Abstract
Background: Aim of our study is to evaluate the role of T‐wave alternans (TWA) to stratify the risk of sudden cardiac death in athletes (Ath) with complex ventricular arrhythmias (VA), and to document a possible correlation between TWA and electrophysiological testing (EPS) results.
Methods: We studied 85 Ath with VA (61 M, mean age 32 ± 11 years). In all cases a cardiological evaluation was performed, including TWA and EPS. The patients were evaluated during a follow‐up of 30 ± 21 months. The end point was the occurrence of sudden death (SD) or malignant ventricular tachyarrhythmias (VT).
Results: TWA was negative in 57 Ath (68%), positive in 15 (18%) and indeterminate in 13 (14%). All subjects with negative TWA did not show induction of VT at EPS, with significant correlation between negative TWA and negative EPS (P < 0.001). All Ath with positive TWA also had VT induced by a EPS, with significant correlation (P < 0.001). By contrast, our data did not show significant correlation between indeterminate TWA and positive or negative EPS. However, there was significant correlation between abnormal TWA test (positive + indeterminate) and inducibility of VT at EPS (P < 0.001). During follow‐up we observed a significant difference in end point occurrence (VT or SD) between Ath with negative or abnormal TWA and between Ath with negative or positive EPS.
Conclusion: TWA confirm its role as a simple and noninvasive test, and it seems useful for prognostic stratification of Ath with VA.
Keywords: T‐wave alternans, sudden death, ventricular arrhythmias, sport activity
Sudden cardiac death (SCD) related to ventricular tachyarrhythmia in young athletes is a dramatic event that has emotional and social impact on the media and the medical community. Ventricular ectopic beats (VEB), even with frequent and/or complex forms, have frequently been described on 24‐hour electrocardiogram (ECG) in 25–65% of athletes, even in absence of underlying structural heart disease. For athletes with ventricular arrhythmias who have no evidence of underlying heart disease or who have an unsuspected cardiovascular disease, identifying subjects at risk of serious ventricular tachyarrhythmias is often problematic, and such cases pose a diagnostic dilemma for physicians called upon to assess the subject's eligibility to practice sport. 1
Systematic diagnostic methods, including ECG, Holter monitoring, stress testing, and echocardiography are sometimes unable to reveal an underlying structural heart disease, and further evaluations are required in order to determine whether the arrhythmia is benign or potentially life‐threatening.
Specific forms of electrocardiographic alternans related to cardiac repolarization abnormalities have recently emerged as a cause of serious ventricular arrhythmias. In the 1980s, Adam and Smith 2 , 3 developed the spectral analysis method for detecting microvolt T‐wave alternans (TWA) in experimental animals and, subsequently, in humans. 4 , 5 These studies showed that microvolt TWA is a reliable noninvasive risk marker of cardiac electrical vulnerability and a predictor of ventricular arrhythmias. 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 The usefulness of TWA in predicting the risk of ventricular tachyarrhythmias has been widely reported in several clinical conditions, including coronary artery disease, nonischemic cardiomyopathy, congestive heart failure and in patients with implantable cardiac defibrillators. 14 Recently, several authors have documented the high‐negative predictive value of TWA in patients with heart disease and low ejection fraction. 15 , 16 , 17
The aim of our study was to evaluate the predictive value of TWA in stratifying the risk of severe ventricular tachyarrhythmias and SCD in athletes with ventricular arrhythmias, and to investigate the relationship between TWA and the results of programmed ventricular stimulation during electrophysiologic endocavitary study.
MATERIAL AND METHODS
Study Protocol
We enrolled 85 consecutive athletes (61 males, 24 females), with no history of cardiovascular disease, with age 32 ± 11 years involved in different types of sports. All subjects had been referred to our Cardiology Department following the detection of ventricular arrhythmias during eligibility screening for competitive sports and first‐level cardiological evaluation. These arrhythmias included frequent VEB, arbitrarily defined as premature ventricular complexes (PVC) ≥2000 (58 athletes) or nonsustained ventricular tachycardia (NSVT) (27 athletes) on 24‐hour ambulatory ECG monitoring. NSVT is defined as a sequence of more than three PVC, with duration less than 30 seconds.
All patients underwent a basic cardiological evaluation at our Cardiology Department, which consisted of anamnesis, physical examination, routine blood tests (including fT3, fT4, TSH), ECG, Holter recording, echocardiogram, and maximal exercise test. Furthermore, ajmaline test for detection of Brugada syndrome, coronary angiogram, right and evaluation left ventriculogram, magnetic resonance imaging and endomyocardial biopsy were performed, depending on the basic cardiological. 18 All athletes underwent electrophysiologic study (EPS) with programmed ventricular stimulation (PVS) and microvolt TWA testing. Informed consent was obtained from each subject.
T‐Wave Alternans Test
TWA was measured always off antiarrhythmic drugs during a bicycle exercise test after careful skin preparation, including mild abrasion, and placement of high‐resolution electrodes (Microvolt Sensors, Cambridge Heart, Inc., Bedford, MA) in order to minimize signal noise. Electrocardiographic leads were placed in the standard 12‐lead positions and in a Frank orthogonal configuration (X,Y,Z). Electrocardiographic signals were amplified and digitalized, and measurements were taken by means of the CH2000 System (Cambridge Heart, Inc.) using the spectral method. This technique involved measuring the amplitudes of corresponding points in 128 consecutive T waves, at the same time after the QRS complex. The amplitude fluctuation was then subjected to spectral analysis. The software then calculated:
-
1)
the alternans voltage (Valt), defined as the difference in voltage between the overall mean beats and either the even‐numbered or odd‐numbered mean beats;
-
2)
the alternans ratio (K), a measure of the significance of microvolt TWA, defined as the ratio of alternans power divided by the standard deviation of the noise in the reference frequency band. A K ≥ 3 was considered to be significant.
TWA was classified as positive if it was sustained (duration ≥1 minutes) with onset at heart rate ≤110 bpm, Valt≥ 1.9 μV and K ≥ 3. TWA was considered negative if it did not meet the positivity criteria and if at least 1 minute of artifact‐free data was available at heart rate ≥105 bpm. Otherwise, the test was classified as indeterminate. 19 Positive or indeterminate TWA test were considered as an abnormal result. 16
Electrophysiologic Study
Electrophysiologic study was performed always off antiarrhythmic drugs. A 6‐French tetrapolar recording and stimulating catheter was inserted through the right femoral vein and positioned in the right ventricular apex. PVS was performed through an automatic stimulator (Micropace EPS 320) with up to three extrastimuli at basic cycle lengths of 600, 500, and 400 msec, down to the ventricular refractory period, but never <180 msec. The same protocol was then repeated with the catheter placed in the right ventricular outflow tract. EPS was considered positive if sustained monomorphic VT was induced.
Follow‐up
All patients were directly evaluated every 6 months and in the event of symptoms. The occurrence of serious ventricular arrhythmias (VT requiring access to emergency room or VF) or SCD was regarded as the end point. SCD was defined as unexpected death occurring within 1 hour of the onset of symptoms or during sleep. The mean follow‐up was 30 ± 21 months.
Statistical Analysis
Continuous variables are expressed as mean value ± standard deviation and were compared using an unpaired t‐test. Categorical variables were compared using Fisher exact test or chi‐square test with Yates correction for continuity, where appropriate. Correlation between results of TWA and EPS were studied by logistic regression analysis. Furthermore, in order to establish a possible significant influence of the TWA and EPS variables on the time free from malignant arrhythmic events, we evaluated the follow‐up data by generating Kaplan–Meier survival curves where the end point was the occurrence of a malignant ventricular arrhythmias and the grouping variables were TWA (positive, negative, and indeterminate) and EPS (positive and negative). A multivariate analysis was performed in order to verify if the presence of structural heart disease was a predictive factor of arrhythmic events during follow‐up.
RESULTS
A structural heart disease was identified in 10 patients (12%). Specifically, arrhythmogenic right ventricular dysplasia was diagnosed in five patients and myocarditis in three. Furthermore, in two patients, the echocardiographic picture was borderline for the diagnosis of early‐stage dilated cardiomyopathy, with left ventricular dilation and left ventricular ejection fraction of about 50%.
Pharmacological antiarrhythmic therapy was started in 25 patients (29%): 10 (40%) were affected by structural heart disease, while in 15 (60%) there was no diagnosis of evident heart disease, therapy being started owing to the complexity of ventricular arrhythmias or to the positive results of electrophysiologic diagnostic tests (TWA or EPS). Antiarrhythmic therapy consisted of amiodarone in 12 patients (48%), beta‐blockers in eight (32%), sotalol in three (12%), and an association of amiodarone and beta‐blockers in two (8%). After cardiological evaluation, an ICD was implanted in six subjects (7%): four affected by arrhythmogenic right ventricular dysplasia and two by myocarditis (Fig. 1). The 25 athletes with heart disease with positive EPS or taking antiarrhythmic drugs were disqualified for sport participation.
Figure 1.
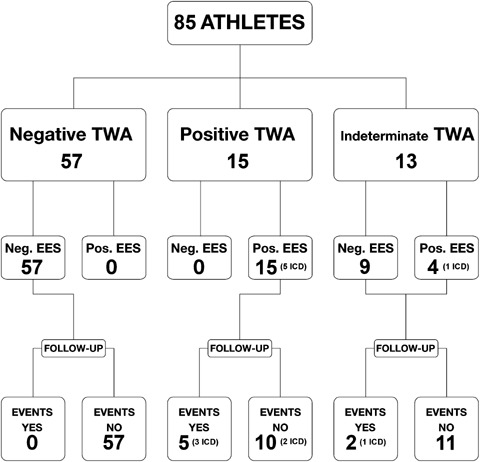
Results of TWA test and EPS and occurrence of spontaneous ventricular arrhythmias during follow‐up in study population. In brackets it is specified the number of patients who received ICD.
Microvolt‐TWA Test
Microvolt‐TWA testing proved negative in 58 patients (68%), positive in 15 (18%), and indeterminate because of excessive number of ectopic beats in 13 (14%). Therefore, 28 patients (32%) showed abnormal microvolt‐TWA testing. There were no significant differences between patients with abnormal or negative TWA in terms of age, sex, ejection fraction, sports activity, and clinical presentation. By contrast, our data show that among patients with abnormal TWA there is a significantly higher prevalence of structural heart disease, prescription of antiarrhythmic therapy and ICD implantations (Table 1).
Table 1.
Comparison of Clinical and Demographic Characteristics between Patients with Negative and Abnormal TWA
| TWA | Negative TWA | Abnormal | P |
|---|---|---|---|
| Age | 31 ± 11 | 33 ± 8 | P = NS |
| Males | 41 (72%) | 20 (71%) | P = NS |
| Sport | |||
| – Soccer | 13 (23%) | 5 (18%) | P = NS |
| – Running | 16 (28%) | 8 (29%) | P = NS |
| – Skiing | 3 (5%) | 3 (11%) | P = NS |
| – Cycling | 9 (16%) | 5 (18%) | P = NS |
| – Motorcycling | 2 (3%) | 0 (0%) | P = NS |
| – Volleyball | 6 (10%) | 4 (14%) | P = NS |
| – Swimming | 7 (12%) | 1 (4%) | P = NS |
| – Climbing | 1 (2%) | 1 (4%) | P = NS |
| – Gymnastics | 0 (0%) | 1 (4%) | P = NS |
| EF (%) | 66 ± 5 | 66 ± 5 | P = NS |
| Arrhythmias | |||
| – VEBs | 38 (67%) | 20 (71%) | P = NS |
| – NSVTs | 19 (33%) | 8 (29%) | P = NS |
| Structural Heart disease | 2 (3%) | 8 (37%) | P< 0.01 |
| Antiarrhythmic therapy | 5 (9%) | 20 (71%) | P < 0.01 |
| ICD | 0 (0%) | 6 (21%) | P < 0.01 |
Electrophysiologic Study
Ventricular tachycardia was induced through programmed ventricular stimulation in 19 patients (22%), and was not induced in 66 (78%). There were no significant differences between these patients in terms of age, sex, clinical presentation, ejection fraction, sports activity. By contrast, our data show that among patients with positive EPS there is a significantly higher prevalence of structural heart disease (42% vs 3%, P < 0.001), prescription of antiarrhythmic therapy (100% vs 9%, P < 0.001) and ICD implantations (34% vs 0%, P < 0.001).
Correlation between TWA Test and Electrophysiologic Study
Malignant ventricular arrhythmias were not inducible during EPS in any of the subjects in whom the TWA test was negative, with significant correlation between a negative microvolt‐TWA test and noninducibility of VT (P < 0.001). In addition, our data showed that VT had been induced in 68% of patients with abnormal TWA, with significant correlation between inducibility of VT and abnormal TWA test (P < 0.001). Specifically, in all patients with positive TWA test there was inducibility of VT, with significant correlation between positive TWA and positive EPS (P < 0.001). By contrast, among patients in whom the TWA test was indeterminate, four (31%) showed inducibility of VT, while in nine (69%) the EPS was negative. So, our data did not show a significant correlation between an indeterminate TWA test and inducibility or non‐inducibility of VT during EPS (P > 0.05). In our study, positive TWA test has a positive predictive value (PPV) of 100% in predicting inducibility of VT during EPS, while PPV of abnormal TWA test for this purpose is 68%. The Negative Predictive Value (NPV) of TWA in predicting the inducibility of VT is 100% (Table 2).
Table 2.
Correlation between TWA Test and Electrophysiologic Study
| Negative EPS | Positive EPS | p | |
|---|---|---|---|
| Negative TWA | 57 (100%) | 0 (0%) | P < 0.001 |
| Indeterminate TWA | 9 (69%) | 4 (31%) | P = NS |
| Positive TWA | 0 (0%) | 15 (100%) | P < 0.001 |
| Abnormal TWA | 9 (32%) | 19 (68%) | P < 0.001 |
Follow‐up
During a mean follow‐up of 30 months, seven patients (8%) experienced an arrhythmic event regarded as the end point of the study (VF in two patients, VT in four and SCD in one). Three patients were affected by arrhythmogenic right ventricular dysplasia and one by myocarditis. By contrast, in three patients no structural heart disease had been detected by cardiological evaluation. An ICD had been implanted in the four patients with diagnosed structural heart disease, and pharmacologic antiarrhythmic therapy had been prescribed in all cases (amiodarone in three patients, beta‐blockers in two and association of amiodarone and beta‐blocker in two). Multivariate analysis identified abnormal TWA as a predictor of the occurrence of ventricular arrhythmias and sudden death during follow‐up. By contrast, in our study group the presence of a previously diagnosed structural heart disease did not result as predictor of outcome.
The difference in end point occurrence rate was significant between patients with a negative TWA test (0%) and those with abnormal TWA test (25%) (P < 0.01). Specifically, six patients had had positive TWA, and one patient indeterminate TWA In our study, the TWA test predicted the occurrence of malignant ventricular arrhythmias with a NPV of 100% and a PPV of 25% (Fig. 2). Furthermore, PPV rises to 40% in the presence of positive TWA.
Figure 2.
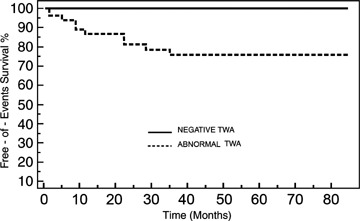
Kaplan–Meier survival curves comparing end point occurrence between patients with negative and abnormal TWA.
Similarly, there was a significant difference in end point occurrence rate between patients in whom the electrophysiologic study proved positive and those in whom it was negative (37% vs 0%) (P < 0.01). The electrophysiologic study predicted the occurrence of malignant ventricular arrhythmias with a NPV of 100% and a PPV of 37% (Fig. 3).
Figure 3.
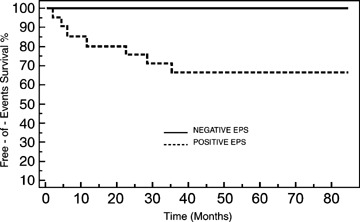
Kaplan–Meier survival curves comparing end point occurrence between patients with negative and positive EPS.
DISCUSSION
VEB have been detected by surface electrocardiogram in 1% of the general population, and by 24‐hour ambulatory ECG Holter monitoring in 40–75% of apparently healthy subjects. 20 , 21 However, athletes constitute a particular population of healthy people, in that they show a high prevalence of morphological cardiac alterations (a condition known as athlete's heart), electrocardiographic abnormalities (wave voltage modification, ST, and T wave changes) and frequent and/or complex ventricular beats. An athlete's heart undergoes morphological adaptations that can mimic a cardiovascular disease. Consequently, it is not easy to make a differential diagnosis with heart diseases at risk of sudden death, such as, for example, hypertrophic and dilated cardiomyopathy and arrhythmogenic right ventricular cardiomyopathy, which are often difficult to diagnose in their initial phase. The onset of these alterations in the athlete's heart can be due to adaptation to dynamic sports activity and the impact of training on cardiac cavity size. Several prospective studies have shown that detraining and physical deconditioning can result in cardiac reverse remodeling, with a reduction in cavity size, and in the reversibility of ventricular arrhythmias, which is complete in about 25% of subjects and partial in 50%. 22 , 23 Biffi et al. 24 reported that the presence of frequent and/or complex ventricular beats in competitive athletes did not indicate an adverse prognosis in subjects without underlying structural heart disease. Nevertheless, the detection of frequent and complex ventricular arrhythmias in a competitive athlete requires particular attention on the part of cardiologists and sports physicians in order to determine whether these arrhythmias have a good prognosis or are potentially life threatening. In recent studies, several authors have reported that competitive athletes with silent arrhythmogenic heart disease have a higher risk of sudden death than sedentary subjects of the same age and with a similar latent cardiac substrate. 25 , 26 , 27
In the recently published European Recommendations for competitive sports participation in athletes with cardiovascular diseases, 18 the authors state that it is mandatory to clarify if ventricular arrhythmias are caused by an undiagnosed structural heart disease. If clinical history and diagnostic screening with noninvasive and invasive tests does not demonstrate the presence of heart disease, there are other issues to consider: the role of intense training on the arrhythmic pattern and probably a different disease progression in young athletes, the potential usefulness of detraining and the criteria of eligibility for sports activity.
The value of TWA in predicting ventricular tachyarrhythmias in patients with congestive heart failure and with previous myocardial infarction has been evaluated in several clinical studies. 12 , 13 , 14 , 15 , 16 , 17 These reports document a highly significant statistical association between positive TWA and ventricular tachyarrhythmic events. Very few data are available on the risk stratification of SCD and severe ventricular tachyarrhythmic events in athletes with ventricular arrhythymias. Heidbuchel et al. 28 demonstrated that in high‐level endurance athletes with ventricular arrhythmias the inducibility of VT at EPS is the only variable correlated with outcome, with a RR 3.4 for VT or SCD during follow‐up. Furthermore, in a multicenter study of 52 competitive athletes with severe arrhythymias, Furlanello et al. 27 found a high negative predictive value of TWA when both programmed ventricular stimulation and follow‐up were used as end points. Unfortunately, the limited number of athletes with positive TWA in this study did not allow to obtain a significant positive predictive value. In our population of athletes undergoing TWA evaluation and programmed ventricular stimulation, we documented a significant correlation between TWA test negativity and noninducibility of ventricular arrhythmias through PVS and between positive or abnormal TWA testing and inducibility of ventricular arrhythmias at EPS.
Comparison between TWA and Programmed Ventricular Stimulation in Different Populations
Review of a number of prospective studies conducted in a variety of clinical patient populations suggests that the occurrence rate of severe ventricular tachyarrhythmias in patients with positive TWA is equivalent to that of ventricular tachyarrhythmias in patients with a positive result on programmed ventricular stimulation during electrophysiologic endocavitary study. The event rate among patients with negative TWA is low, and in many cases lower than the event rate in patients with a negative response to programmed ventricular stimulation. 12 , 13 , 14 , 15 , 16 , 17 In many studies, TWA has proved to be equivalent to programmed ventricular stimulation and better than SAECG in the risk stratification of patients for life‐threatening arrhythmias. 4 , 7 , 12 , 13 , 14 , 15 , 16 , 17
In our study we found a significant difference between patients with abnormal and negative TWA tests with regard to the occurrence of sudden death, ventricular fibrillation and sustained ventricular tachycardia during an average follow‐up of 30 months. Kaplan–Meier survival analysis showed a 100% rate of end point‐free survival in patients with a negative TWA test and a 75% rate in subjects with an abnormal TWA test, suggesting that TWA can also predict spontaneous malignant arrhythmic events in athletes with ventricular arrhythmias. By contrast, in our study group the presence of structural heart disease did not result as a predictor of SCD or ventricular arrhythmias during follow‐up. Therefore, in our study TWA might be useful in order to identify patients with complex ventricular arrhythmias who are particularly prone to develop life‐threatening ventricular arrhythmias, even in the absence of a structural heart disease clearly detectable by routinely performed invasive and non‐invasive diagnostic tests. However, in our patients we did not perform genetic analysis in order to detect the presence of genetically determined arrhythmogenic diseases, so we were not able to surely rule out the presence of such diseases, even in the absence of typical electrocardiographic pattern. The NPV (100%) and PPV (25% for abnormal TWA test, rising to 40% for positive TWA test), obtained in our study are comparable to those reported by Gehi et al. 29 in different populations.
TWA and Programmed Ventricular Stimulation in Competitive Athletes
Few studies have evaluated TWA as a predictor of arrhythmic events in competitive athletes, and little informations are available on the comparison of TWA and programmed ventricular stimulation in arrhythmia risk stratification in this population. 27 In our study, the predictive accuracy of invasive programmed ventricular stimulation during EPS was similar to that of TWA. These results, which were obtained in a single‐center experience, are in line with those from a multicenter study by Furlanello et al. 27 on 52 competitive athletes, and suggest that TWA may play an important role in the prognostic stratification of athletes with ventricular arrhythmias. Unlike electrophysiological study, which is an invasive procedure requiring hospitalization, TWA testing is noninvasive, cheap, easy to perform and repeatable during follow‐up.
Study Limitations
A limitation of our study is the presence of patients taking antiarrhythmic drugs. In fact, the antiarrhythmic therapy may interfere with the arrhythmias occurrence during follow‐up and with clinical outcome with unknown mechanisms. Furthermore, in our study multivariate analysis did not demonstrate a predictive value of the presence of structural heart disease with regard to the occurrence of ventricular arrhythmias and sudden death during follow‐up. However, we cannot rule out the presence of arrhythmogenic diseases with apparently intact heart in patients without diagnosis of structural heart disease who had ventricular arrhythmias or sudden death during follow‐up.
CONCLUSION
TWA is an effective noninvasive predictor of the risk of severe ventricular tachyarrhythmias and SCD in competitive athletes, its efficacy being at least comparable to that of invasive programmed ventricular stimulation. In particular, TWA has a high negative predictive value. In athletes with ventricular arrhythmias TWA seems to be useful for improving risk stratification for SCD.
REFERENCES
- 1. Corrado D, Basso C, Rizzoli G, et al Does sports activity enhance the risk of sudden death in adolescents and young adults? J Am Coll Cardiol 2003;42:1959–1963. [DOI] [PubMed] [Google Scholar]
- 2. Adam D, Smith J, Askelrod S, et al Fluctuations in T‐wave morphology and susceptibility to ventricular fibrillation. J Electrocardiol 1984;17:209–218. [DOI] [PubMed] [Google Scholar]
- 3. Smith JM, Clancy EA, Valeri R, et al Electrical alternans and cardiac electrical instability. Circulation 1988;77:110–121. [DOI] [PubMed] [Google Scholar]
- 4. Rosenbaum DS, Jackson LE, Smith JM, et al Electrical alternans and vulnerability to ventricular arrhythmias. N Engl J Med 1994;330:235–241. [DOI] [PubMed] [Google Scholar]
- 5. Nearing B, Huang AH, Verrier RL. Dynamic tracking of cardiac vulnerability by complex demodulation of the T‐wave. Science 1991;252:437–440. [DOI] [PubMed] [Google Scholar]
- 6. Sutton PMI, Taggart P, Lab M, et al Alternans of epicardial repolarization as a localized phenomenon in man. Eur Heart J 1991;12:70–78. [DOI] [PubMed] [Google Scholar]
- 7. Gold MR, Bloomfield DM, Anderson KP, et al A comparison of T‐wave alternans, signal averaged electrocardiography and programmed ventricular stimulation for arrhythmia risk stratification. J Am Coll Cardiol 2000;36:2247–2253. [DOI] [PubMed] [Google Scholar]
- 8. Platt SB, Vijgen JM, Albrecht P, et al Occult T wave alternans in long QT syndrome. J Cardiovasc Electrophysiol 1996;7:144–148. [DOI] [PubMed] [Google Scholar]
- 9. Momiyama Y, Hartikainen J, Nagayoshi H, et al Exercise‐induced T‐wave alternans as a marker of high risk in patients with hypertrophic cardiomyopathy. Jpn Circ J 1997;61:650–656. [DOI] [PubMed] [Google Scholar]
- 10. Armoundas AA, Rosenbaum DS, Ruskin JN, et al Prognostic significance of electrical alternans versus signal averaged electrocardiography in predicting the outcome of electrophysiological testing and arrhythmia‐free survival. Heart 1998;80:251–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hohnloser SH, Klingenheben T, Li YG, et al T wave alternans as a predictor of recurrent ventricular tachyarrhythmias in ICD recipients: Prospective comparison with conventional risk markers. J Cardiovasc Electrophysiol 1998;9:1258–1268. [DOI] [PubMed] [Google Scholar]
- 12. Ikeda T, Saito H, Tanno K, et al T‐wave alternans as a predictor for sudden death after myocardial infarction. Am J Cardiol 2002;89:79–82. [DOI] [PubMed] [Google Scholar]
- 13. Klingenheben T, Zabel M, D'Agostino RB, et al Predictive value of T‐wave alternans for arrhythmic events in patients with congestive heart failure. Lancet 2000;356:651–652. [DOI] [PubMed] [Google Scholar]
- 14. Narayan SV. T‐Wave Alternans and the Susceptibility to Ventricular Arrhythmias. J Am Coll Cardiol 2006;47:269–281. [DOI] [PubMed] [Google Scholar]
- 15. Hohnloser SH, Ikeda T, Bloomfield DM, et al T‐wave alternans negative coronary patients with low ejection fraction and benefit from defibrillator implantation. Lancet 2003;362:125–126. [DOI] [PubMed] [Google Scholar]
- 16. Bloomfield DM, Steinman RC, Namerow PB, et al Microvolt T‐wave alternans distinguishes between patients likely and patients not likely to benefit from implanted cardiac defibrillator therapy. A solution to the Multicenter Automatic Defibrillator Implantation Trial (MADIT) II conundrum. Circulation 2004;110:1885–1889. [DOI] [PubMed] [Google Scholar]
- 17. Bloomfield DM, Bigger JT, Steinman RC, et al Microvolt T‐wave alternans and the risk of death or sustained ventricular arrhythmias in patients with left ventricular dysfunction. J Am Coll Cardiol 2006;47:456–463. [DOI] [PubMed] [Google Scholar]
- 18. Pelliccia A, Fagard R, Bjornstad HH, et al Recommendations for competitive sports participation in athletes with cardiovascular disease. Eur Heart J 2005;26:1422–1445. [DOI] [PubMed] [Google Scholar]
- 19. Bloomfield DM, Hohnloser SH, Cohen RJ. Interpretation and classification of microvolt T‐wave alternans tests. J Cardiovasc Electrophysiol 2002;13:502–512. [DOI] [PubMed] [Google Scholar]
- 20. Bjerregaard P. Premature beats in healthy subjects 40–79 years of age. Eur Heart J 1982;3:493–503. [DOI] [PubMed] [Google Scholar]
- 21. Fleg JL, Kennedy HL. Cardiac arrhythmias in a healthy elderly population: Detection by 24‐hour ambulatory electrocardiography. Chest 1982;81:302–307. [DOI] [PubMed] [Google Scholar]
- 22. Spirito P, Pelliccia A, Proschan MA, et al Morphology of the “athlete's heart” assessed by echocardiography in 947 elite athletes representing 27 sports. Am J Cardiol 1994;74:802–806. [DOI] [PubMed] [Google Scholar]
- 23. Biffi A, Maron BJ, Verdile L, et al Impact of physical deconditioning on ventricular tachyarrhythmias in trained athletes. J Am Coll Cardiol 2004;44:1053–1058. [DOI] [PubMed] [Google Scholar]
- 24. Biffi A, Pelliccia A, Verdile L, et al Long‐term clinical significance of frequent and complex ventricular tachyarrhythmias in trained athletes. J Am Coll Cardiol 2002;40:446–452. [DOI] [PubMed] [Google Scholar]
- 25. Furlanello F, Bertoldi A, Dallago M, et al Cardiac arrest and sudden death in competitive athletes with arrhythmogenic right ventricular dysplasia. Pacing Clin Electrophysiol 1998;21:331–335. [DOI] [PubMed] [Google Scholar]
- 26. Thiene G, Basso C, Corrado D. Pathology of sudden death in young athletes: European experience In: Bayes de Luna A, Furlanello F, Maron BJ, Zipes DP. (eds.): Arrhythmias and sudden death in athletes. Dordrecht , Kluwer Academic Publisher, 2001; Chap. 5, pp. 49–69. [Google Scholar]
- 27. Furlanello F, Galanti G, Manetti P, et al Microvolt T‐wave alternans as predictor of electrophysiological testing results in professional competitive athletes. ANE 2004;9:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Heidbuchel H, Hoogsteen J, Fagard R, et al High prevalence of right ventricular involvement in endurance athletes with ventricular arrhythmias. Eur Heart J 2003;24:1473–1480. [DOI] [PubMed] [Google Scholar]
- 29. Gehi AK, Stein RH, Metz LD, et al Microvolt T‐wave alternans for the risk stratification of ventricular tachyarrhythmic events. J Am Coll Cardiol 2005;46:75–82. [DOI] [PubMed] [Google Scholar]


