Abstract
This case demonstrates the use of QRS scoring to quantify myocardial scar in a patient with cardiac sarcoidosis and left bundle branch block who progressively received an implantable defibrillator, cardiac resynchronization therapy (CRT), left ventricular assist device and cardiac transplantation. QRS scoring has been shown to correlate with magnetic resonance imaging measurements of scar, identify arrhythmogenic substrate and predict response to CRT, but had not previously been compared to pathology‐documented scar in nonischemic cardiomyopathies. Further study is warranted to assess the ability of QRS scoring to guide therapy for individual patients.
Ann Noninvasive Electrocardiol 2011;16(2):219–222
Keywords: noninvasive techniques ‐ electrocardiography; clinical, implantable devices ‐ biventricular pacing/defibrillation; Clinical, electrophysiology ‐ ventricular tachycardia; clinical
CASE STUDY TEXT
A 54‐year‐old woman underwent cardiac transplantation for severe congestive heart failure (CHF). She was diagnosed with nonischemic dilated cardiomyopathy 3 years previously after a viral illness. Cardiac catheterization showed no significant coronary artery disease. She received an implantable cardioverter‐defibrillator (ICD) at that time for primary prevention of sudden cardiac death. Subsequently, she was upgraded to a biventricular pacemaker for cardiac resynchronization therapy (CRT‐D), and she received mitral and tricuspid valve repair. Despite these interventions, her CHF worsened, she experienced ventricular tachycardia, received a left ventricular assist device, and underwent cardiac transplantation.
This case illustrates the number of invasive therapies available for CHF patients and the need for better diagnostic methods to risk‐stratify patients and guide appropriate therapy. Recent studies have shown that patients with increased myocardial scar detected by contrast‐enhanced magnetic resonance imaging (MRI) have an increased occurrence of ventricular tachyarrhythmias, while having a decreased response to CRT. 1 Although contrast‐enhanced MRI is promising as a risk‐stratifying tool, it is not widely available, especially in patients with implanted devices. Alternatively, a 12‐lead electrocardiographic (ECG) scoring system (QRS scoring) to quantify myocardial scar in the presence or absence of hypertrophy and conduction defects has been proposed and validated in comparison to contrast‐enhanced MRI. 2 QRS scoring has been shown to identify arrhythmogenic substrate 2 , 3 and predict response to CRT. 4
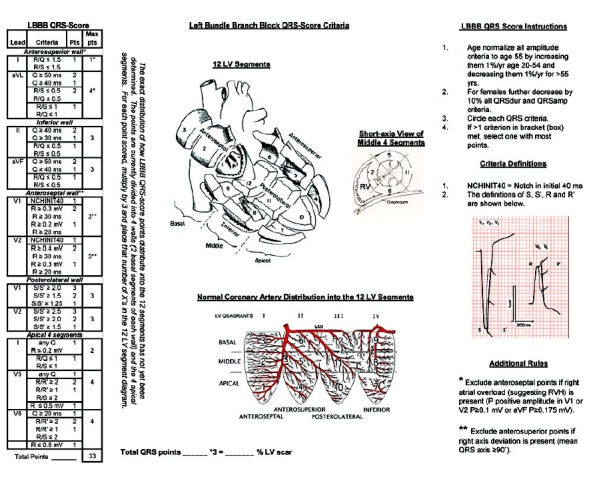
In this case, the patient's 12‐lead ECG shows a left bundle branch block (LBBB) (Fig. 1). The LBBB QRS‐scoring system contains Q‐, R‐, and S‐wave duration, amplitude, amplitude ratio, and notch criteria in eight of 12 leads. There are 33 possible points with each point representing 3% of the left ventricle (LV) infarcted or scarred (see Appendix). Using the LBBB QRS‐scoring system, the patient receives two QRS points in leads II and aVF suggesting inferior LV scar, three points in leads V1‐V2 suggesting anteroseptal LV scar, and four points in V5‐V6 suggesting apical LV scar. The nine total QRS points estimate that 27% of the LV is scarred.
Figure 1.
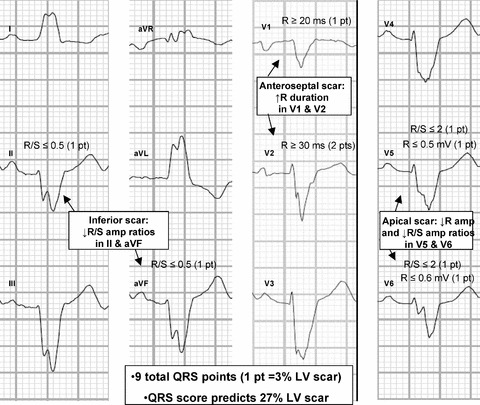
A 12‐lead ECG with LBBB QRS‐score points. The ECG received QRS‐score points in leads II and aVF (two points) suggesting inferior LV scar, leads V1‐V2 (three points) suggesting anteroseptal LV scar, and V5‐V6 (four points) suggesting apical LV scar. The nine total points estimate that 27% of the LV is scarred. ECG signs of scar in LBBB differ most significantly from normal conduction in V1‐V2. Because ventricular depolarization in LBBB proceeds through the septum only from the right ventricle to the LV, scar causes pathological R waves in V1‐V2 in LBBB rather than pathological Q waves in V1‐V2 in normal conduction. This is demonstrated in more detail in a prior publication. 2 The Appendix shows the complete QRS scoring system for LBBB.
Gross examination of the explanted heart showed extensive focal scar not typical of epicardial coronary artery disease (Fig. 2 and Fig. S1). This included scar in the subepicardium with subendocardial sparring, scar near the insertion points of right ventricle, and scar in the septum extending to the apex that varied from being transmural, limited to the midwall, and sparring the midwall at different points. While the patient was presumed to have viral myocarditis, clinically, microscopic examination revealed giant cells with asteroid bodies and noncaseating granulomas (Fig. 2 and Fig. S2) typical of cardiac sarcoidosis. Extensive replacement fibrosis (scar) was present with areas of interspersed live myocardium, which can create the substrate for reentrant tachyarrhythmias.
Figure 2.
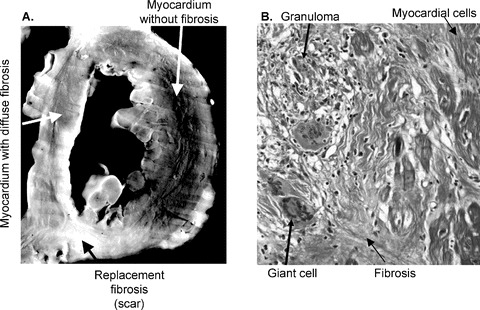
(A) The left image shows a mid‐ventricular short‐axis section of the explanted LV showing extensive scarring. This black and white image accentuates the differences in tissue composition between normal myocardium without fibrosis (black), myocardium with diffuse fibrosis (gray), and complete replacement fibrosis/scar (white). The Online Supplement Figure S1 contains enlarged color images. (B) The right image shows a microscopic image of the septum with Masson trichrome staining with differential staining of fibrosis (blue) and myocardial cells (red). The septum exhibits transmural mature scarring with interspersed viable myocytes in the midwall along with foci of inflammation, granulomas, and giant cells, throughout. Online Supplement Figure S2 shows the full‐size 1× and 20× magnifications of the septum.
Increased scar burden by contrast‐enhanced MRI and QRS scoring has been shown to correlate with increased ICD shocks and decreased response to CRT. This case demonstrates that pathologically documented scar in cardiac sarcoidosis can be detected by the 12‐lead ECG, even in the presence of LBBB. In line with recent studies, this patient with high‐scar burden developed ventricular tachycardia, but did not respond to CRT. Further study is warranted to assess the ability of widely available and inexpensive ECG algorithms to risk‐stratify patients and guide‐appropriate therapy.
Supporting information
Figure S1. Midventricular short‐axis section of the explanted LV showing extensive scarring. The left image is in color, while the right black and white image accentuates the differences in tissue composition between normal myocardium without fibrosis (black), myocardium with diffuse fibrosis (gray) and complete replacement fibrosis/scar (white).
Figure S2. Microscopic images of the interventricular septum at 1× (A) and 20× (B) magnification with Masson trichrome staining with differential staining of fibrosis (blue) and myocardial cells (red). The septum exhibits transmural mature scarring with interspersed viable myocytes in the midwall along with foci of inflammation, granulomas and giant cells throughout.
Supporting info item
Supporting info item
Supporting info item
Funding: None.
Conflicts of interest: The authors report no conflicts of interest.
REFERENCES
- 1. White JA, Yee R, Yuan X, et al Delayed enhancement magnetic resonance imaging predicts response to cardiac resynchronization therapy in patients with intraventricular dyssynchrony. J Am Coll Cardiol 2006;48:1953–1960. [DOI] [PubMed] [Google Scholar]
- 2. Strauss DG, Selvester RH, Lima JA, et al ECG quantification of myocardial scar in cardiomyopathy patients with or without conduction defects: Correlation with cardiac magnetic resonance and arrhythmogenesis. Circ Arrhythm Electrophysiol 2008;1:327–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Strauss DG, Poole JE, Wagner GS, et al An ECG index of myocardial scar enhances prediction of defibrillator shocks: An analysis of the Sudden Cardiac Death in Heart Failure Trial. Heart Rhythm 2011;8:38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sweeney MO, van Bommel RJ, Schalij MJ, et al Analysis of ventricular activation using surface electrocardiography to predict left ventricular reverse volumetric remodeling during cardiac resynchronization therapy. Circulation 2010;121:626–634. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Midventricular short‐axis section of the explanted LV showing extensive scarring. The left image is in color, while the right black and white image accentuates the differences in tissue composition between normal myocardium without fibrosis (black), myocardium with diffuse fibrosis (gray) and complete replacement fibrosis/scar (white).
Figure S2. Microscopic images of the interventricular septum at 1× (A) and 20× (B) magnification with Masson trichrome staining with differential staining of fibrosis (blue) and myocardial cells (red). The septum exhibits transmural mature scarring with interspersed viable myocytes in the midwall along with foci of inflammation, granulomas and giant cells throughout.
Supporting info item
Supporting info item
Supporting info item


