Abstract
Background: This study aimed to compare the circadian distribution of the onset, maintenance and termination of paroxysmal atrial fibrillation (PAF) between structural and non‐structural heart diseases (SHD and NSHD, respectively) in the untreated state.
Subjects and Methods: We included 217 patients with 338 PAF (79 SHD patients with 131 episodes; 138 NSHD patients with 207 episodes). The probabilities for the onset, maintenance and termination of PAF for each hour were analyzed using Holter monitoring data and harmonic models being fitted into a cosinusoidal function.
Results: The SHD group had a triphasic circadian pattern at the onset with higher peaks at midnight, in the early morning and in the late afternoon (p < 0.05), whereas the NSHD group showed a single peak at midnight (p < 0.01). The probability of maintenance revealed a single peak during midnight (SHD, p < 0.0001; NHD, p < 0.01). The termination showed a peak at noon in the SHD group (p < 0.05), whereas there was a double peak at 10:00 am and 8:00 pm in the NSHD group (p = 0.06). RR intervals just after the PAF onset showed marked shortening in the daytime initiation PAF as compared to the nighttime initiation PAF in both SHD and NSHD groups (p < 0.01).
Conclusion: These observations suggest that the SHD group has very complex onset hours, whereas the NSHD group shows complex termination hours. Reflexly accelerated sympathetic tone just after the PAF onset is suggested in the daytime initiation PAF.
Keywords: circadian distribution, paroxysmal atrial fibrillation (PAF), onset, termination, structural and nonstructural heart diseases
With respect to the time of occurrence, early morning hours have been known to be the peak time for the incidence of acute myocardial infarction, 1 , 2 sudden cardiac death, 3 , 4 transient myocardial ischemia, 5 , 6 and stroke. 7 Regarding arrhythmias, premature ventricular contractions occur with definite onset hours throughout the day with high reproducibility in patients: daytime, nighttime, both daytime and nighttime, and irregular occurrences. 8 , 9 The incidence of paroxysmal supraventricular tachycardia is reportedly higher during the daytime than the period from midnight to the early morning hours. 10
Paroxysmal atrial fibrillation (PAF) is one of the most common arrhythmias in patients with structural and nonstructural heart diseases (SHD and NSHD, respectively). 11 , 12 Yamashita et al. 13 found a double peak for the onset with increases after lunch and midnight, and an interesting circadian variation in the total duration of PAF with a peak at midnight and a nadir at 11:00 AM. However, all their patients had NSHDs.
Although PAF may not be fatal by itself, it increases the incidence of stroke, especially at a higher risk in patients with SHDs. 14 , 15 Moreover, PAF is often associated with coronary artery disease, which may result in sudden cardiac death in relation to circadian variation with a peak incidence in the morning. 1 , 2 , 3 , 4 , 5 , 6 Therefore, a direct investigation of the circadian distribution of the onset of PAF in patients with SHDs should be considered.
The purpose of this study was to analyze the circadian distributions of the onset, maintenance, and termination of PAF associated with SHDs and to compare the corresponding values in NSHD patients in a drug‐free state.
SUBJECTS AND METHODS
Study Population
A total of 217 patients with 338 episodes of PAF in a drug‐free state were enrolled in this study (170 males [mean age, 62.1 ± 12.0 years] and 47 females [mean age, 68.1 ± 10.9 years]). PAF episodes that lasted for ≧1 minute were selected from the 19,148 consecutive Holter recordings obtained from October 1986 to September 1998. The clinical characteristics of the study population are summarized in Table 1. The first recording Holter monitoring was analyzed when the patients recorded two or more Holter monitoring within this period in a drug‐free state. Patients with PAF within this period were selected to allow an investigation of the prognosis or changes into chronic atrial fibrillation of these patients, which was intended as the topic of other study. Of the 217 patients, there were 79 patients (62 males [mean age, 64.9 ± 11.4 years] and 17 females [mean age, 69.6 ± 8.8 years]) with SHDs; these included 21 patients with sick sinus syndrome, 21 patients with hypertensive heart disease, 16 with coronary heart disease, 15 with essential hypertension, 7 with mitral valve diseases, 6 with cerebral vascular diseases, 4 with hyperthyroidism, 3 with dilated cardiomyopathy, and 8 with miscellaneous other heart diseases (the total number exceeds 79 because some patients had more than one disease). The remaining 138 patients (108 males [mean age, 59.2 ± 12.5 years] and 30 females [mean age, 66.5 ± 13.0 years]) had no apparent SHD. Whether a patient had an SHD or NSHD depended on the presence or absence of abnormal findings in a physical examination, 12‐lead electrocardiography, chest radiography, echocardiography, exercise testing, and laboratory tests, as well as the results of the coronary angiography, when needed.
Table 1.
Clinical Characteristics of the Study Population
| SHD (n = 79) | NSHD (n = 138) | P | |
|---|---|---|---|
| Number of episodes | 131 | 207 | |
| Age, years | 67.2 ± 10.1 | 62.8 ± 12.8 | |
| Male | 64.9 ± 11.4 | 59.2 ± 12.5 | P < 0.01 |
| Female | 69.6 ± 8.8 | 66.5 ± 13.0 | NS |
| Sex (male/female) | 62/17 | 108/30 | |
| Underlying diseases (%) | |||
| Sinus node dysfunction | 21 | ||
| Hypertensive heart disease | 21 | ||
| Coronary heart disease | 16 | ||
| Hypertension | 15 | ||
| Mitral valve diseases | 7 | ||
| Stroke | 6 | ||
| Hyperthyroidism | 4 | ||
| Dilated cardiomyopathy | 3 | ||
| Unknown | 8 | ||
| Blood pressure (BP) | |||
| Systolic BP (mmHg) | 146.2 ± 12.9 | 135.6 ± 7.7 | P < 0.05 |
| Diastolic BP (mmHg) | 88.2 ± 9.4 | 82.9 ± 7.4 | P < 0.01 |
| Echocardiographic findings | |||
| LVEF (%) | 65.3 ± 13.3 | 69.3 ± 9.8 | NS |
| LAD (mm) | 44.8 ± 6.1 | 36.0 ± 5.3 | P < 0.01 |
| LVPWth | 11.5 ± 2.5 | 10.0 ± 1.2 | P < 0.05 |
| IVSth | 12.2 ± 2.2 | 10.9 ± 1.4 | P < 0.05 |
| ECG findings | |||
| QRS duration (ms) | 96.8 ± 13.5 | 90.8 ± 14.2 | NS |
| QT interval (ms) | 377.7 ± 34.2 | 363.9 ± 90.9 | NS |
| QTc interval (ms) | 397.5 ± 43.0 | 385.2 ± 92.1 | NS |
Data represent mean ± SD or frequency. LVEF = left ventricular ejection fraction; LAD = left atrial dimension; QTc = rate‐corrected QT interval; LVPWth = posterior wall thickness of the left ventricle; IVSth = thickness of the interventricular septum.
Data Analysis
The duration of each episode of PAF was clasified into the following eight categories: ≧1 and <5 minutes, ≧5 and <10 minutes, ≧10 and <30 minutes, ≧30 and <60 minutes, ≧60 and <120 minutes, ≧120 and <600 minutes, ≧600 minutes, and undetermined duration. For each category, a comparison was made between SHD and NSHD groups. The undetermined duration reflected the existence of PAF at the beginning or termination of the Holter monitor recording; therefore, it was impossible to measure the duration of the PAF.
The method described by Yamashita et al. 13 , 16 was used to analyze data for the SHD and NSHD groups. Briefly, first, the hourly total recorded duration of PAF for the patients in each group was determined. Then, the hourly numbers for the onset, maintenance, and termination of PAF were determined and normalized according to the equation of Yamashita et al. 14 , 17 In brief, the probability of an onset at N o’clock equals the number of recordings that developed PAF between N and N + 1 o’clock divided by the number of recordings in sinus rhythm at N o’clock. The probability of maintenance at N o’clock equals the number of recordings with sustained PAF from N to N + 1 o’clock divided by the number of patients with PAF at N o’clock. The probability of termination at N o’clock equals the number of recordings in which the PAF terminated between N and N + 1 o’clock divided by the number of recordings that demonstrated PAF at N o’clock or developed it between N and N + 1 o’clock.
Average and minimum RR intervals for 5 beats just after the onset and just before the termination of PAF were measured to compare them between the daytime and nighttime initiation PAF and between just after the PAF onset and just before the PAF termination. The day was divided into two 12‐hour periods according to the time of PAF initiation: daytime initiation PAF (onset from 6:00 AM to 5:59 PM) and nighttime initiation PAF (onset from 6:00 PM to 5:59 AM).
The execution of this study was approved by the Institutional Review Board for Clinical Research of Tokai University School of Medicine.
Statistical Analysis
We used a chi‐square test to compare the differences in gender and the duration of PAF between the SHD and NSHD groups. The hourly data on the recorded duration of PAF and the data on the probabilities of onset, maintenance, and termination were analyzed using an algorithm for least‐squares cosine‐curve fitting. Single‐, double‐, or triple‐harmonic models with the hour of the day as the independent variable were fitted into a cosinusoidal function with a 24‐hour period: g(t) = M + A cos (ωt +ϕ), where M is the midline estimator statistic of rhythm, a rhythm‐adjusted 24‐hour time series mean. A is the amplitude (one‐half of the peak‐to‐trough difference by the fit of the single cosine curve approximation), ϕ is the acrophase (crest time of the fitted approximation referenced to local midnight), and t reflects the time of day in hours. 17 A circadian rhythm was considered to exist when the null hypothesis regarding the significance of rhythm was < 0.05. An unpaired Student's t‐test was used to compare the results of average and minimum RR intervals measurements for 5 beats just after the onset and just before the termination of PAF between different categories (the daytime vs nighttime initiation PAF; just after the PAF onset vs just before the PAF termination). P < 0.05 was used as cutoff for statistical significance.
RESULTS
Gender and Age Distribution
The study population comprised more male patients than female patients (P < 0.001), as shown in Figure 1. PAF occurrence was related to an increase in age and was most frequently documented in patients in their sixties for both genders.
Figure 1.
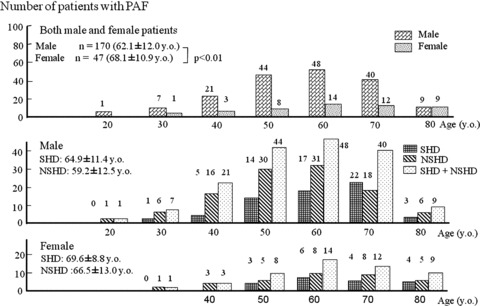
Age distribution of paroxysmal atrial fibrillation (PAF) in patients with structural and nonstructural heart diseases (NHD and NSHD, respectively).
Duration of PAF
There were no significant differences in each category of PAF duration between the SHD and NSHD groups, although the duration categories, namely, ≧120 and <600 minutes and ≧600 minutes showed a tendency of higher incidence in the NSHD group than in the SHD group (P < 0.1)(Fig. 2).
Figure 2.
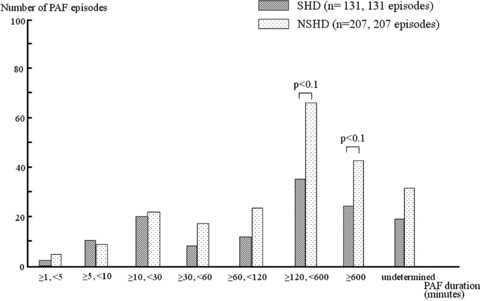
Distribution of the episode duration of PAF—its comparison between patients with SHD and NSHD, respectively.
Both SHD and NSHD groups showed a simple distribution of hourly total durations with a peak during the night at around 3:00–4:00 AM and with a nadir in the afternoon at around 4:00–5:00 PM (Fig. 3). Each distribution well fitted into a single harmonic curve: g(t) = 1419.960 + 993.192 × cos (ωt – 4.050) with the acrophase corresponding to 4:03 AM in the SHD group (P < 0.001), and g(t) = 2363.960 + 1335.192 × cos (ωt – 3.029) with the acrophase corresponding to 3:01 AM in the NSHD group (P < 0.001).
Figure 3.
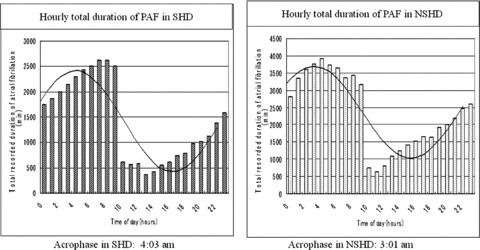
Hourly total duration of PAF in SHD and NSHD, respectively. Very similar single harmonic fit of the data with a peak (longest duration) around 3:00–4:00 AM and with a nadir (shortest duration) around 4:00–5:00 PM in PAF is observed in both groups.
Circadian Distribution of Onset, Maintenance, and Termination of PAF
Onset of PAF
The circadian distributions of the probability of PAF onset during the day showed a marked difference between the two groups (Fig. 4A). The SHD group had a triphasic circadian pattern with higher peaks between late evening and midnight, in the early morning, and in the late afternoon (Fig. 4A, left). It fitted into the triple harmonic curves: g(t) = 0.054 + 0.015 × cos (2ωt – 19.760) + 0.017 × cos (3ωt – 40.600) + 0.016 × cos (4ωt – 52.100)(P < 0.05), and each coefficient was significant (P < 0.05), except that of the third harmonic segment (P = 0.056).
Figure 4.
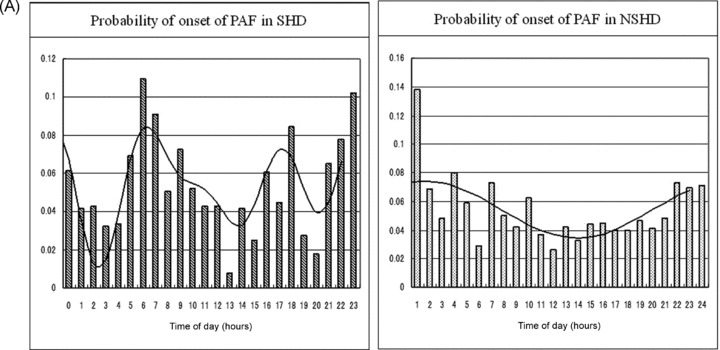
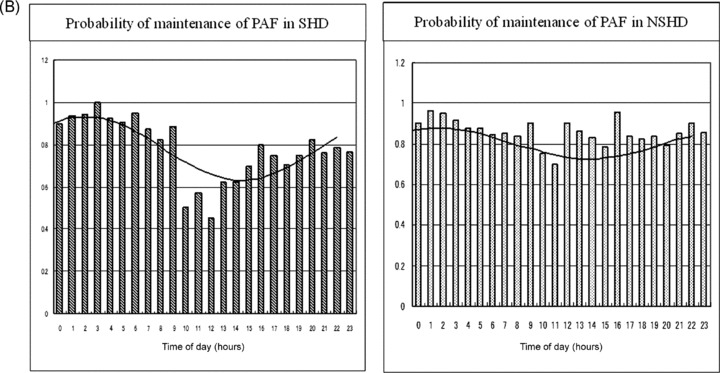
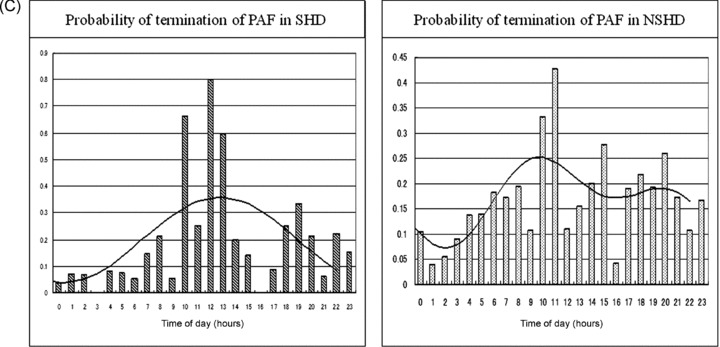
(A) Hourly probabilities of the onset of PAF in patients with SHD and NSHD, respectively. A marked difference in the circadian distribution is documented between the two groups. The details are described in the text. (B) Hourly probabilities of maintenance of PAF in patients with SHD and NSHD, respectively. The circadian distribution reveals a single harmonic fitting in both groups. The details are described in the text. (C) Hourly probabilities of termination of PAF in patients with SHD and NSHD, respectively. The distribution is in a mirror image of the distribution of maintenance in the SHD group, whereas it shows a double harmonic curve without a mirror image in the NSHD group. The details are described in the text.
In contrast, the NSHD group showed a simple single peak between late evening and midnight (Fig. 4A, right). The cosinusoidal function fitted into g(t) = 0.054 + 0.021 × cos (ωt – 0.559) with the acrophase corresponding to 0:33 AM (P < 0.01).
Maintenance of PAF
The distribution of the probability of maintenance revealed a single peak at around midnight and a nadir in the early afternoon in both groups: g(t) = 0.781 + 0.159 × cos (ωt − 2.037) with the acrophase corresponding to 2:02 AM in the SHD group (P < 0.0001), and g(t) = 0.803 + 0.075 × cos (ωt − 1.438) with the acrophase corresponding to 1:25 AM in the NSHD group (P < 0.01) (Fig. 4B).
Termination of PAF
The circadian distribution of the probability of termination was a mirror image of the probability of maintenance in the SHD group, and it significantly well fitted into g(t) = 0.199 + 0.160 × cos (ωt − 12.767) with the acrophase corresponding to 12:46 PM and a nadir corresponding to around midnight (P < 0.05)(Fig. 4C, left). On the other hand, the probability of termination in the NSHD group tended to fit with the presence of a double harmonic curve: g(t) = 0.170 + 0.061 × cos (2ωt − 12.940) + 0.0278 × cos (3ωt − 8.737) with peak incidences corresponding to around 10:00 AM (P = 0.052) and 8:00 PM (P = 0.181) (total: P = 0.063)(Fig. 4C, right).
RR Intervals Just after the Onset and Just before the Termination of PAF
Average and minimum RR intervals for 5 beats just after the onset of PAF were more shortened in the daytime initiation PAF than in the nighttime initiation PAF both in groups NSHD and SHD (P < 0.01 each). There were, however, no significant differences regarding RR intervals just before the PAF termination between the daytime and nighttime initiation PAFs in both groups (Fig. 5A, B). In the NSHD group, average and minimum RR intervals just after the PAF onset were shorter than those just before the termination in the daytime initiation PAF (P < 0.05 each), whereas no significant differences were found in the nighttime initiation PAF. In the SHD group, there were no significant differences in average and minimum RR intervals between just after the onset and just before the termination of PAF both in the daytime and nighttime initiation PAFs (Fig. 5A, B).
Figure 5.
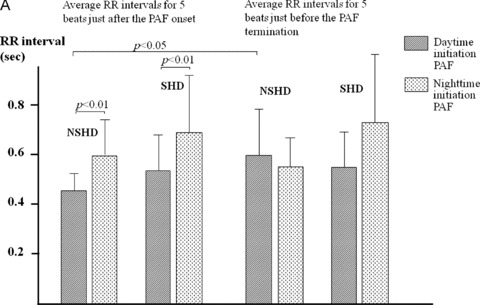
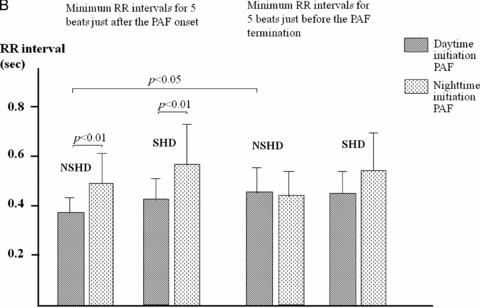
(A) Average RR intervals for 5 beats just after the onset and just before the termination of PAF in the daytime and nighttime initiation PAF. Details are described in the text. (B) Minimum RR intervals for 5 beats just after the onset and just before the termination of PAF in the daytime and nighttime initiation PAF. Details are described in the text.
DISCUSSION
Our main findings were as follows: (1) very similar single harmonic fits of the circadian distributions of the hourly total PAF duration with a peak during the night at around 3:00–4:00 AM in both the SHD and NSHD groups; (2) a marked difference in the distributions of the PAF onset between the two groups, with a triphasic circadian pattern in the SHD group and a simple single maximum in the NSHD group; and (3) a distribution of the PAF termination with a peak at around noon in the SHD group, which was a mirror image of the distribution of the maintenance, and a relative double peak that was not a mirror image of the probability of maintenance in the NSHD group.
PAF is well known to have a higher occurrence in men than in women. This study supported this finding. The NSHD group showed a tendency for higher incidence of long PAF duration. Yamashita et al. 13 demonstrated much shorter duration of PAF beginning during the day and much longer duration PAF beginning in the night in NSHD patients. Our data showed that most PAF (58%) occurred during the night in NSHD patients, whereas less than half occurred at night in SHD patients. The reason for the tendency of a higher incidence of longer PAFs in NSHD patients may be explained by a greater incidence of episodes at night in NSHD patients than in SHD patients.
A previous study has reported a circadian variation in the total hourly duration of PAF with a peak prolongation at midnight and a minimal prolongation at about 11:00 AM in NSHD patients. 13 This study supports the above finding for the NSHD patients. In addition, our data suggest that SHD patients also show a longer hourly total PAF duration at night, although the hourly duration was relatively shorter in SHD patients than in NSHD patients.
Some studies demonstrates a double‐peak occurrence of PAF during nighttime and morning hours, 18 , 19 whereas other studies have shown a peak incidence during the daytime, 20 no circadian distribution 21 or triple peaks at noon to 2:00 PM, 6:00 PM to 2:00 AM, and 4:00 AM to 6:00 AM. 22 However, most of these studies do not seem to reflect the real circadian distribution of PAF, because they only included patients with symptomatic episodes; 18 , 19 , 21 those in special circumstances such as in an emergency room 18 or mobile coronary care unit; 19 those with transtelephonic transmission of ECG; 21 those taking antiarrhythmic drugs including beta‐blockers; 18 , 20 and those who had undergone simultaneous analysis for SHDs and NSHDs. 18 , 19
One study precisely considered the characteristics of the NSHD patients, and absence of drug therapy, and included both symptomatic and asymptomatic subjects. 13 However, the results of the circadian distribution of the PAF onset for the NSHD patients in this previous study were different from our results: the peak incidence only at midnight was noted in our study whereas a double peak with increases after lunch and at midnight was reported in their study.
The circadian distribution pattern for the PAF onset can be attributed to sympatovagal imbalance, especially in patients with NSHDs. Coumel 23 clinically studied the selective effects of vagal and sympathetic activity on PAF, and distinguished vagally induced PAF from sympathetically induced PAF. According to a part of his description, vagally induced PAF was always idiopathic (NSHD) and it most often occurred at night. In contrast, sympathetically induced PAF was considerably less frequent than PAF of vagal origin, and it often occurred during the daytime in association with a myocardial disease rather than with a normal heart. The NSHD patients in the study of Yamashita et al. 13 included patients with essential hypertension (38 of 150 patients), whereas the NHSD group in our study did not include such patients. The plasma concentration was highly elevated during the daytime from 8:00 AM to 6:00 PM than during the nighttime in patients with essential hypertension. 24 Therefore, in our NSHD patients, PAF is believed to be induced by vagal stimulation, whereas in their patients, PAF occurred in response to either vagal or sympathetic stimuli.
Some studies have shown no significant difference in the time of PAF occurrence between SHD and NSHD groups. 18 , 19 In this study, however, the SHD group showed a triphasic circadian pattern and the NSHD group, a simple harmonic curve. The reason for the difference between the previous studies and ours is unclear. The previous study failed to mention data on treatment with antiarrhythmic agents, or whether the groups, SHD or NSHD comprised patients with hypertension. The distribution with much more frequent onset peaks during the day in the SHD group in our study appear reasonable, since not only autonomic influence but also cardiac load by itself is considered to initiate episodes of PAF in this group.
The SHD group in this study showed a distribution of PAF termination with a peak between 10:00 AM and 01:00 PM. This is very similar to the phenomenon in older patients that was studied by Yamashita et al. 16 They reported substantially greater sympathetic augmentation in the elderly than in younger patients. The much greater incidence of PAF termination between the late morning and noon observed in our study may be due to sympathetic tone augmentation during the daytime. The termination of PAF in the NSHD group may also be influenced by an increase in sympathetic tone, since a relatively higher incidence of the distribution of termination was seen in the late morning and in the afternoon in this study.
Gabathuler and Adamec 25 observed that heart rate of 40 beats/min just before the onset of PAF rapidly increased to that of 94 beats/min just after the beginning of PAF. We compared average and minimum RR intervals just after the PAF onset and those just before the PAF termination between the daytime and nighttime initiation PAFs. As a result, in both SHD and NSHD groups, those RR intervals just after the PAF onset showed significant shortening in the daytime initiation PAF as compared to the nighttime initiation PAF, whereas no significant differences in RR intervals just before the termination between both initiation PAFs were found. In addition, RR intervals just after the PAF onset were much more shortened than those just before the PAF termination in the NSHD group. These observations, together with the findings of Gabathuler and Adamec, 25 suggest that reflexly accelerated sympathetic tone just after the onset of PAF replaces vagal tone predominance just before the PAF onset, especially in the daytime initiation PAF.
This study, however, had some limitations. First, it was difficult to determine the real duration of some PAF episodes because the PAF was already present before the beginning of the Holter recording (PAF termination could, however, be evaluated) or PAF started after the completion of the recording (the onset could be evaluated). Second, the selection of the patients was limited because of inclusion of only opportunities of Holter recording for the evaluation of cardiovascular disease including arrhythmia. Nevertheless, this study demonstrated new findings regarding the differences in the clinical characteristics of the onset, maintenance, and termination of PAF between SHD and NSHD patients.
REFERENCES
- 1. Muller JE, Stone PH, Turi ZG, et al. MILIS Study Group . Circadian variation in the frequency of onset of acute myocardial infarction. N Engl J Med 1985;313:1315–1322. [DOI] [PubMed] [Google Scholar]
- 2. Hjalmarson A, Gilpin EA, Nicod P, et al Differing circadian patterns of symptoms onset: In subgroups of patients with acute myocardial infarction. Circulation 1989;80:267–275. [DOI] [PubMed] [Google Scholar]
- 3. Muller JE, Ludmer PL, Willich SN, et al Circadian variation in the frequency of sudden cardiac death. Circulation 1987;75:131–138. [DOI] [PubMed] [Google Scholar]
- 4. Willich SN, Levy D, Rocco MB, et al Circadian variation in the incidence of sudden cardiac death in the Framingham Heart Study Population. Am J Cardiol 1987;60:801–806. [DOI] [PubMed] [Google Scholar]
- 5. Rocco MB, Barry J, Campbell S, et al Circadian variation of transient myocardial ischemia in patients with coronary artery disease. Circulation 1987;75:395–400. [DOI] [PubMed] [Google Scholar]
- 6. Mulcahy D, Cunningham D, Crean P, et al Circadian variation of total ischaemic burden and its alteration with anti‐anginal agents. Lanset 1988;2:755–759. [DOI] [PubMed] [Google Scholar]
- 7. Tsementzis SA, Gill JS, Hichcock ER, et al Diurnal variation of and activity during the onset of stroke. Neurosurgery 1985;17:901–904. [DOI] [PubMed] [Google Scholar]
- 8. Lown B, Tykocivski M, Garfein A, et al Sleep and ventricular premature beats. Circulation 1973;48:691–701. [DOI] [PubMed] [Google Scholar]
- 9. Tanabe T, Yoshikawa H, Tagawa R, et al Evaluation of antiarrhythmic drug efficacy using Holter electrocardiographic technique. Jpn Circ J 1985;49:337–344. [DOI] [PubMed] [Google Scholar]
- 10. Irwin JM, McCarthy EA, Wilkinson WE, et al Circadian occurrence of symptomatic proxysmal supraventricular tachycardia in untreated patients. Circulation 1988;77:298–300. [DOI] [PubMed] [Google Scholar]
- 11. Kopecky SL, Gersh BJ, McGoon MD, et al The natural history of lone atrial fibrillation: A population‐based study over three decades. N Engl J Med 1987;317:669–674. [DOI] [PubMed] [Google Scholar]
- 12. Fleinberg WM, Blacxkshear JL, Laupacis R, et al Prevalence, age distribution and gender of patients with atrial fibrillation. Analysis and implication. Arch Intern Med 1995;155:469–478. [PubMed] [Google Scholar]
- 13. Yamashita T, Murakawa Y, Sezaki K, et al Circadian variation of atrial fibrillation. Circulation 1997;96:1537–1541. [DOI] [PubMed] [Google Scholar]
- 14. Kannel WB, Abbott RD, Savage DD, et al Epidemiologic features of atrial fibrillation: The Framingham Study. N Engl J Med. 1982;306:1018–1022. [DOI] [PubMed] [Google Scholar]
- 15. Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation: A major contributor to stroke in the elderly. Arch Intern Med. 1987;147:1561–1564. [PubMed] [Google Scholar]
- 16. Yamashita T, Murakawa Y, Hayami N, et al Relation between aging and circadian variation of paroxysmal atrial fibrillation. Am J Cardiol 1998;11:1364–1367. [DOI] [PubMed] [Google Scholar]
- 17. Brigham C, Arbogart B, Guillaume GC, et al Inferential statistical methods for estimating and comparing cosinor parameters. Chronobiology 1982;9:397–439. [PubMed] [Google Scholar]
- 18. Kupari M, Koskinen P, Leinonen H. Double‐peaking circadian variation in the occurrence of sustained supraventricular tachyarrhythmias. Am Heart J 1990;120:1364–1369. [DOI] [PubMed] [Google Scholar]
- 19. Rostagno C, Taddei T, Paladini B, et al The onset of symptomatic atrial fibrillation and paroxysmal supraventricular tachycardia is characterized by different circadian rhythm. Am J Cardiol 1993;71:453–455. [DOI] [PubMed] [Google Scholar]
- 20. Rawles JM, Metcalfe MJ, Jennings K. Time of occurrence, duration, and ventricular rate of paroxysmal atrial fibrillation: Effect of digoxin. Br Heart J 1990;63:225–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Clair WK, Wilkinson WE, McCarthy EA, et al Spontaneous occurrence of symptomatic paroxysmal atrial fibrillation and paroxysmal supraventricular tachycardia in untreated patients. Circulation 1993;87:1114–1122. [DOI] [PubMed] [Google Scholar]
- 22. Vincenti A, Brambilla R, Fumagalli MG, et al Onset mechanism of paroxysmal atrial fibrillation detected by ambulatory Holter monitoring. Europace 2006;8:204–210. [DOI] [PubMed] [Google Scholar]
- 23. Coumel P. Neural aspects of paroxysmal atrial fubrillation In: Falk RH, Podrid PJ. (eds.): Atrial Fibrillation. Mechanisms and Management. New York , Raven Press, 1992, pp 109–125. [Google Scholar]
- 24. Prinz PN, Halter J, Benedetti C, et al Circadian variation of plasma catecholamine in young and old men: Relation to rapid eye movement and slow wave sleep. J Clin Endocrinol Metab 1979;49:300–304. [DOI] [PubMed] [Google Scholar]
- 25. Gabauthler J, Adamec R. Triggering of paroxysmal auricular fibrillation. Study using continuous electrocardiographic recording (Holter system). Arch Mal Coeur Vaiss 1985;78:1255–1262. [PubMed] [Google Scholar]


