Abstract
Background: Biphasic pacing is a novel mode of pacing that was suggested to increase cardiac conduction velocity as compared with cathodal monophasic pacing. We aimed to evaluate the safety and efficacy of rapid atrial pacing to convert atrial fibrillation (AF) to normal sinus rhythm.
Methods: Multiple biphasic (anodal/cathodal), reverse biphasic (cathodal/anodal), and monophasic (cathodal) atrial pacing therapies were performed among 12 patients undergoing left atrial catheter ablation for AF. The efficacy end point was successful conversion of AF to sinus rhythm, and safety end point no induction of ventricular arrhythmias. Patients were paced at three cycle lengths (100, 200, and 333 msec) for 60 seconds at three locations (right and left atrial appendages and coronary sinus).
Results: Among the 66 biphasic (anodal/cathodal) pacing procedures one procedure in a patient with chronic AF, which involved pacing at the left atrial appendage with a cycle length of 200 msec, led to conversion of AF to sinus rhythm.
None of the 66 monophasic pacing procedures or the 66 reverse biphasic (cathodal/anodal) pacing procedures was associated with AF termination. None of the biphasic pacing procedures was associated with induction of ventricular arrhythmias.
Conclusions: Rapid atrial pacing using a variety of waveforms at the cycle length and output used in the current study was found to be safe. There was a single success in converting a chronic AF to sinus rhythm.
Ann Noninvasive Electrocardiol 2012;17(1):22–27
Keywords: atrial fibrillation/atrial arrhythmias; basic, cellular electrophysiology/electropharmocology
Atrial fibrillation (AF) is the most common sustained cardiac rhythm disturbance encountered in clinical practice. It affects 1–2% of the general population, and this figure is likely to increase as the population ages. 1 , 2 AF is associated with increased risk for death, stroke, heart failure, cognitive dysfunction, and poorer quality of life; mortality from AF‐related strokes is almost double that of strokes unrelated to AF, and functional deficits after AF‐related strokes are more likely to be severe. 2
Clinical management of patients with AF involves anticoagulation, rate, and rhythm control. Despite major advances in ablation therapy, radiofrequency catheter ablation (RFCA) has been offered mainly as a second line of therapy for patients with AF who remain symptomatic despite optimal medical therapy including antiarrhythmic drugs. This recommendation is due to the high recurrence rate of AF after RFCA ranging from 11% to 44% at 1 year and the complex ablation procedure that may be associated with severe complications such as cardiac tamponade, atrioesophageal fistula, and stroke. 2 Given the increasing prevalence of AF and the lack of treatment efficacy with ablation and/or antiarrhythmic drugs, there is a growing interest in developing more innovative, efficacious, and safer therapies.
In particular, there is considerable interest in the use of device‐based technologies for the prevention and treatment of AF. Sophisticated algorithms have been developed to increase the percentage of atrial pacing and suppress the onset of AF. 3 , 4 In addition, providing bursts of rapid atrial pacing, known as antitachycardia pacing (ATP), has been successful in the termination of organized rhythms such as atrial flutter and atrial tachycardias and was suggested to prevent the development of AF episodes. Commercially available implantable devices utilizing these algorithms have shown a greater than 50% ability to terminate episodes of atrial tachycardia, although the clinical impact on AF prevention has been variable. 4 , 5 , 6 , 7 , 8 , 9 Other optional electrically based therapies for terminating AF include pulsed low‐energy far‐field stimulation 10 and cardioversion. 11 To date, there is no pacing algorithm that was shown to successfully terminate AF.
Novel pacing waveforms containing some anodal content seem to increase cardiac conduction velocity as compared with cathodal monophasic pacing, 12 as well as having additional hemodynamic effects. 13 , 14 Burst atrial pacing with anodal and biphasic waveforms has been successfully used to revert induced AF in an acetylcholine‐induced canine model of that rhythm which has some electrophysiologic similarities in chronic AF in man. 15 , 16
The purpose of this pilot study is to evaluate (1) the efficacy of a new pacing mode employing biphasic pacing as compared with the conventional cathodal monophasic pacing to convert AF to sinus rhythm and (2) the safety of this pacing mode in three cardiac locations with regard to ventricular proarrhythmia risk.
METHODS
Study Population
Consecutive patients referred to the University of Rochester Medical Center for a pulmonary vein isolation/left atrial catheter ablation for AF were enrolled in the study. Patients had either paroxysmal or persistent AF; paroxysmal AF was defined as episodes of AF that self‐terminated in <7 days, and persistent AF was defined as AF episodes that lasted >7 days and/or required intervention for termination. Demographic and clinical information was obtained for all patients. Patients were excluded if they met any one of the following criteria: under 18 years of age, pregnant or lactating women, have active myocardial ischemia or had prior myocardial infarction, on intravenous inotropic or vasopressor medications, require mechanical hemodynamic support, are intubated, with renal failure requiring hemodialysis, baseline SBP <80 mmHg or DBP <30 mmHg, baseline pulse oximetry <90%, serum sodium <130 mEq/L or >150 mEq/L, serum potassium <3.3 mEq/L or >5.5 mEq/L, serum magnesium <1.8 mg/dL or >2.5 mg/dL, have severe (stenotic or regurgitant) structural valvular heart disease, congenital heart disease, or are unable to sign informed consent. The study protocol was approved by the local Institutional Review Board and all patients signed a written informed consent for the electrophysiology study, ablation procedure, and the pacing study.
Electrophysiology Study and Pacing Protocol
During the EPS and pacing study patients were continuously monitored on telemetry. Blood pressure was monitored and recorded utilizing an automated blood pressure cuff measurement prior to pacing, at minute one of pacing and upon completion of pacing. Oxygen saturation levels were followed using a continuous pulse oximeter. Heparin anticoagulation was used during the procedure to maintain an activated clotting time >350 seconds because of the recognized risk of spontaneous soft thrombus on sheaths/catheters.
All patients underwent a trans‐septal puncture performed with intracardiac echo guidance to access the left atrium. Diagnostic catheters were placed in the right atrium and coronary sinus and an open‐irrigated ablation catheter was placed in the left atrium for mapping and ablation purposes. If patients presented in normal sinus rhythm, rapid coronary sinus pacing was performed to induce AF. The diagnostic right atrial, coronary sinus and ablation catheter cables were attached in sequential fashion to the Multiphasic Slave Stimulator Model 71006 (Rivertek Medical Systems, St. Paul, MN, USA) which can be manually operated in order to deliver either monophasic or biphasic pacing. Pacing within the coronary sinus was performed with an octapolar diagnostic catheter using the most distal electrodes (SteeroCath‐Dx, Boston Scientific, Natick, MA, USA). Pacing within the left atrial appendage was performed with a 20 pole circular mapping catheter (Lasso, Biosense Webster, Diamond Bar, CA, USA). Pacing was performed from the electrode pair with the largest amplitude atrial electro gram. Pacing from the right atrial appendage was performed with a bidirectional irrigated tip ablation catheter (EZ Steer Thermocool, Biosense Webster).
Among 10 patients, we carried out pacing at a cycle length of 200 msec and repeated pacing at a cycle length of 100 msec, both for 60 seconds using the following different modes of pacing: (1) cathodal monophasic pacing at a pulse width of 1.7 msec and amplitude of 5 volts; (2) biphasic pacing (anodal/cathodal phases), both phases at 1.7 msec and 5 volts; and (3) reverse biphasic pacing (cathodal/anodal phases), both phases at 1.7 msec and 5 volts. Among two patients, we carried out pacing at a cycle length of 333 msec for 60 seconds using the following different modes of pacing: (1) Cathodal monophasic pacing at 0.3 msec and 5 volts; (2) biphasic pacing, anodal (0.8 msec) and cathodal (0.3 msec) at 5 volts; (3) reverse biphasic pacing, cathodal (0.3 msec) and anodal (0.8 msec) at 5 volts. These modes of pacing were repeated in all patients at three locations: right atrial appendage, proximal coronary sinus, and in the left atrial appendage. Thus, overall this study comprised 66 monophasic pacing procedures, and 132 different biphasic pacing procedures, each procedure lasting 60 seconds. Figure 1 describes the pacing protocol, summarizing different modes of pacing, cycle length, output, and pacing locations.
Figure 1.
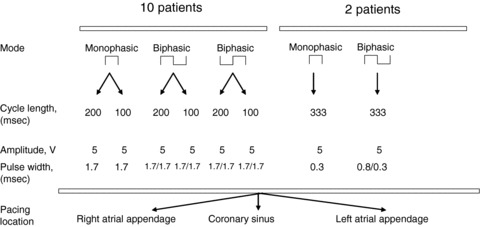
Summary of the pacing protocol.
End Points
The primary end point was conversion to normal sinus rhythm which was confirmed by the electrophysiologist as per standard electrocardiographic criteria. Secondary end points included ventricular premature beats, nonsustained and sustained ventricular tachycardia occurring during or immediately after the pacing procedure.
RESULTS
The present pilot study included 12 consecutive patients with AF; their clinical characteristics are described in Table 1. All patients were males, with a median (IQR) age of 61 (55–66) years, and a median LVEF of 52% (40–56%). Eighty‐three percent of patients had persistent AF and the reminder (17%) paroxysmal AF. Most patients had a long history of AF with a median duration of 2 years. Although most of them (83%) were treated with warfarin, overall they had a low risk for stroke with the vast majority (92%) having CHADS2 score of <2. Two patients (17%) were in sinus rhythm during the procedure and therefore underwent rapid coronary sinus pacing to induce AF.
Table 1.
Patient Characteristics
| n = 12 | |
|---|---|
| Age, years | 61 (55–66) |
| Male | 100% |
| HTN | 50% |
| Diabetes mellitus | 0% |
| CHF | 8% |
| TIA/CVA | 8% |
| Obstructive sleep apnea | 33% |
| CHADS2 score | |
| 0 | 42% |
| 1 | 50% |
| 2+ | 8% |
| LVEF,% | 52 (40–56) |
| Left atrial diameter, cm | 4.8 (4.3–5.1) |
| Left atrial volume, cm3 | 103 (74–124) |
| Persistent AF | 83% |
| Duration of AF, months | 24 (16–39) |
| Antiplatelet agents | 58% |
| Warfarin | 83% |
| Beta‐blockers | 67% |
| ACEI | 58% |
| Digoxin | 33% |
| Diuretics | 42% |
| Antiarrhythmic drugs | 50% |
Data are presented as median (interquartile range) and percentage.
No one of the 66 monophasic pacing procedures (22 procedures at three different locations) or the 66 reverse biphasic (cathodal followed by anodal) pacing procedures was associated with AF termination. Among the 66 biphasic (anodal followed by cathodal) pacing procedures one procedure, pacing at the left atrial appendage at a cycle length of 200 msec, led to the termination of AF. This occurred in a 31‐year‐old patient who had persistent AF for 4 years. It should be noted that monophasic or biphasic (cathodal followed by anodal) pacing in the same patient at the left atrial appendage at a cycle length of 200 msec using the same output was not associated with the termination of AF.
We have also evaluated the risk for ventricular arrhythmias, particularly because a third of the pacing procedures were performed in the proximal coronary sinus which could activate the left ventricle. There was no evidence of any ventricular arrhythmias (including VPBs, nonsustained VT, or sustained VT) during any of the monophasic or biphasic pacing procedures.
DISCUSSION
In the present pilot study we have evaluated the efficacy and safety of a novel pacing modality, employing biphasic pacing to convert AF to normal sinus rhythm among 12 patients. No one of the 66 monophasic or the 66 biphasic (cathodal/anodal) pacing procedures was associated with AF termination, whereas one procedure out of the 66 Biphasic (anodal/cathodal) pacing attempts terminated AF. No pacing procedure was associated with ventricular arrhythmias. Thus, overall the new mode of biphasic pacing appears to be safe, but pacing at the specific output and frequency used at the current study was not demonstrated to be useful for terminating AF.
Previous studies have shown that ATP has been successful in the termination of organized rhythms such as atrial flutter and atrial tachycardias. 4 , 5 , 6 , 7 , 8 , 9 , 17 In addition, the existence of an “excitable gap” and the resultant ability to capture at least a portion of the fibrillating atrium have been demonstrated in several models. 18 , 19 However, the ability to terminate the more disorganized and chaotic rhythm of AF by ATP is less certain. In the Jewel AF study, 7 pacing therapies successfully terminated 62% of atrial tachycardia episodes and 24% of AF episodes. In this study, the device discriminated atrial tachycardia from AF based on detection from a single site in the right atrium, using overlapping cycle length detection zones; thus the apparent effects of ATP on AF may reflect the inclusion of fast atrial tachycardia that were classified as AF and possibly the spontaneous termination of some episodes. 8
Other studies failed to show that conventional rapid pacing can effectively terminate AF. 20 , 21 Presumably an insufficient area of atrial myocardium to terminate the AF is depolarized as a result of collision with multiple wavelets and encroachment on the refractory period of the surrounding atrial myocytes.
Current pacemaker technology utilizes monophasic cathodal waveforms for pacing. The region of cardiac tissue excitation has a dog bone shape suggested to occur due to the distinct conducting properties of the intracellular and extracellular spaces. 22 Thus, cathodal stimulation of the heart leads to an excitation wave front which begins by direct depolarization of the cells in the region under the electrode. However, anodal stimulation can also pace the heart despite hyperpolarizing the cells in the region under the electrode with a dog bone shape; the cells at the convexity of the dog bone shape become depolarized, creating virtual cathodes, beginning the excitation wave front. Likewise, the mechanisms of myocardial excitation after the end of the pulse (break stimulation) involve virtual electrodes, but with reverse polarity as compared to cathodal and anodal make stimulation. 22 , 23
Anodal pacing was found to be associated with more vigorous action potential upstrokes and enhanced conduction as compared with cathodal pacing in Langendorff‐perfused rabbit hearts. 12 However, anodal pacing is associated with higher pacing thresholds and shorter refractory periods as compared with cathodal pacing which may predispose to ventricular tachyarrhythmias. Anodal pacing may also cause corrosion of the platinum contained in the pacemaker's metal wiring. 24 In a rabbit heart model, by combining both anodal and cathodal stimuli into a biphasic waveform, the high stimulation thresholds observed with sole anodal stimulation decrease while at the same time, preserving the beneficial effects of enhanced conduction. 12 Because the triggered depolarization travels down the myocardium faster using biphasic stimulation, there is the potential to capture an area of myocardium large enough to interrupt the sustainability of the AF and thereby terminate the arrhythmia. Evidence from this hypothesis comes from several preclinical trials which have demonstrated that anodal beats have a higher conduction velocity than cathodal beats in the canine atrium and that there is a higher likelihood of conversion to sinus rhythm from AF with rapid atrial pacing using anodal or biphasic pacing waveforms. 15 , 16 , 25 An alternate mechanism by means of which reversion might occur is the presence of a larger virtual electrode effect associated with anodal stimulation. 26
In the present study we have used two biphasic waveforms and a cathodal monophasic waveform to pace the atria at three locations including the coronary sinus. None of the pacing procedures was associated with an increased risk for ventricular arrhythmias. Based on animal studies exploring the effect of biphasic waveforms on AF termination 15 , 16 , 25 we paced at three different cycle lengths and several different outputs; however, in the present study only one AF episode was successfully converted to AF. Several factors might account for the lesser effectiveness in the present study compared to previous animal studies. It is possible that the transseptal puncture may have altered the ability to revert. Acetylcholine‐induced AF may not really be similar to the chronic human variety. It is also possible that other biphasic pacing waveforms using different cycle lengths and outputs or alternatively monophasic anodal pacing might confer greater success rates in AF conversion to sinus rhythm.
REFERENCES
- 1. Feinberg WM, Blackshear JL, Laupacis A, et al Prevalence, age distribution, and gender of patients with atrial fibrillation. Analysis and implications. Arch Intern Med 1995;155:469–473. [PubMed] [Google Scholar]
- 2. Camm AJ, Kirchhof P, Lip GY, et al Guidelines for the management of atrial fibrillation: The Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Eur Heart J 2010;31:2369–2429. [DOI] [PubMed] [Google Scholar]
- 3. Mitchell AR, Sulke N. How do atrial pacing algorithms prevent atrial arrhythmias? Europace 2004;6:351–362. [DOI] [PubMed] [Google Scholar]
- 4. Lee MA, Weachter R, Pollak S, et al The effect of atrial pacing therapies on atrial tachyarrhythmia burden and frequency: Results of a randomized trial in patients with bradycardia and atrial tachyarrhythmias. J Am Coll Cardiol 2003;41:1926–1932. [DOI] [PubMed] [Google Scholar]
- 5. Purerfellner H, Ruiter JH, Widdershoven JW, et al Reduction of atrial tachyarrhythmia episodes during the overdrive pacing period using the post‐mode switch overdrive pacing (PMOP) algorithm. Heart Rhythm 2006;3:1164–1171. [DOI] [PubMed] [Google Scholar]
- 6. Mont L, Ruiz‐Granell R, Martinez JG, et al Impact of anti‐tachycardia pacing on atrial fibrillation burden when added on top of preventive pacing algorithms: Results of the prevention or termination (POT) trial. Europace 2008;10:28–34. [DOI] [PubMed] [Google Scholar]
- 7. Friedman PA, Dijkman B, Warman EN, et al Atrial therapies reduce atrial arrhythmia burden in defibrillator patients. Circulation 2001;104:1023–1028. [DOI] [PubMed] [Google Scholar]
- 8. Gold MR, Leman RB, Euler DE. Effectiveness of rapid atrial pacing for termination of drug refractory atrial fibrillation: Results of a dual chamber implantable cardioverter defibrillator trial. Card Electrophysiol Rev 2003;7:341–344. [DOI] [PubMed] [Google Scholar]
- 9. Gillis AM, Unterberg‐Buchwald C, Schmidinger H, et al Safety and efficacy of advanced atrial pacing therapies for atrial tachyarrhythmias in patients with a new implantable dual chamber cardioverter‐defibrillator. J Am Coll Cardiol 2002;40:1653–1659. [DOI] [PubMed] [Google Scholar]
- 10. Fenton FH, Luther S, Cherry EM, et al Termination of atrial fibrillation using pulsed low‐energy far‐field stimulation. Circulation 2009;120:467–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Geller JC, Reek S, Timmermans C, et al Treatment of atrial fibrillation with an implantable atrial defibrillator—long term results. Eur Heart J 2003;24:2083–2089. [DOI] [PubMed] [Google Scholar]
- 12. Thakor NV, Ranjan R, Rajasekhar S, et al Effect of varying pacing waveform shapes on propagation and hemodynamics in the rabbit heart. Am J Cardiol 1997;79:36–43. [DOI] [PubMed] [Google Scholar]
- 13. Ravazzi A, Priolo C, Provera FP, et al Improvement of inter‐ventricular activation time using biphasic pacing pulses on right ventricle septal wall. Pacing Clin Electrophysiol 1999;22:A216 (abstr). [Google Scholar]
- 14. Lyon X, Sedmera D, Raddatz E, et al Comparison of effects of anodic versus cathodic pacing on ejection fraction through in ovo pacing of the chick embryo heart. Pacing Clin Electrophysiol 1999;22:A119 (abstr). [Google Scholar]
- 15. Oke L, Mower MM, Cothran LV, et al Pacing thresholds and apparent conduction velocities of anodal induced beats. J Invest Med 2000;48:101A (abst). [Google Scholar]
- 16. Oke L, Mower MM, Cothran LV, et al Comparison of simple and complex pacing waveforms for reversion of atrial fibrillation. J Invest Med 2000;8:108A (abstr). [Google Scholar]
- 17. Vollmann D, Kollet E, Luthje L, et al Comparison of immediate and delayed automatic antitachycardia pacing for the termination of atrial tachyarrhythmias. Europace 2005;7:248–54. [DOI] [PubMed] [Google Scholar]
- 18. Kirchhof C, Chorro F, Scheffer GJ, et al Regional entrainment of atrial fibrillation studied by high‐resolution mapping in open‐chest dogs. Circulation 1993;88:736–749. [DOI] [PubMed] [Google Scholar]
- 19. Daoud EG, Pariseau B, Niebauer M, et al Response of type I atrial fibrillation to atrial pacing in humans. Circulation 1996;94:1036–1040. [DOI] [PubMed] [Google Scholar]
- 20. Paladino W, Bahu M, Knight BP, et al Failure of single‐ and multisite high‐frequency atrial pacing to terminate atrial fibrillation. Am J Cardiol 1997;80:226–227. [DOI] [PubMed] [Google Scholar]
- 21. Everett THt, Akar JG, Kok LC, et al Use of global atrial fibrillation organization to optimize the success of burst pace termination. J Am Coll Cardiol 2002;40:1831–1840. [DOI] [PubMed] [Google Scholar]
- 22. Wikswo JP, Jr. , Lin SF, Abbas RA. Virtual electrodes in cardiac tissue: A common mechanism for anodal and cathodal stimulation. Biophys J 1995;69:2195–2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ranjan R, Chiamvimonvat N, Thakor NV, et al Mechanism of anode break stimulation in the heart. Biophys J 1998;74:1850–1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Thakral A, Stein LH, Shenai M, et al Effects of anodal vs. cathodal pacing on the mechanical performance of the isolated rabbit heart. J Appl Physiol 2000;89:1159–1164. [DOI] [PubMed] [Google Scholar]
- 25. Oke L, Mower MM, Cothran LV, et al Anodal pacing reversion in short‐term canine atrial fibrillation model. FASEB J 2000;14:A697 (abstr). [Google Scholar]
- 26. Ortega DF, Chirife R, Barja LD, et al Large virtual electrode and high output pacing: Effect on QRS duration. Pacing Clin Electrophysiol 1999;22:A12. [Google Scholar]


