Abstract
Background: Prolonged duration of the QRS complex is a prognostic marker in patients with heart failure (HF), whereas electrocadiographic markers in HF with narrow QRS complex remain unclear. We evaluated the prognostic value of the T‐wave amplitude in lead aVR in HF patients with narrow QRS complexes.
Methods: We examined 331 patients who were admitted to our hospital for worsening HF (68 ± 15 years, mean ± standard deviation) from January 2000 to October 2004 who had sinus rhythm and QRS complex <120 ms. The patients were categorized into three groups according to the peak T‐wave amplitude from baseline in lead aVR: negative (<–0.1 mV; n = 209, 63%), flat (–0.1–0.1 mV; n = 64, 19%), and positive (>0.1 mV; n = 58, 18%).
Results: During a mean follow‐up of 33 months, 113 (34%) patients had all‐cause death, the primary end point. After adjusting for clinical covariates, flat T wave (hazard ratio [HR] 1.86, 95% confidence interval [CI] 1.42–2.46), and positive T wave (HR 6.76, 95% CI 3.92–11.8) were independent predictors of mortality, when negative T wave was considered a reference.
Conclusions: As the peak T‐wave amplitude in lead aVR becomes less negative, there was a progressive increase in mortality. The T wave in lead aVR provides prognostic information for risk stratification in HF patients with narrow QRS complexes.
Ann Noninvasive Electrocardiol 2011;16(3):250–257
Keywords: electrocardiogram, mortality, hospitalization, arrhythmia
Heart failure (HF) is a major public health problem in developed countries. 1 , 2 Despite significant improvement in drug therapies, morbidity and mortality of HF remain high, with 1‐year mortality >30% and 1‐year readmission rates >50%. The search for a better risk predictor is still under way. 1 , 2 Risk stratification in HF using the 12‐lead electrocardiogram (ECG) has been extensively studied. 2 , 3 , 4 Certain ECG abnormalities, such as wide QRS complex, 5 prolonged QT interval, 6 , 7 and low QRS voltage, 8 have been associated with higher mortality in HF patients. Recent studies focused on prolonged QRS duration in patients with reduced left ventricular ejection fraction (LVEF) because electrical ventricular dyssynchrony manifests in ECG as a prolonged duration of the QRS, and cardiac resynchronization therapy reduces the occurrence of death or worsening HF requiring hospitalization. 9 However, prognostic ECG markers in HF patients with narrow QRS complexes remain unclear.
Lead aVR is largely ignored in interpreting 12‐lead ECG, whereas it has been reported to provide useful diagnostic, as well as prognostic information in various myocardial diseases. 10 , 11 ST elevation in lead aVR in acute coronary syndrome indicates left main coronary artery obstruction or unstable angina in three‐vessel disease, and this finding is predictive of poor prognosis. 12 , 13 , 14 , 15 , 16 , 17 , 18 It has been reported that P‐wave morphology and polarity helps to differentiate the origin of the atrial tachycardia, 19 and larger R‐wave amplitude in lead aVR correlated with a higher risk of recurrent arrhythmia in patients with Brugada‐type ECG. 20 Positive T wave in lead aVR is an uncommon finding with unclear significance in the absence of bundle branch block. Recently, Tan et al. demonstrated that positive T wave in lead aVR correlated with cardiovascular death in male veterans. 21 Little published data are available as to T‐wave amplitude in lead aVR offer prognostic value to hospitalized HF patients with narrow QRS complexes. The aim of the present study was to determine the independent contribution of the T‐wave amplitude in lead aVR to the risk of mortality and readmission to the hospital in HF patients compared to other established markers of HF severity.
METHODS
Patient Population
Consecutive patients hospitalized for overt clinical decompensation of HF from January 2000 to October 2004 were considered for the study. To identify eligible patients, admissions were screened daily in two phases. 22 First, patients were identified by an admission diagnosis or radiographic signs of HF on the admission chest x‐ray. Second, patients who met the aforementioned conditions had their medical records reviewed within 2 days of admission to verify the presence of HF, based on the published criteria. 23 We excluded patients in whom HF developed after admission (i.e., in‐hospital complication), who were ≥95 years or <20 years of age, and those with severe primary pulmonary disease, congenital heart disease, active myocarditis, severe hepatic or renal disease, or malignancy. Patients were also excluded if they had undergone coronary revascularization or had had an acute myocardial infarction, unstable angina, or cerebral ischemic event within the previous 2 months. Patients with atrial fibrillation or flutter, QRS duration ≥120 ms, implanted pacemaker or defibrillator, and preexcitation syndrome were also excluded. 21 The study protocol was approved by the ethical committee of our institution. All patients provided written informed consent.
Analysis of 12‐Lead ECG
The 12‐lead ECG recording was made before discharge from the hospital at a paper speed of 25 mm/s (Fukuda Denshi, Tokyo, Japan). QRS duration and left ventricular hypertrophy were evaluated using the Sokoloff‐Lyon criteria determined by the ECG computer. Physicians unaware of the clinical outcomes (EW and KS) performed ECG analyses using digital calipers on a 12‐lead ECG and magnified to 200% of normal size. The T‐wave amplitude was measured as the value of the largest deflection above and below the baseline in a window spanning from 80 ms after the end of QRS to the end of the T wave. Patients were classified into three groups according to the T‐wave amplitude; negative (<–0.1 mV), flat (–0.1–0.1 mV), or positive (>0.1 mV) (Fig. 1). The QT interval was defined as the time between QRS onset and the point at which the isoelectric line intersected a line drawn tangentially to the maximal downslope of the T wave and was heart rate corrected using Bazett's formula. A mean QTc interval was calculated from all QTc intervals measured. When the T wave was interrupted by the U wave, the end of the T wave was defined as the nadir between the T wave and U wave. To assess the interobserver (EW and KS) and intraobserver reliability of the ECG analysis, the same recordings of 100 ECGs were interpreted at an interval of 1 month; the correlation coefficients for T‐wave amplitude measurement were 0.99 and 0.98, respectively.
Figure 1.
T‐wave morphology in lead aVR. The patients were categorized into three groups according to the peak T‐wave amplitude from baseline: negative T wave, <–0.1 mV; flat T wave, –0.1 mV–0.1 mV; and positive T wave, >0.1 mV.
Blood Test and Echocardiography
Blood test and two‐dimensional echocardiography were performed before discharge from the hospital. The plasma B‐type natriuretic peptide (BNP) level was determined using the Shionoria kit (Shionogi, Tokyo, Japan). A single echocardiographer who was blinded to the patients’ clinical information performed offline echocardiographic analysis using a Sonos 5500 (Hewlett Packard, Palo Alto, CA, USA). Left ventricular systolic function was assessed by the LVEF calculated by the biplane Simpson's method of discs.
Follow‐Up and End Points
Research coordinators and physicians recorded baseline data for all patients at the time of enrollment including patient demographics, past medical conditions, and current medication. During the follow‐up period, patients or their families were periodically sent a questionnaire and interviewed by telephone. The primary end point of the study was all‐cause death, and the secondary end point was a composite of cardiovascular death or unplanned rehospitalization for worsening HF. The cause of death was determined from medical charts or by direct communication with patients’ general practitioners or families. Cardiovascular death includes death due to HF, acute myocardial infarction, aortic dissection, stroke, and systemic embolism. Hospitalization for HF was defined as requiring intravenous administration of diuretics.
Statistical Analysis
We performed a full data set analysis for all outcomes without imputation of missing values. Differences in the baseline characteristics of the three groups were tested using the one‐way analysis of variance, and categorical data were evaluated by chi‐square tests with Yates’ correction. Associations of the T‐wave amplitude in lead aVR with the end points were analyzed using the Cox proportional‐hazards regression model and presented as a hazard ratio (HR) and 95% confidence interval (CI). The HR for a continuous variable refers to the HR per unit of the analyzed variable unless otherwise specified. In the multivariate models, HRs were adjusted for the age; sex; ischemic etiology of HF; body mass index (BMI); New York Heart Association (NYHA) class II/III or IV; LVEF; diastolic blood pressure; BNP; and the use of angiotensin‐converting enzyme inhibitor (ACE‐I) or angiotensin II receptor blocker (ARB), or beta‐blocker at discharge from hospital. These covariates were selected based on the significance in baseline clinical characteristics of the patients or possible confounding factors. Time‐to‐event curves describing the proportion of patients, remaining alive during the follow‐up period were calculated by the Kaplan‐Meier method and compared with the log rank test. Quantitative data were expressed as mean ± standard deviation. The BNP level was transformed to natural logarithms because of the skewed distribution. We used the JMP 8 program (SAS Institute, Cary, NC, USA) for statistical analyses. A value of P < 0.05 was considered significant.
RESULTS
During the recruitment period, 432 consecutive patients were assessed for enrollment eligibility. We excluded 101 patients due to predetermined criteria, resulting in a total of 331 patients enrolled in the study.
Baseline Clinical Characteristics of the Patients
The baseline clinical characteristics of the three groups of patients are listed in Table 1. The patients included in the final analysis were aged 68 ± 15 (range, 21–94) years, and 188 (54%) were male. No significant differences were found between the groups in regard to age, medical history and behavior, HF etiology, LVEF, and ln BNP. Significant differences were found in regard to sex, BMI, NYHA class, and diastolic blood pressure.
Table 1.
Baseline Characteristics of Patients
| Negative T Wave | Flat T Wave | Positive T Wave | P‐Value | |
|---|---|---|---|---|
| n (%) | 209 (63) | 64 (19) | 58 (18) | |
| Age (years) | 67 ± 15 | 67 ± 17 | 71 ± 14 | 0.166 |
| Sex (%male /%female) | 63/37 | 45/55 | 47/53 | 0.009 |
| Medical history and behavior (%) | ||||
| Diabetes mellitus | 41 | 41 | 34 | 0.683 |
| History of hypertension | 60 | 55 | 60 | 0.714 |
| Prior myocardial infarction | 51 | 47 | 57 | 0.541 |
| Prior CABG or PCI | 50 | 45 | 51 | 0.748 |
| Stroke | 2 | 1 | 1 | 0.787 |
| Current or past smoker | 35 | 31 | 40 | 0.581 |
| Prior hospitalization for HF | 8 | 7 | 9 | 0.881 |
| HF etiology, % ischemic | 51 | 47 | 57 | 0.541 |
| Physiological and functional assessments | ||||
| BMI (kg/m2) | 22.8 ± 3.4 | 21.6 ± 4.3 | 21.4 ± 1.6 | 0.002 |
| NYHA class II, III/IV (%) | 92/8 | 80/20 | 55/45 | <0.001 |
| LVEF (%) | 46 ± 15 | 44 ± 14 | 42 ± 13 | 0.212 |
| LAD (mm) | 37 ± 7 | 37 ± 7 | 37 ± 6 | 0.765 |
| Systolic blood pressure (mmHg) | 113 ± 19 | 113 ± 26 | 109 ± 21 | 0.309 |
| Diastolic blood pressure (mmHg) | 65 ± 11 | 59 ± 12 | 60 ± 11 | <0.001 |
| ECG findings | ||||
| QRS duration (ms) | 100 ± 11 | 98 ± 11 | 99 ± 11 | 0.371 |
| QT interval (ms) | 407 ± 53 | 396 ± 55 | 411 ± 56 | 0.306 |
| QTc interval (ms) | 447 ± 49 | 456 ± 55 | 453 ± 58 | 0.431 |
| LVH (%) | 64 | 62 | 53 | 0.285 |
| ln BNP (pg/mL) | 5.16 ± 1.24 | 5.32 ± 1.10 | 5.44 ± 1.33 | 0.243 |
| Medications (%) | ||||
| Beta‐blocker | 76 | 74 | 70 | 0.646 |
| ACE‐I/ARB | 78 | 72 | 62 | 0.135 |
| Loop diuretics | 84 | 80 | 74 | 0.391 |
| Spironolactone | 54 | 53 | 40 | 0.318 |
| Digoxin | 17 | 20 | 19 | 0.784 |
| Calcium‐channel blocker | 26 | 25 | 26 | 0.990 |
| Nitrate | 35 | 39 | 33 | 0.882 |
| Statin | 30 | 27 | 24 | 0.804 |
Negative T wave indicates T‐wave amplitude in lead aVR <–0.1 mV, flat T wave:–0.1–0.1 mV, positive T wave: >0.1 mV. CABG = coronary artery bypass graft; PCI = percutaneous coronary intervention; HF = heart failure; BMI = body mass index; NYHA = New York Heart Association; LVEF = left ventricular ejection fraction; LAD = left atrial dimension; QTc = rate‐corrected QT interval; LVH = left ventricular hypertrophy; BNP = B‐type natriuretic peptide; ACE‐I = angiotensin‐converting enzyme inhibitor; ARB = angiotensin II receptor blocker. Data represent mean ± standard deviation or frequency.
Survival Analysis
Over the follow‐up period of 33 ± 23 months (range, 1–75 months), there were 113 (34%) all‐cause deaths, 91 (27%) cardiovascular deaths, and 185 (56%) unplanned rehospitalizations due to worsening HF.
Primary End Point
The survival duration of the 113 nonsurvivors was 15 ± 16 months. The incidence of all‐cause death was as follows: negative T wave, 14%; flat T wave, 56%; and positive T wave, 83% (P < 0.0001). Cox proportional‐hazards regression analysis of the flat T wave in lead aVR was shown in Table 2. Multivariate analysis revealed that flat T wave, age, NYHA class III or IV, diastolic blood pressure, ln BNP, and the use of beta‐blocker or ACE‐I/ARB were independent predictors of the primary end point when negative T wave was taken as a reference. Table 3 presented Cox proportional‐hazards regression analysis of the positive T wave in lead aVR. Multivariate analysis revealed that independent predictors of the primary end point were positive T wave, age, NYHA class III or IV, diastolic blood pressure, ln BNP, and the use of ACE‐I/ARB, when negative T wave was taken as a reference. Figure 2A shows the Kaplan‐Meier survival curves of the three groups.
Table 2.
Hazard Ratios of the Primary End Point of Flat T Wave
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Hazard Ratio (95% CI) | P‐Value | Hazard Ratio (95% CI) | P‐Value | |
| Negative T wave | 1 | – | 1 | – |
| Flat T wave | 2.30 (1.80–2.95) | <0.001 | 1.86 (1.42–2.46) | <0.001 |
| Age | 1.03 (1.02–1.06) | 0.001 | 1.02 (1.00–1.04) | 0.036 |
| Sex | 0.80 (0.49–1.30) | 0.360 | 1.05 (0.59–1.88) | 0.875 |
| Ischemic etiology of HF | 1.46 (0.89–2.39) | 0.131 | 1.23 (0.69–2.21) | 0.490 |
| BMI | 0.92 (0.53–1.10) | 0.429 | 1.01 (0.47–1.78) | 0.778 |
| NYHA class III or IV | 11.7 (4.81–24.7) | <0.001 | 5.69 (2.08–14.2) | 0.001 |
| LVEF | 0.99 (0.98–1.01) | 0.521 | 0.98 (0.90–1.01) | 0.539 |
| Diastolic blood pressure | 0.94 (0.91–0.96) | <0.001 | 0.95 (0.93–0.98) | 0.006 |
| ln BNP | 1.76 (1.40–2.21) | <0.001 | 1.22 (1.20–1.92) | <0.001 |
| Beta‐blocker | 0.55 (0.32–0.91) | 0.019 | 0.52 (0.29–0.90) | 0.020 |
| ACE‐I/ARB | 0.51 (0.31–0.88) | 0.015 | 0.43 (0.24–0.79) | 0.007 |
Abbreviations as in Table 1. Negative T wave in lead aVR was taken as a reference. CI = confidence interval.
Table 3.
Hazard Ratios of the Primary End Point of Positive T Wave
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Hazard Ratio (95% CI) | P‐Value | Hazard Ratio (95% CI) | P‐Value | |
| Negative T wave | 1 | – | 1 | – |
| Positive T wave | 10.3 (6.45–16.6) | < 0.001 | 6.76 (3.92–11.8) | < 0.001 |
| Age | 1.04 (1.02–1.06) | <0.001 | 1.02 (1.01–1.05) | 0.027 |
| Sex | 0.72 (0.46–1.12) | 0.148 | 0.99 (0.58–1.68) | 0.978 |
| Ischemic etiology of HF | 1.69 (1.07–2.70) | 0.023 | 0.90 (0.51–1.58) | 0.709 |
| BMI | 0.77 (0.40–1.16) | 0.178 | 1.06 (0.78–1.60) | 0.772 |
| NYHA class III or IV | 35.2 (16.5–74.3) | <0.001 | 5.91(2.49–14.1) | <0.001 |
| LVEF | 0.99 (0.98–1.01) | 0.446 | 0.99 (0.97–1.01) | 0.195 |
| Diastolic blood pressure | 0.95 (0.92–0.97) | <0.001 | 0.97 (0.95–0.99) | 0.013 |
| ln BNP | 1.79 (1.46–2.20) | <0.001 | 1.45 (1.17–1.83) | <0.001 |
| Beta‐blocker | 0.49 (0.29–0.78) | 0.003 | 0.42 (0.25–1.29) | 0.338 |
| ACE‐I/ARB | 0.34 (0.21–0.54) | <0.001 | 0.42 (0.26–0.71) | 0.001 |
Abbreviations as in Table 1. Negative T wave in lead aVR was taken as a reference. CI = confidence interval.
Figure 2.
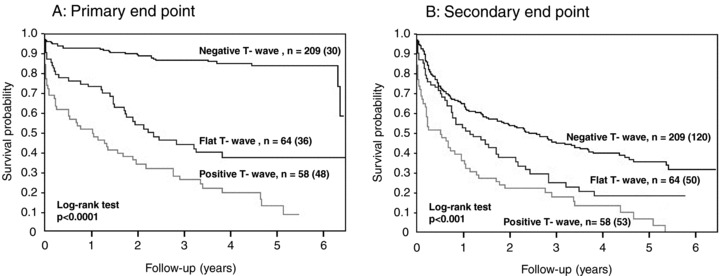
Kaplan‐Meier estimates of the survival rates. (A) Primary end point. As the peak T‐wave amplitude in lead aVR becomes less negative, there was a progressive increase in all‐cause death. (B) Secondary end point. As the peak T‐wave amplitude in lead aVR becomes less negative, there was a progressive increase in the combined risk of cardiovascular death and unplanned rehospitalization for worsening heart failure. The n denotes the number of the patients in a subgroup, and the number of patients who reached the end point during the observation period is in parentheses.
Secondary End Point
The incidence of the secondary end point was as follows: negative T wave, 57%; flat T wave, 78%; and positive T wave, 91% (P < 0.001). Cox proportional‐hazards regression analysis of the flat T wave in lead aVR was shown in Table 4. Multivariate analysis showed that flat T wave, age, NYHA class III or IV, diastolic blood pressure, and ln BNP were independent predictors of the secondary end point when negative T wave was taken as a reference. Table 5 presented Cox proportional‐hazards regression analysis of the positive T wave in lead aVR. Multivariate analysis revealed that independent predictors of the secondary end point were positive T wave, age, NYHA class III or IV, ln BNP, and the use of ACE‐I/ARB, when negative T wave was taken as a reference. Figure 2B shows the Kaplan‐Meier survival curves of the three groups.
Table 4.
Hazard Ratios of the Secondary End Point of Flat T Wave
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Hazard Ratio (95% CI) | P‐Value | Hazard Ratio (95% CI) | P‐Value | |
| Negative T wave | 1 | – | 1 | – |
| Flat T wave | 1.30 (1.10–1.53) | 0.002 | 1.21 (1.01–1.43) | 0.040 |
| Age | 1.02 (1.00–1.03) | 0.002 | 1.01 (0.99–1.02) | 0.124 |
| Sex | 0.98 (0.72–1.34) | 0.919 | 0.91 (0.64–1.30) | 0.601 |
| Ischemic etiology of HF | 1.63 (1.21–2.22) | 0.001 | 1.36 (0.98–1.92) | 0.068 |
| BMI | 0.93 (0.65–1.11) | 0.955 | 0.90 (0.74–1.36) | 0.731 |
| NYHA class III or IV | 7.67 (3.41–14.9) | <0.001 | 6.18 (2.56–13.3) | 0.002 |
| LVEF | 0.99 (0.98–1.00) | 0.323 | 0.99 (0.98–1.00) | 0.243 |
| Diastolic blood pressure | 0.97 (0.95–0.98) | <0.001 | 0.98 (0.96–0.99) | 0.003 |
| ln BNP | 1.32 (1.16–1.50) | <0.001 | 1.26 (1.10–1.44) | <0.001 |
| Beta‐blocker | 0.90 (0.67–1.22) | 0.516 | 0.81 (0.58–1.13) | 0.225 |
| ACE‐I/ARB | 0.79 (0.56–1.13) | 0.200 | 0.70 (0.48–1.02) | 0.064 |
Abbreviations as in Table 1. Negative T wave in lead aVR was taken as a reference. CI = confidence interval.
Table 5.
Hazard Ratios of the Secondary End Point of Positive T wave
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Hazard Ratio (95% CI) | P‐Value | Hazard Ratio (95% CI) | P‐Value | |
| Negative T wave | 1 | – | 1 | – |
| Positive T wave | 2.63 (1.88–3.62) | <0.001 | 1.79 (1.21–2.61) | 0.003 |
| Age | 1.02 (1.01–1.04) | <0.001 | 1.02 (1.01–1.03) | 0.002 |
| Sex | 0.87 (0.65–1.18) | 0.374 | 0.88 (0.63–1.22) | 0.437 |
| Ischemic etiology of HF | 1.79 (1.27–2.35) | <0.001 | 1.27 (0.90–1.81) | 0.160 |
| BMI | 0.88 (0.57–1.17) | 0.731 | 0.90 (0.55–1.29) | 0.536 |
| NYHA class III or IV | 19.2 (9.88–35.3) | <0.001 | 7.38 (3.42–15.2) | <0.001 |
| LVEF | 0.99 (0.98–1.01) | 0.156 | 0.99 (0.98–1.00) | 0.130 |
| Diastolic blood pressure | 0.98 (0.97–0.99) | <0.001 | 0.99 (0.97–1.00) | 0.151 |
| ln BNP | 1.34 (1.19–1.52) | <0.001 | 1.20 (1.06–1.37) | 0.004 |
| Beta‐blocker | 0.80 (0.58–1.08) | 0.138 | 0.90 (0.65–1.24) | 0.533 |
| ACE‐I/ARB | 0.57 (0.41–0.81) | 0.002 | 0.60 (0.42–0.87) | 0.007 |
Abbreviations as in Table 1. Negative T wave in lead aVR was taken as a reference. CI = confidence interval.
DISCUSSION
In the present study, we examined whether the T‐wave amplitude in lead aVR provides prognostic information for hospitalized HF patients with narrow QRS complexes. Our novel finding based on the 12‐lead ECG was that the T‐wave amplitude in lead aVR is predictive of total mortality in HF patients. This result holds true for the combined risk of cardiovascular death and rehospitalization due to worsening HF. Our findings highlight the need to consider patients with positive deflection of the T wave in lead aVR for assessment of the therapeutic options that might modify the prognosis.
The positive T wave in lead aVR has been reported to have prognostic significance by Tan et al. 21 In an observational study of 24,270 male veterans, they investigated the association between abnormal findings in all 12 leads of the ECG (i.e., LVH, Q waves, QRS duration, QT interval, and ST depression) and cardiovascular mortality after excluding hospitalized patients, atrial fibrillation, and paced rhythms. A positive T wave in lead aVR had a prevalence of 7.3% and the relative risk for cardiovascular mortality came out to be 5.0 when negative T wave was taken as a reference. They also showed that T‐wave amplitude in lead aVR was an independent predictor of cardiovascular death in the multivariate analysis, whereas ST elevation in lead aVR was not. Our study confirmed and extended the prognostic value of T‐wave amplitude in lead aVR for hospitalized HF patients irrespective of sex or etiology of HF. We also showed that the T‐wave amplitude became less negative, mortality progressively increased. The T‐wave morphology in lead aVR, in addition to conventional predictors such as age, NYHA class, or BNP, has been shown to provide significant prognostic information.
Previously, van Domburg et al. 24 reported the prognostic importance of the cardiac infarction injury score (CIIS) in 3395 postmyocardial infarction patients enrolled in the ASPECT trial. 25 The CIIS is an ECG scoring system, originally designed by Rautaharju 26 and constructed to increase the diagnostic yield of the ECG in patients with suspected acute myocardial infarction. The CIIS is composed of selected ECG characteristics and combined into a single score. Notably, Rautaharju et al. showed that the T‐wave amplitude in lead aVR enhances the predictive accuracy in acute myocardial infarction. van Domburg et al. 24 found that the prognostic value of the CIIS persists after adjusting for the clinical and demographic variables by multivariate analysis, and also noted that the amplitude of the T wave in aVR makes a substantial contribution to the improvement in overall survival and infarct‐free survival.
Lead aVR, which gives information from the right upper side of the heart, has been considered to provide reciprocal information from the left lateral side of the heart, being already covered by leads aVL, II, V5, and V6. 27 Because of the observational nature of the present study, whether flat or positive T waves in lead aVR are a part of the mechanisms underlying increased mortality in HF patients, or if it is merely a marker of poor prognosis among them, is not clear. The prognostic value of T‐wave amplitude in lead aVR was independent of LVEF, BNP, and HF etiology. Thus, this finding is not a simple reflection of impaired ventricular performance or the characteristics of known cardiac diseases, though we cannot exclude the possibility that the T‐wave amplitude identifies patients with other unmeasured differences in disease severity that influence survival in this population.
Study Limitations
Several important limitations exist for our results. First, the small number of patients and the fact that our patients were recruited among those admitted to university hospital for HF, which may constitute a selection bias. Second, the exclusion of patients with clinical conditions potentially associated with a higher mortality, such as atrial fibrillation or cardiac pacemaker, may also add bias to our results. In HF patients, hemodynamic or electrophysiological variables can be modified by several factors, such as the etiology and stage of HF, and the therapeutic interventions applied over time. Whether serial measurement of the T wave in lead aVR is useful in predicting the subsequent clinical course or patient survival is not known.
CONCLUSIONS
Our study shows that T‐wave amplitude in lead aVR is associated with an increased risk of mortality and readmission to the hospital for HF. This simple and useful ECG finding provides complementary prognostic information for the risk stratification of HF patients with narrow QRS complexes.
This work was supported by the 25th Memorial Grant from Suzuken Memorial Foundation.
We do not have any potential conflict of interest to disclose.
REFERENCES
- 1. Rosamond W, Flegal K, Furie K, et al Heart disease and stroke statistics–2008 update: A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 2008;117:e25–e146. [DOI] [PubMed] [Google Scholar]
- 2. Hunt SA, Abraham WT, Chin MH, et al 2009 Focused Update Incorporated Into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines Developed in Collaboration With the International Society for Heart and Lung Transplantation. J Am Coll Cardiol 2009;53:e1–e90. [DOI] [PubMed] [Google Scholar]
- 3. Cowburn PJ, Cleland JG, Coats AJ, et al Risk stratification in chronic heart failure. Eur Heart J 1998;19:696–710. [DOI] [PubMed] [Google Scholar]
- 4. Deedwania PC. The key to unraveling the mystery of mortality in heart failure: An integrated approach. Circulation 2003;107:1719–1721. [DOI] [PubMed] [Google Scholar]
- 5. Iuliano S, Fisher SG, Karasik PE, et al QRS duration and mortality in patients with congestive heart failure. Am Heart J 2002;143:1085–1091. [DOI] [PubMed] [Google Scholar]
- 6. Brendorp B, Elming H, Jun L, et al QTc interval as a guide to select those patients with congestive heart failure and reduced left ventricular systolic function who will benefit from antiarrhythmic treatment with dofetilide. Circulation 2001;103:1422–1427. [DOI] [PubMed] [Google Scholar]
- 7. Watanabe E, Arakawa T, Uchiyama T, et al Prognostic significance of circadian variability of RR and QT intervals and QT dynamicity in patients with chronic heart failure. Heart Rhythm 2007;4:999–1005. [DOI] [PubMed] [Google Scholar]
- 8. Kamath SA, Meo Neto Jde P, Canham RM, et al Low voltage on the electrocardiogram is a marker of disease severity and a risk factor for adverse outcomes in patients with heart failure due to systolic dysfunction. Am Heart J 2006;152:355–361. [DOI] [PubMed] [Google Scholar]
- 9. Abraham WT, Fisher WG, Smith AL, et al Cardiac resynchronization in chronic heart failure. N Engl J Med 2002;346:1845–1853. [DOI] [PubMed] [Google Scholar]
- 10. Siddiqui MA, Khan IA. Role of lead aVR in evaluation of 12‐lead electrocardiogram. Angiology 2002;53:709–713. [DOI] [PubMed] [Google Scholar]
- 11. Kireyev D, Arkhipov MV, Zador ST, et al Clinical utility of aVR—The neglected electrocardiographic lead. Ann Noninvas Electrocardiol 2010;15:175–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gorgels AP, Vos MA, Mulleneers R, et al Value of the electrocardiogram in diagnosing the number of severely narrowed coronary arteries in rest angina pectoris. Am J Cardiol 1993;72:999–1003. [DOI] [PubMed] [Google Scholar]
- 13. Engelen DJ, Gorgels AP, Cheriex EC, et al Value of the electrocardiogram in localizing the occlusion site in the left anterior descending coronary artery in acute anterior myocardial infarction. J Am Coll Cardiol 1999;34:389–395. [DOI] [PubMed] [Google Scholar]
- 14. Kosuge M, Kimura K, Ishikawa T, et al ST‐segment depression in lead aVR predicts predischarge left ventricular dysfunction in patients with reperfused anterior acute myocardial infarction with anterolateral ST‐segment elevation. Am Heart J 2001;142:51–57. [DOI] [PubMed] [Google Scholar]
- 15. Yamaji H, Iwasaki K, Kusachi S, et al Prediction of acute left main coronary artery obstruction by 12‐lead electrocardiography. ST segment elevation in lead aVR with less ST segment elevation in lead V(1). J Am Coll Cardiol 2001;38:1348–1354. [DOI] [PubMed] [Google Scholar]
- 16. Barrabes JA, Figueras J, Moure C, et al Prognostic value of lead aVR in patients with a first non‐ST‐segment elevation acute myocardial infarction. Circulation 2003;108:814–819. [DOI] [PubMed] [Google Scholar]
- 17. Kosuge M, Kimura K, Ishikawa T, et al Predictors of left main or three‐vessel disease in patients who have acute coronary syndromes with non‐ST‐segment elevation. Am J Cardiol 2005;95:1366–1369. [DOI] [PubMed] [Google Scholar]
- 18. Kühl JT, Berg RMG. Utility of lead aVR for identifying the culprit lesion in acute myocardial infarction. Ann Noninvas Electrocardiol 2009;14:219–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kistler PM, Roberts‐Thomson KC, Haqqani HM, et al P‐wave morphology in focal atrial tachycardia: Development of an algorithm to predict the anatomic site of origin. J Am Coll Cardiol 2006;48:1010–1017. [DOI] [PubMed] [Google Scholar]
- 20. Babai Bigi MA, Aslani A, Shahrzad S. aVR sign as a risk factor for life‐threatening arrhythmic events in patients with Brugada syndrome. Heart Rhythm 2007;4:1009–1012. [DOI] [PubMed] [Google Scholar]
- 21. Tan SY, Engel G, Myers J, et al The prognostic value of T wave amplitude in lead aVR in males. Ann Noninvas Electrocardiol 2008;13:113–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vaccarino V, Kasl SV, Abramson J, et al Depressive symptoms and risk of functional decline and death in patients with heart failure. J Am Coll Cardiol 2001;38:199–205. [DOI] [PubMed] [Google Scholar]
- 23. Schocken DD, Arrieta MI, Leaverton PE, et al Prevalence and mortality rate of congestive heart failure in the United States. J Am Coll Cardiol 1992;20:301–306. [DOI] [PubMed] [Google Scholar]
- 24. van Domburg RT, Klootwijk P, Deckers JW, et al The cardiac infarction injury score as a predictor for long‐term mortality in survivors of a myocardial infarction. Eur Heart J 1998;19:1034–1041. [DOI] [PubMed] [Google Scholar]
- 25. Effect of long‐term oral anticoagulant treatment on mortality and cardiovascular morbidity after myocardial infarction . Anticoagulants in the Secondary Prevention of Events in Coronary Thrombosis (ASPECT) Research Group. Lancet 1994;343:499–503. [PubMed] [Google Scholar]
- 26. Rautaharju PM, Warren JW, Jain U, et al Cardiac infarction injury score: An electrocardiographic coding scheme for ischemic heart disease. Circulation 1981;64:249–256. [DOI] [PubMed] [Google Scholar]
- 27. Gorgels AP, Engelen DJ, Wellens HJ. Lead aVR, a mostly ignored but very valuable lead in clinical electrocardiography. J Am Coll Cardiol 2001;38:1355–1356. [DOI] [PubMed] [Google Scholar]


