Abstract
Background: Sudden cardiac death and myocardial infarction have a circadian variation with a peak incidence in the early morning hours. Increased dispersion of repolarization facilitates the development of conduction delay necessary to induce sustained arrhythmia. Both QT‐dispersion and T‐wave peak to T‐wave end (TpTe) have been proposed as markers of dispersion of myocardial repolarization.
Methods: Forty healthy adults (20 women), age 35–67 years old, with normal EKGs, echocardiograms, stress tests, and tilt‐table tests were analyzed during a 27‐hour hospital stay. EKGs were done at eight different time points. QT‐intervals, QT‐dispersion, and TpTe were measured at each time point. Harmonic regression was used to model circadian periodicity, P < 0.05 was considered significant.
Results: The composite QT‐interval was longer in women than in men (416 ± 17 msec vs 411 ± 20 msec, respectively, P = 0.006). The QT‐dispersion among all leads was greater in men than women (37 ± 13 msec vs 30 ± 11 msec, respectively, P < 0.0001); a similar difference was found in the precordial leads. Harmonic regression showed that QT‐dispersion had a significant circadian variation, primarily in men. In men, the maximum QT‐dispersion occurred at 6 AM (45 ± 15 msec). TpTe also had a significant circadian variation that was not affected by gender in the majority of leads.
Conclusions: A circadian variation exists in the dispersion of myocardial repolarization, as measured by both TpTe and QT‐dispersion. Men and women have a different circadian variation pattern. Further studies regarding the mechanisms and clinical implications are needed.
Ann Noninvasive Electrocardiol 2010;15(1):3–10
Keywords: repolarization, circadian variation, QT‐dispersion
Sudden cardiac death and myocardial infarction exhibit a circadian variation with a peak incidence in the early morning hours, 1 and men have a greater incidence of sudden cardiac death compared to women. 2 Increased dispersion of repolarization facilitates the development of conduction delay necessary to induce sustained arrhythmia by a premature stimulus. 3 Recent interest on ventricular repolarization has focused on the descending limb of the T wave as an indicator for differences in repolarization, whether transmural 4 , 5 or apicobasal. Alternatively, dispersion of the QT‐interval has been previously proposed as a marker for the regional differences in repolarization 6 and prior studies have shown gender and age differences in this marker. 7 , 8 , 9
A circadian variation in repolarization has been previously reported, 10 , 11 , 12 , 13 , 14 and the cycle‐by‐cycle variability in ventricular repolarization has been found to be lower during the night and exhibits a peak shortly after awakening in the morning. 15 However, previous studies of circadian variation in ventricular repolarization have not used harmonic regression analysis, a published technique used in modeling variation throughout a 24‐hour cycle. 15 , 16
The purpose of this study is to use harmonic regression analysis to model the circadian periodicity of multiple indicators of dispersion in cardiac repolarization, and to analyze the effect of gender on this model.
METHODS
Study Population
The study protocol was approved by the Northwestern University Institutional Review Board. The study consists of 40 healthy adults (20 men, 20 women) between the ages of 35–67 years, with a mean age of 42.7 ± 9.0 years in women and 42.9 ± 7.0 years in men. Men were significantly taller, heavier, and had greater body mass index (BMI) than did women (Table 1). Sixteen of the 20 women were premenopausal, and four were postmenopausal. None of the postmenopausal women were on hormone replacement therapy. All subjects had a normal electrocardiogram (EKG), echocardiogram, stress test, and a tilt‐table test to rule out structural disease and autonomic dysfunction. Exclusion criteria consisted of: (1) major ECG abnormalities – QRS duration >110 msec, left or right ventricular hypertrophy, evidence of Q‐wave myocardial infarction, long QT syndrome (QT‐interval > 480 msec) or severe T‐wave abnormalities (T‐wave flattening or biphasic T waves); (2) the presence of heart disease by stress test or two‐dimensional echo including evidence left ventricular hypertrophy (ventricular wall thickness >1.2 cm), (3) therapy with drugs known to affect ventricular repolarization such as antiarrhythmic agents, phenothiazines, or terfenadine, (4) subjects with conditions altering the autonomic nervous system including diabetes or autonomic dysfunction evidenced by tilt table test.
Table 1.
Demographics of Subjects
| Men | Women | |
|---|---|---|
| Age (years) | 42.9 ± 7.1 | 42.7 ± 9.2 |
| Height (cm) | 178.4 ± 5.8* | 161.8 ± 5.5 |
| Weight (kg) | 85.7 ± 16.8* | 67.5 ± 14.6 |
| BSA (m2) | 2.03 ± 0.20* | 1.71 ± 0.17 |
*P < 0.05.
BSA = body surface area.
Subjects were admitted to Northwestern Memorial Hospital's Clinical Research Center and serial 12‐lead ECGs were recorded at a paper speed of 25 mm/sec with a gain of 10 mm/mV during a 27‐hour hospital stay. All rooms had windows facing open environments. Subjects abstained from caffeine during the hospital stay. Subjects were required to rest supine for 15 minutes prior to each recording, and were instructed to sleep from 11 PM to 7 AM. Each subject had a total of 10 EKG recordings beginning at 8 AM on day 1 and ending at 6 AM on day 2. EKG recordings were taken at 6 AM, 8 AM, 10 AM, 12 noon, 6 PM, 8 PM, 10 PM, and 12 midnight.
Indicators of Repolarization
An automated computer software program QT Guard (GE Healthcare, Chalfont St. Giles, UK) was used to analyze the ECG variables. 17 The QT Guard analysis software analyzes the QRS complexes in all 12 leads and develops a filtered complex that is then used for the estimation of the Q‐wave onset. The Q‐wave onset is identical in each lead. The T‐wave peak in each lead is determined by the “regional centering method” that detects the maximum point of the T wave and for T‐plateau the center of the plateau is taken as T‐wave peak. T‐wave offset was defined as the intersection of the maximal slope of the terminal T wave with a baseline threshold defined by the T‐P segment using a least‐square fitting method, reported to have the most reproducibility. 18 The QT‐interval was defined as the Q‐wave onset to the T‐wave offset. Bazett's formula 19 [QT/(RR∧1/2)] was used to correct for heart rate. T‐peak to T‐end (TpTe) was computed as the differences from T‐wave peak to the T‐wave offset. 5 QT‐interval dispersion (QTD) was computed as the difference between the maximum and the minimum QT‐intervals between the 12 leads on a standard EKG.
The automated computer measurements were manually adjusted for gross T‐wave misidentification by an investigator blinded to the sex of the patient and the time of day of the recording. As reported previously, a minimum of nine leads with a discrete T‐wave offset point were required to allow measurement of QTD. 20 The majority of subjects (97%) had ECGs that had 10–12 valid leads.
Statistical Analysis
ANOVA with repeated measures was used to evaluate overall differences taking into account multiple data points from each individual, and to detect the differences in repolarization variables at different times of day. A P value < 0.05 was considered statistically significant.
Harmonic regression analysis within a hierarchical mixed effects model was used to model circadian periodicity. 15 , 16 First, an unconditional means base model was computed, which contained no predictors other than an individual's grand mean:
Then, sex differences in the overall mean QTD values were assessed without a model for changes that occur during the day. Next, the circadian variation in QTD values for the entire sample was modeled by means of harmonic slopes:
A circadian variation in a given variable was considered to be present if the combined impact of β1 and β2 was significant. The results of this step were then examined for the influence of Sex using means‐and‐slopes‐as‐outcomes approach in a multilevel regression context. Exploratory analysis of QTD revealed that the effects of BMI and BMI by Sex interaction on the lower level harmonic regression parameters were not statistically significant. Analysis of TpTe showed that for some leads BMI and Age were also significant predictors though in general the effects of Sex remained unchanged even when these two additional predictors were introduced into the model. Therefore, results of Sex are presented uncontrolled for other variables. A P < 0.05 was considered significant, and results are presented as means ± standard deviation.
RESULTS
QT‐Interval, QTD
In a multivariate model including sex and time of heart rate measurement (time of EKG recording), a significant difference in heart rate was found between men and women (66.2 bpm men vs 63.9 bpm women, P = 0.016). Time of heart rate measurement was also a significant factor (P = 0.0001). The composite heart rate‐corrected QT‐interval was longer in women than in men (416 ± 17 msec in women vs 411 ± 20 msec in men, P = 0.006). The QTD among all measurable leads was significantly greater in men (37 ± 13 msec) than women (30 ± 11 msec), P < 0.0001; a similar difference in QTD was found using only the precordial leads (29 ± 15 msec in men vs 24 ± 12 msec in women, P = 0.0012).
In men, the maximum QTD was at 6 AM (45 ± 15 msec) and lowest at both 6 PM (34 ± 14 msec, P ≤ 0.02 compared to 6 AM) and 8 PM (33 ± 12 msec, P < 0.02 compared to 6 AM). In women the maximum QTD was at midnight (32 ± 11 msec) and the minimum at both 8 PM (28 ± 13 msec) and 10 PM (28 ± 11 msec), though the differences compared to midnight were not significant.
Harmonic Regression Analysis
QTD
QTD data from 40 subjects were available for eight discrete time points: 6 AM, 8 AM, 10 AM, noon, 6 PM, 9 PM, 10 PM, and midnight. Two subjects had one missing data point each, one at 6 AM and the other at noon. Therefore, a total of 318 data points were analyzed.
Multilevel regression strategy was used for the present analysis with EKG measurements throughout the day nested within subjects. The mean QTD was estimated from all available data points for each subject; 33.18 ± 1.68 msec (95% CI = 29.89–36.47). Individual subject means varied significantly around this grand mean, which suggested that subject‐level predictors may help explain this variance. The intraclass correlation coefficient of 0.64 for these indicates that 64% of the variation in the sample data occurs between individuals, while the rest is attributable to the variation of individual values across multiple repeated measurements. Subject sex was a significant predictor of the overall mean, and the women's QTD mean (29.55 ± 2.26 msec) was 7.26 ± 3.19 msec shorter than the mean for men (36.18 ± 2.13 msec), P = 0.03.
Averaging across all subjects, harmonic slopes explained 22.56% of within‐subject variation in QTD over the base model, P < 0.001 signifying that there is a circadian variation in QTD (Fig. 1). Sex had a significant combined impact on the harmonic slopes (men‐women difference: sin = 3.5 ± 1.59, cos = 0.51 ± 1.62, P = 0.047) indicating that women exhibited less diurnal variation of QTD than men (Fig. 2). In fact, the cumulative impact of harmonic terms was not significant for women indicating that the circadian variation in QTD is found primarily in men. Mean QTD levels at four representative times of the day were also compared by sex: 6 AM, noon, 6 PM, and midnight. QTD in men was greater than QTD in women during all times; however, the difference was only significant at 6 AM and midnight (P < 0.05) with a trend at noon (P = 0.06). No other covariates affected QTD or circadian variation on QTD.
Figure 1.
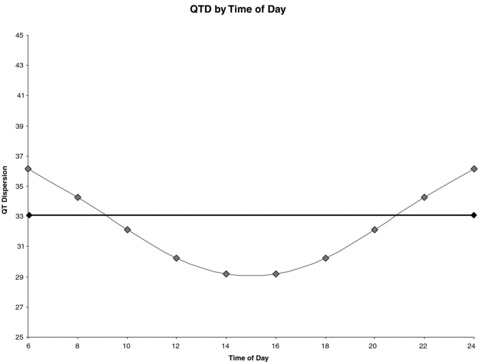
Harmonic Regression of QT‐Dispersion (QTD, in milliseconds). QTD had a significant circadian variation with a peak near the early morning hours.
Figure 2.
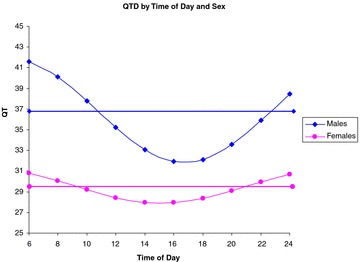
Harmonic regression of QT‐dispersion (QTD, in milliseconds) with Sex as a Variable. When divided by Sex, men but not women had a significant circadian variation in QTD.
TpTe
Analysis of Variance. The mean TpTe intervals ranged from 16.43 to 25.82 msec in the precordial leads (21.76–24.88 msec from V3 to V6) (Table 2). The intraclass correlation coefficients ranged from 0.49 to 0.79, suggesting that 49–79% of the variation of the sample data occurred between individuals, while the rest was due to the variation of the TpTe intervals across multiple repeated measurements for the same individual. The longest TpTe were observed in the afternoon in most leads with a peak noted from noon to 6 PM. An example is shown in Figure 3 (lead V6). Subject sex was a significant predictor of the overall TpTe means in leads V3–V6 (P < 0.05); sex was not a significant predictor in leads V1–V2 (P = 0.10, 0.20). The women's mean TpTe interval for V3–V6 were significantly shorter than men ranging from 21.02 to 23.16 msec versus 22.50–26.60 msec (P < 0.05). In summary, men were found to have significantly longer TpTe intervals in the precordial leads V3–V6.
Table 2.
Longest and Shortest TpTe Values (msec ± 2 SD) for Each Lead and Time at Which It Occurred
| Longest Value* (Time) | Shortest Value(s)** (Time) | |
|---|---|---|
| TpTe avF | 20.10 ± 3.72 (4 PM) | 19.12 ± 3.99 (8 PM) |
| TpTe avL | 17.96 ± 5.19 (noon) | 16.65 ± 5.65 (6 AM) |
| TpTe avR | 22.91 ± 1.72 (noon) | 21.76 ± 3.43 (6 AM) |
| TpTe I | 22.15 ± 3.30 (4 PM) | 21.20 ± 3.10 (6 AM) |
| TpTe II | 22.76 ± 2.09 (4 PM) | 21.60 ± 2.08 (10 AM), |
| 21.60 ± 3.01 (6 PM) | ||
| TpTe III | 16.37 ± 5.06 (noon) [no significant longer or shorter values] | |
| TpTe v1 | 18.10 ± 4.09 (midnight) | 16.14 ± 5.83 (8 AM) |
| TpTe v2 | 26.93 ± 2.62 (4 PM) | 25.78 ± 2.14 (6 AM) |
| TpTe v3 | 25.32 ± 2.91 (4 PM) | 24.37 ± 4.56 (10 AM) |
| TpTe v4 | 24.50 ± 3.44 (8 PM) | 23.38 ± 2.68 (10 AM) |
| TpTe v5 | 23.60 ± 2.27 (4 PM) | 21.66 ± 3.15 (10 AM) |
| TpTe v6 | 22.47 ± 2.60 (noon) | 21.12 ± 2.76 (10 AM) |
The longest TpTe intervals mainly occurred at 4 PM, while the shortest TpTe intervals mainly occurred at 6 AM or 10 AM.
*Longest TpTe value compared to at least one other measurement in the same lead, P < 0.05.
**Shortest TpTe value compared to at least one other measurement in the same lead, P < 0.05.
Figure 3.
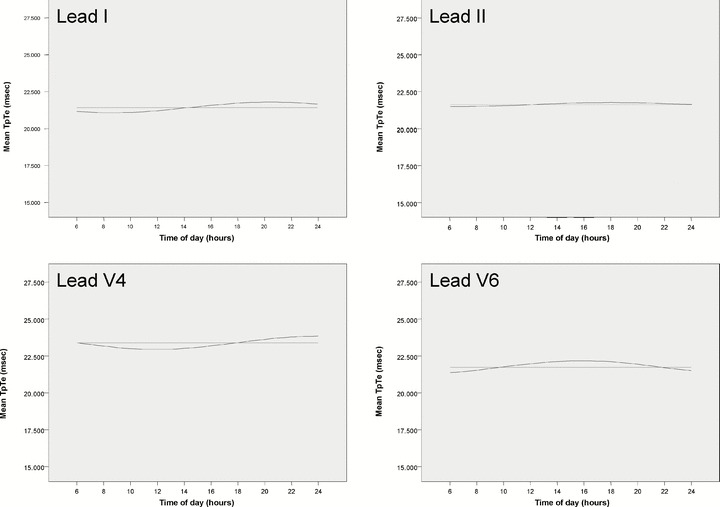
Harmonic regression of TpTe for leads I, II, V4, and V6. Horizontal line (light) represents the mean TpTe across all time points. Sinusoidal line (dark) represents circadian variation found with harmonic regression. No circadian variation is present in lead II.
Harmonic Regression. Averaging across all subjects, harmonic slopes explained 15.38–24.8% of the within‐subject variation in TpTe intervals over the base model in all leads except the inferior leads (P < 0.05) (Tables 2 and 3, Fig. 3). Of the inferior leads, only aVF showed a trend toward circadian variation based on harmonic regression (P = 0.19, 0.19, 0.05 for leads II, III, aVF, respectively). Female sex was found to have a significant negative impact on the harmonic slope in leads V1 and aVR but not in any other lead. Older age was found to have a significant impact on the harmonic slopes in leads V3 (negative impact), V6 (negative), aVR (positive); greater BMI negatively affected harmonic slopes in leads V6, and aVR; greater weight positively affected the harmonic slope of aVR independent of BMI effects (P < 0.05 for all effects). Mean TpTe intervals were evaluated at four representative times of the day based on the harmonic model and compared by sex: 6 AM, noon, 6 PM, and midnight. The TpTe interval was longer for men at all time points for leads V3–V5 (P < 0.05).
Table 3.
Results of Harmonic Regression (Model Using Sin and Cos) for TpTe in Each Lead
| Lead | Sigma2 | Delta R2 | P |
|---|---|---|---|
| TpTe V1 | 4.95 | 0.15 | 0.009 |
| TpTe V2 | 5.11 | 0.21 | <0.001 |
| TpTe V3 | 4.51 | 0.15 | 0.01 |
| TpTe V4 | 3.77 | 0.25 | <0.001 |
| TpTe V5 | 5.27 | 0.22 | <0.001 |
| TpTe V6 | 3.98 | 0.19 | 0.001 |
| TpTe I | 4.38 | 0.12 | 0.03 |
| TpTe II | 2.69 | 0.13 | 0.19 |
| TpTe III | 6.93 | 0.13 | 0.19 |
| TpTe AVR | 2.30 | 0.21 | <0.001 |
| TpTe AVL | 5.43 | 0.22 | <0.001 |
| TpTe AVF | 3.42 | 0.14 | 0.05 |
A significant circadian variation was found in all except the inferior leads.
Sigma2= variance within an individual subject's measurements; Delta R2= percentage of intrasubject variance explained by Sin and Cos over the base model; P = Likelihood ratio test, reduction in error variance due to using Sin and Cos in the model.
In summary, the TpTe intervals in all but the inferior leads were found to exhibit significant circadian variation and, with the exception of leads V1 and aVR, this variation was not found to be significantly different based on sex.
DISCUSSION
Summary of Main Findings
A circadian variation was found in two measures of dispersion of myocardial repolarization. QTD shows a significant circadian variation that is found primarily in men. The largest QTD was found in the early morning hours at a time when arrhythmias are more common. Men were found to have significantly longer TpTe intervals in the precordial leads V3–V6. The TpTe intervals were found to exhibit significant circadian variation in all but the inferior leads. For the majority of leads, this variation was not found to be significantly different based on sex. Thus, ventricular repolarization shows circadian variation.
QT‐Interval, TpTe, and QTD
Prolongation of the QT‐interval has been associated with an increased risk of malignant arrhythmias 21 and sudden cardiac death. 22 In addition, the interval from the peak of the T wave to the end (TpTe) has been suggested to be a marker in myocardial repolarization. As early as the 1980s O’Donnell et al. suggested that TpTe measurements could reflect changes in repolarization in exercise‐induced ischemia. 23 There is evidence that the TpTe interval reflects the transmural dispersion of repolarization, and that the precordial leads (particularly V3 and V4) are the most sensitive for transmural dispersion given their proximity to the ventricular myocardium. 24 , 25 There is evidence that a prolonged TpTe may be arrhythmogenic. Lubinski et al. showed congenital long QT syndrome is associated with a prolonged TpTe, and recently Watanabe et al. found that a lengthened TpTe interval was associated with inducibility of ventricular tachycardia at electrophysiology study. 24 , 26 Smetana et al. demonstrated that women have significantly shorter TpTe intervals and that this sex difference is decreased with longer cycle lengths. 5
Dispersion of ventricular repolarization has been associated with ventricular arrhythmias. Prior studies have suggested that surface ECG measurements can approximate transmural TpTe or horizontal QTD dispersion of repolarization. Using a canine left ventricular wedge preparation and simultaneous floating microelectrodes in the epicardial, M‐region and endocardial sites, Yan and Antzelevich 25 were able to record action potentials in each region while concurrently recording a transmural ECG. Repolarization of the epicardial action potential, the earliest to repolarize, coincided with the peak of the T wave while repolarization of the M cells, the last to repolarize, marked the end of the T wave. Thus, TpTe in the canine wedge model represented the maximum difference in repolarization times and was a measure of dispersion of repolarization across the ventricular myocardium. However, whether TpTe on the body surface ECG represents transmural depolarization or apicobasal heterogeneity may still be under debate. QTD has been hypothesized to reflect spatial differences in myocardial recovery time. 6 Day et al. 6 showed that among those with prolonged QT‐intervals, QTD was able to distinguish between subjects with ventricular arrhythmias and those without, proposing that QTD should be used in determining risk.
QTD
Prior studies have suggested that QTD may have a circadian variation. Batur et al. studied circadian variation in QTc‐dispersion in subjects with coronary artery disease compared to controls and found a significant circadian variation in QTc‐dispersion in the coronary artery disease subgroup. 27 Hansen et al. reported a circadian variation in QT‐dispersion in healthy subjects 13 and in a subsequent analysis showed that while a circadian variation in QT‐dispersion exists in healthy subjects and those with coronary artery disease, it was absent in those who had a prior myocardial infarction or heart failure. 14 Ishida et al. studied healthy subjects with 24‐hour ambulatory monitoring and found QT‐dispersion values were greatest during the day (1–5 PM), and lowest at night (1–5 AM), reaching statistical significance. 28 Also, Michelucci and coworkers studied the circadian pattern among normal subjects using three‐lead ECGs and found no diurnal variation. 29
Prior studies from our group have shown a significant circadian variation in ventricular refractory periods with the maximal shortening between hourly refractory periods as well as the shortest ventricular refractory periods occur in the early morning hours. 15 This response was abolished by beta‐blockade.
In this study, men were found to have a greater overall QTD than women. The QTD was found to exhibit a significant circadian variation, and men were shown to exhibit more diurnal variation than women. In addition, men but not women exhibited significant changes in their QTD during waking hours, consistent with prior findings by Molnar et al. 11 QTD magnitude was found to be greatest early in the morning hours as well as right before sleep and was reduced between these times of the day, in contrast to findings by Ishida et al. 28 However, the study by Ishida involved a continuous three‐lead EKG, and this study did not evaluate EKGs from the period of 1–5 AM. The levels of QTD were statistically equivalent for both sexes in the late afternoon hours. Finally, though overall QTD was greater in men than in women, the absolute QT‐intervals were shorter in men.
TpTe
This study demonstrated a sex difference in the mean TpTe values in leads V3–V6. Consistent with prior research, men had significantly longer TpTe intervals in the precordial leads. By harmonic regression analysis, TpTe values for all leads showed significant circadian variation with the exceptions of II, III, and aVF. Except for leads V1 and aVR, there were no sex difference in this circadian variation. Since it has been suggested that the TpTe interval in the precordial leads (particularly V3 and V4) most reflect the transmural heterogeneity of ventricular repolarization, it is relevant that these leads were found to have a significant circadian variation. However, the circadian variation in TpTe was more variable than that of QTD with the peak value occurring at different times for different leads. Further studies will be required to clarify the mechanism of these differences. No previous studies have used harmonic regression to analyze the circadian variation of the TpTe interval.
The findings of this study show that ventricular repolarization has a significant circadian variation, lending further insight into the predilection for sudden cardiac death to occur in the early morning hours. In addition, the different circadian pattern in men and women to some variables suggests a complex interaction between gender and repolarization that requires further study.
Limitations
One limitation is that the results may be subject to the inherent limitations in automated measurement software, although the least square fitting method of measuring QTD used in this study has been shown to have the highest reproducibility. 18 A second limitation is that QTD may have been determined in a different set of leads for each recording as a result of occasional leads with poor data. However, 97% of subjects had at least 10 of 12 leads of sufficient quality and therefore QTD should not be significantly affected. Another limitation is that data across several days were not analyzed to determine the day‐to‐day variability; further study will be needed to assess this variation. Finally, beat‐to‐beat variability in repolarization was not evaluated in this study.
This work was supported in part by grant 5R01HL075382 from the NHLBI.
REFERENCES
- 1. Muller JE, Stone PH, Turi ZG, et al Circadian variation in the frequency of onset of acute myocardial infarction. N Engl J Med 1985;313:1315–1322. [DOI] [PubMed] [Google Scholar]
- 2. Lerner DJ, Kannel WB. Patterns of coronary heart disease morbidity and mortality in the sexes: A 26‐year follow‐up of the Framingham population. Am Heart J 1986;111:383–390. [DOI] [PubMed] [Google Scholar]
- 3. Kuo CS, Munakata K, Reddy CP, et al Characteristics and possible mechanism of ventricular arrhythmia dependent on the dispersion of action potential durations. Circulation 1983;67:1356–1367. [DOI] [PubMed] [Google Scholar]
- 4. Smetana P, Malik M. Gender differences in ventricular repolarization: Terminal T wave interval was shorter in women than men. Pacing Clin Electrophysiol 2003;26:2350; author reply 2351. [DOI] [PubMed] [Google Scholar]
- 5. Smetana P, Batchvarov V, Hnatkova K, et al Sex differences in the rate dependence of the T wave descending limb. Cardiovasc Res 2003;58:549–554. [DOI] [PubMed] [Google Scholar]
- 6. Day CP, McComb JM, Campbell RW. QT dispersion: An indication of arrhythmia risk in patients with long QT intervals. Br Heart J 1990;63:342–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cheng J. Evidences of the gender‐related differences in cardiac repolarization and the underlying mechanisms in different animal species and human. Fundam Clin Pharmacol 2006;20:1–8. [DOI] [PubMed] [Google Scholar]
- 8. Taneja T, Larsen J, Goldberger J, et al Age, gender, and autonomic tone effects on surface electrocardiographic indices of ventricular repolarization. Ann Noninvasive Electrocardiol 2001;6:290–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mayuga KA, Parker M, Sukthanker ND, et al Effects of age and gender on the QT response to exercise. Am J Cardiol 2001;87:163–167. [DOI] [PubMed] [Google Scholar]
- 10. Molnar J, Zhang F, Weiss J, et al Diurnal pattern of QTc interval: How long is prolonged? Possible relation to circadian triggers of cardiovascular events. J Am Coll Cardiol 1996;27:76–83. [DOI] [PubMed] [Google Scholar]
- 11. Molnar J, Rosenthal JE, Weiss JS, et al QT interval dispersion in healthy subjects and survivors of sudden cardiac death: Circadian variation in a twenty‐four‐hour assessment. Am J Cardiol 1997;79:1190–1193. [DOI] [PubMed] [Google Scholar]
- 12. De Leonardis V, Cinelli P, Capacci F, et al Circadian rhythms in dynamic electrocardiography. J Electrocardiol 1983;16:351–354. [DOI] [PubMed] [Google Scholar]
- 13. Hansen S, Rasmussen V, Larsen K, et al Circadian variation in QT dispersion determined from a 12‐lead Holter recording: A methodological study of an age‐ and sex‐stratified group of healthy subjects. Ann Noninvasive Electrocardiol 2007;12:185–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hansen S, Rasmussen V, Torp‐Pedersen C, et al QT intervals and QT dispersion determined from a 12‐lead 24‐hour Holter recording in patients with coronary artery disease and patients with heart failure. Ann Noninvasive Electrocardiol 2008;13:22–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kong TQ, Jr , Goldberger JJ, Parker M, et al Circadian variation in human ventricular refractoriness. Circulation 1995;92:1507–1516. [DOI] [PubMed] [Google Scholar]
- 16. Lampert R, Rosenfeld L, Batsford W, et al Circadian variation of sustained ventricular tachycardia in patients with coronary artery disease and implantable cardioverter‐defibrillators. Circulation 1994;90:241–247. [DOI] [PubMed] [Google Scholar]
- 17. Hnatkova K, Gang Y, Batchvarov VN, et al Precision of QT interval measurement by advanced electrocardiographic equipment. Pacing Clin Electrophysiol 2006;29:1277–1284. [DOI] [PubMed] [Google Scholar]
- 18. Xue Q, Reddy S. Algorithms for computerized QT analysis. J Electrocardiol 1998;30(Suppl.):181–186. [DOI] [PubMed] [Google Scholar]
- 19. Bazett HC. An analysis of the time relations of electrocardiograms. Heart 1920;7:353–367. [Google Scholar]
- 20. Zabel M, Portnoy S, Franz MR. Electrocardiographic indexes of dispersion of ventricular repolarization: An isolated heart validation study. J Am Coll Cardiol 1995;25:746–752. [DOI] [PubMed] [Google Scholar]
- 21. Moss AJ. Measurement of the QT interval and the risk associated with QTc interval prolongation: A review. Am J Cardiol 1993;72:23B–25B. [DOI] [PubMed] [Google Scholar]
- 22. Algra A, Tijssen JG, Roelandt JR, et al QTc prolongation measured by standard 12‐lead electrocardiography is an independent risk factor for sudden death due to cardiac arrest. Circulation 1991;83:1888–1894. [DOI] [PubMed] [Google Scholar]
- 23. O’Donnell J, Lovelace DE, Knoebel SB, et al Behavior of the terminal T wave during exercise in normal subjects, patients with symptomatic coronary artery disease and apparently healthy subjects with abnormal ST segment depression. J Am Coll Cardiol 1985;5:78–84. [DOI] [PubMed] [Google Scholar]
- 24. Watanabe N, Kobayashi Y, Tanno K, et al Transmural dispersion of repolarization and ventricular tachyarrhythmias. J Electrocardiol 2004;37:191–200. [DOI] [PubMed] [Google Scholar]
- 25. Yan GX, Antzelevitch C. Cellular basis for the normal T wave and the electrocardiographic manifestations of the long‐QT syndrome. Circulation 1998;98:1928–1936. [DOI] [PubMed] [Google Scholar]
- 26. Lubinski A, Kornacewicz‐Jach Z, Wnuk‐Wojnar AM, et al The terminal portion of the T wave: A new electrocardiographic marker of risk of ventricular arrhythmias. Pacing Clin Electrophysiol 2000;23:1957–1959. [DOI] [PubMed] [Google Scholar]
- 27. Batur MK, Aksoyek S, Oto A, et al Circadian variations of QTc dispersion: Is it a clue to morning increase of sudden cardiac death? Clin Cardiol 1999;22:103–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ishida S, Nakagawa M, Fujino T, et al Circadian variation of QT interval dispersion: Correlation with heart rate variability. J Electrocardiol 1997;30:205–210. [DOI] [PubMed] [Google Scholar]
- 29. Michelucci A, Lazzeri C, Conti AA, et al The dispersion of ventricular repolarization diagnosed by Holter: Limitations and possibilities. Cardiologia 1998;43(Suppl. 1):25–28. [PubMed] [Google Scholar]


