardia heart failure dogs. J Am Coll Cardiol 2009;54:840–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Piccirillo G, Magnanti M, Matera S, et al. Age and QT variability index during free breathing, controlled breathing and tilt in patients with chronic heart failure and healthy control subjects. Transl Res 2006;148:72–78. [DOI] [PubMed] [Google Scholar]
- 16. Piccirillo G, Cacciafesta M, Lionetti M, et al. The influence of age, the autonomic nervous system and anxiety on QT interval variability. Clin Sci 2001;101:429–438. [PubMed] [Google Scholar]
- 17. Piccirillo G, Nocco M, Lionetti M, et al. Effects of sildenafil [Viagra] on cardiac repolarization and on autonomic control in subjects with heart failure. Am Heart J 2002;143:703–710. [DOI] [PubMed] [Google Scholar]
- 18. Piccirillo G, Quaglione R, Nocco M, et al. Effects of long‐term β‐blocker [metoprolol or carvedilol] therapy on QT variability in subjects with chronic heart failure secondary to ischemic cardiomyopathy. Am J Cardiol 2002;90:1113–1117. [DOI] [PubMed] [Google Scholar]
- 19. Piccirillo G, Magrì D Matera S, et al. A effect of pink grapefruit juice on QT variability in patients with dilatated or hypertensive cardiomyopathy and healthy subjects. Trasl Res 2008;151:267–272. [DOI] [PubMed] [Google Scholar]
- 20. Antzelevitch C, Shimizu W, Yan GX, et al. The M‐cell: Its contribution to the ECG and to normal and abnormal electrical function of the heart. J Cardiovasc Electrophysiol 1999;10:1124–1152. [DOI] [PubMed] [Google Scholar]
- 21. Antzelevitch C. Heterogeneity and cardiac arrhythmias: An overview. Heart Rhythm 2007;4:964–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Task force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology . Heart rate variability. Standard of measurements, physiological interpretation and clinical use. Circulation 1996;93:1043–1065. [PubMed] [Google Scholar]
- 23. Robbe HWJ, Mulder LJM, Rüddel H, et al. Assessment of baroreceptor reflex sensitivity by means of spectral analysis. Hypertension 1987;10:538–543. [DOI] [PubMed] [Google Scholar]
- 24. Pagani M, Somers V, Furlan R, et al. Changes in autonomic regulation induced by physical training in mild hypertension. Hypertension 1988;12:600–610. [DOI] [PubMed] [Google Scholar]
- 25. Piccirillo G, Cacciafesta M, Viola E, et al. Influence of ageing on cardiac baroreflex sensitivity determined non‐invasively by power spectral analysis. Clin Sci 2001;100:267–274. [PubMed] [Google Scholar]
- 26. Piccirillo G, Leonetti L R, Luparini R, et al. Effects of Carvedilol on heart and blood pressure variability in subjects with chronic heart failure. Am J Cardiol 2000;86;1392–1395. [DOI] [PubMed] [Google Scholar]
- 27. Piccirillo G, Di Giuseppe V, Nocco M, et al. Influence of aging and other cardiovascular risk factors on baroreflex sensitivity. J Am Geriatr Soc 2001;49:1059–1065. [DOI] [PubMed] [Google Scholar]
- 28. Guzzetti S, La Rovere MT, Pinna GD, et al. Different spectral components of 24 h heart rate variability are related to different modes of death in chronic heart failure. Eur Heart J 2005;26:357–362. [DOI] [PubMed] [Google Scholar]
- 29.La Rovere MT, Pinna GD, Maestri R, et al. Short‐term heart rate variability strongly predicts sudden cardiac death in chronic heart failure patients. Circulation 2003;107:565–570. [DOI] [PubMed] [Google Scholar]
- 30. Piccirillo G, Magrì D, Di Carlo S, et al. Influence of cardiac‐resynchronization therapy on heart rate and blood pressure variability: 1‐Year follow‐up. Eur J Heart Fail 2006;8:718–722. [DOI] [PubMed] [Google Scholar]
- 31. Piccirillo G, Ogawa G, Song J, et al. Power spectral analysis of heart rate variability and autonomic nervous system activity measured directly in healthy dogs and dogs with tachycardia‐induced heart failure. Heart Rhythm 2009;6:546–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Xia Y, Liang Y, Kongstad O, et al. In vivo validation of the coincidence of the peak and end of the T wave with full repolarization of the epicardium and endocardium in swine. Heart Rhythm 2005;2:162–169. [DOI] [PubMed] [Google Scholar]
- 33. Xia Y, Liang Y, Kongstad O, et al. Tpeak‐Tend interval as an index of global dispersion of ventricular repolarization: Evaluations using monophasic action potential mapping of the epi‐ and endocardium in swine. J Interv Cardiac Electrophysiol 2005;14:79–87. [DOI] [PubMed] [Google Scholar]
- 34. Opthof T, Coronel R, Wilms‐Schopman FJG, et al. Dispersion of repolarization in canine ventricle and the electrocardiographic T wave: Tp‐e interval does not reflect transmural dispersion. Heart Rhythm 2007;4:341–348. [DOI] [PubMed] [Google Scholar]
- 35. Jin H, Lyon AR, Akar FG. Arrhythmias mechanisms in the failing heart. Pacing Clin Electrophysiol 2008;31:1048–1058. [DOI] [PubMed] [Google Scholar]
- 36. Kurokawa J, Abriel H. Neurohormonal regulation of cardiac ion channels in chronic heart failure. J Cardiovasc Pharmacol 2009;54:98–105. [DOI] [PubMed] [Google Scholar]
- 37. Piccirillo G, Magrì D, Naso C, et al. Factors influencing heart rate variability power spectral analysis during controlled breathing in patients with chronic heart failure or hypertension and in healthy normotensive subjects. Clin Sci 2004;107:183–190. [DOI] [PubMed] [Google Scholar]
- 38. Sandercock GR, Brodie DA. The role of heart rate variability in prognosis for different modes of death in chronic heart failure. Pacing Clin Electrophysiol 2006;29:892–904. [DOI] [PubMed] [Google Scholar]


