Abstract
Background: Cigarette smoking increased the risk of acute cardiac events related with endothelial dysfunction and increased sympathetic activity. Impaired autonomic nervous activity is recognized as a considerable symptom of cardiac dysfunction and is strongly associated with increased risk overall mortality.
Methods: A total of 75 healthy habitual smokers (40 female, 35 male, mean age 36.5 ± 8.5 years), and 73 non‐smokers subjects (45 female, 28 male, mean age 34.6 ± 7.2 years) were studied. LF and LF/HF ratio were significantly higher in smokers than in non‐smokers. On the contrary, SDNN, SDANN, RMSSD, and HF values were lower in smokers compared to those in non‐smokers. Not the duration of smoking but the number of cigarettes smoked per day was correlated with the HRV parameters and NT‐pro BNP. Furthermore, the average levels of NT‐pro BNP were found to be positively correlated with LF, LF/HF and inversely correlated with SDNN, SDANN, RMSSD and HF.
Results: As a result, smoking impaires sympathovagal balance and decreases the heart rate variability in healthy subjects. And even a one cigarette smoking leads to overt sympathetic excitation. Furthermore, smoking results in an increase in NT‐proBNP levels and the changes in adrenergic nervous system and NT‐proBNP levels are well correlated.
Conclusion: These findings could contribute to the higher rate of cardiovascular events in smokers.
Keywords: electrophysiology, autonomic nervous system and clinical, noninvasive techniques, heart rate variability and clinical
Cigarette smoking may lead to acute cardiac events such as myocardial infarction, ventricular fibrillation, and sudden death especially in the presence of preexisting coronary artery disease. 1 The underlying trigger mechanisms are endothelial dysfunction, increased platelet aggregation, increased sympathetic activity, and coronary vasoconstriction. 2 , 3 , 4 , 5 Assessment of heart rate variability (HRV) may provide quantitative information about the modulation of cardiac sympathetic and parasympathetic nerve activities. 6 Impaired autonomic nervous activity has been recognized as a considerable symptom of cardiac dysfunction and is strongly associated with an increased risk of overall mortality. 7 , 8 Studies have documented alterations of autonomic function during smoking. 9 , 10 , 11 Acute cigarette smoking reduces baseline levels of vagal‐cardiac nerve activity and increases peripheral sympathetic nerve activity. 12 , 13 Plasma brain‐natriuretic peptide (BNP) and its biologically inactive fragment N‐terminal pro‐BNP (NT‐pro‐BNP) are essential serum markers of cardiac disease and have been reported to be useful in the diagnosis of ventricular dysfunction including isolated diastolic dysfunction and in the assessment of prognosis in heart failure. 14 , 15 Although the effect of cigarette smoking on autonomic nervous system has been demonstrated, the relationship between HRV and plasma NT‐pro‐BNP levels has not been studied in healthy smokers. The aim of this study was to assess the effects of cigarette smoking on autonomic nerve activity by HRV analysis and plasma NT‐pro‐BNP levels and we also aimed to determine whether there is any relationship between HRV and NT‐pro‐BNP levels in smoker subjects.
METHODS
Subjects
A total of 75 healthy habitual smokers (40 females, 35 males, with mean age of 36.5 ± 8.5 years), and 73 nonsmokers (45 females, 28 males, with mean age of 34.6 ± 7.2 years) were included in the study. All subjects were selected among outpatients in our institute. None of the subjects was taking any medication at the moment of study nor had any history of chronic disease. The participants were instructed to behave in a normal manner with usual daily physical activity. They were not allowed to have alcoholic beverages or beverages containing caffeine during the analysis. An informed written consent was obtained from all subjects and the trial was approved by the local ethic committee.
Heart Rate Variability Analysis
All subjects underwent 3‐channel 24‐hour Holter ambulatory ECG monitoring (Biomedical System Century 2000/3000 Holter System, Version 1.32). Recordings were analyzed by “Biomedical Systems Century 2000/3000 HRV Package System,” following manual adjustment of RR intervals. Analog data were digitized at 200 Hz and edited by a cardiologist. The validation procedure consisted of beat labeling and tagging of noisy regions. The continuous series of RR (NN) intervals (tachogram) was obtained and all 5‐minute segments with at most five isolated ectopic beats were retained for spectral analysis. Recordings with <18 hour of data or <85% of qualified sinus beats were excluded. The time and frequency‐domain analyses of HRV were performed according to the recommendation of the task force. 6 The mean heart rate, standard deviation of all NN intervals (SDNN), the standard deviation of the 5‐minute mean RR intervals (SDANN), root mean square of successive differences (RMSSD) were measured in the time‐domain analysis of HRV. A reduced SDNN has been considered reflecting diminished vagal and increased sympathetic modulation of sinus node. The power spectrum of HRV was measured using fast‐Fourier transform analysis in four frequency bands: <0.0033 Hz (ultra low frequency, ULF), 0.0033 to 0.04 (very low frequency, VLF), 0.04 to 0.15 (low frequency, LF), and 0.15 to 0.40 (high frequency, HF). HF was used as a marker of parasympathetic nervous system and LF was used as a marker of parasympathetic nervous system and sympathetic activity. 6 The ratio of low‐to‐high frequency power (LF/HF) reflecting the sympathovagal balance was also measured. High values indicate dominant sympathetic activity. 16 For frequency‐domain parameters, three circadian periods (the complete 24 hour, the diurnal, and the nocturnal periods defined on the basis of patient diaries) were considered. Diurnal periods were kept at a minimum of 6 hours to a maximum of 10 hours; nocturnal periods were kept at a minimum of 4 hours to a maximum of 6 hours.
Measurement of NT‐Pro‐BNP Plasma Levels
Peripheral venous blood samples were collected into tubes containing ethylenediamine‐tetra‐acetic acid (EDTA) for each subject at rest. The samples were centrifuged within 20 minutes at +4°C. The plasma was stored at –80°C until analysis. Serum NT‐Pro‐BNP was measured by a double antibody sandwich technique using electrochemiluminescence immunoassay kit (Elecsys NT‐proBNP, Roche Diagnostics, Mannheim, Germany). The results were reported as picogram per milliliter (pg/mL). The clinicians involved in the study were blinded to the NT‐pro‐BNP values obtained.
STATISTICAL ANALYSIS
The results are expressed as mean ± SD. Comparisons between the two groups were performed with the Student's t‐test for numerical variables and the chi‐square test for categorical data. The relation between the number of years of smoking and the number of cigarettes smoked per day and HRV parameters, NT‐pro‐BNP levels, and hemodynamic parameters were assessed by Pearson's correlation coefficient. Linear logistic regression analysis was used to assess the independent effect of smoking on HRV parameters and NT‐pro‐BNP levels. A P value < 0.05 was considered statistically significant.
RESULTS
No significant difference was found between two groups with respect to age, gender, and body mass index (BMI, kg/m2). Mean heart rate, systolic blood pressure, diastolic blood pressure, the average levels of NT‐pro‐BNP, and number of average ventricular premature contractions (VPCs) were significantly higher in smokers as compared to nonsmokers (Table 1). A comparison of HRV analysis results obtained from two groups indicates that LF (day, night, and 24 hours) and LF/HF ratio (day, night, and 24 hours) were significantly higher in the smoker group, while lower values of SDNN, SDANN, RMSSD, and HF (day, night, and 24 hours) were found in the same group (Table 2). A correlation analysis demonstrated a significant association between the number of cigarettes smoked per day (number/day) and SDNN (P < 0.0001, r =−0.484), LF day (P < 0.0001, r = 0.607), LF night (P < 0.0001, r = 0.606), LF 24 hours (P < 0.0001, r = 611), LF/HF day (P < 0.0001, r = 0.429), LF/HF night (P < 0.0001, r = 450), LF/HF 24 hours (P < 0.0001, r = 0.465), VPCs (P = 0.03, r = 0.243), and NT‐pro‐BNP (P < 0.001, r = 0.579) (Figs A–C). No correlation was found between the duration of smoking and changes in HRV parameters in smoker subjects, while a significant positive correlation was observed between the duration of smoking and
Table 1.
Demographic Characteristics of the Study Subjects
| Variables | Smokers (n = 75) | Nonsmokers (n = 73) | P |
|---|---|---|---|
| Age (years) | 36 ± 8 | 34 ± 7 | 0.1 |
| Gender (females/males) | 40/35 | 45/28 | 0.2 |
| Body mass index (kg/m2) | 20.5 ± 1.6 | 20.8 ± 1.6 | 0.3 |
| Duration of smoking (years) | 7 ± 5 | ‐ | ‐ |
| Number of cigarettes/day | 26 ± 7 | ‐ | ‐ |
| Systolic blood pressure (mmHg) | 128 ± 6 | 116 ± 9 | <0.001 |
| Diastolic blood pressure (mmHg) | 80 ± 5 | 73 ± 5 | <0.001 |
| Mean heart rate | 79 ± 9 | 76 ± 11 | <0.001 |
| Ventricular premature contraction/day | 336 ± 324 | 36 ± 5 | <0.001 |
| NT‐pro‐BNP | 70 ± 16 | 36 ± 20 | <0.001 |
NT‐pro‐BNP = N‐Terminal‐ pro‐B‐type natriuretic peptide (pg/mL).
Table 2.
Heart Rate Variability Parameters of the Study Subjects
| Variables | Smokers (n = 75) | Nonsmokers (n = 73) | P |
|---|---|---|---|
| SDNN | 54.2 ± 31 | 138.2 ± 61.7 | <0.0001 |
| SDANN | 45.3 ± 20.2 | 116.6 ± 46.2 | <0.0001 |
| RMSSD | 36.9 ± 18.1 | 48.7 ± 30.8 | 0.006 |
| LF day | 1520 ± 506 | 505 ± 431 | <0.0001 |
| LF night | 1087 ± 416 | 543 ± 127 | <0.0001 |
| LF 24 hours | 1313 ± 467 | 428 ± 325 | <0.0001 |
| HF day | 263 ± 102 | 448 ± 404 | <0.0001 |
| HF night | 319 ± 113 | 616 ± 138 | 0.003 |
| HF 24 hours | 278 ± 98 | 438 ± 154 | 0.005 |
| LF/HF day | 6.4 ± 3.0 | 1.4 ± 1.2 | <0.0001 |
| LF/HF night | 3.6 ± 1.5 | 1.0 ± 1.2 | <0.0001 |
| LF/HF 24 hours | 4.9 ± 1.9 | 1.2 ± 0.7 | <0.0001 |
SDNN = standard deviation of all NN intervals; SDANN = standard deviation of the 5‐minute mean RR intervals; RMSSD = root mean square of successive differences; LF = low frequency; HF = high frequency.
Figure 1.
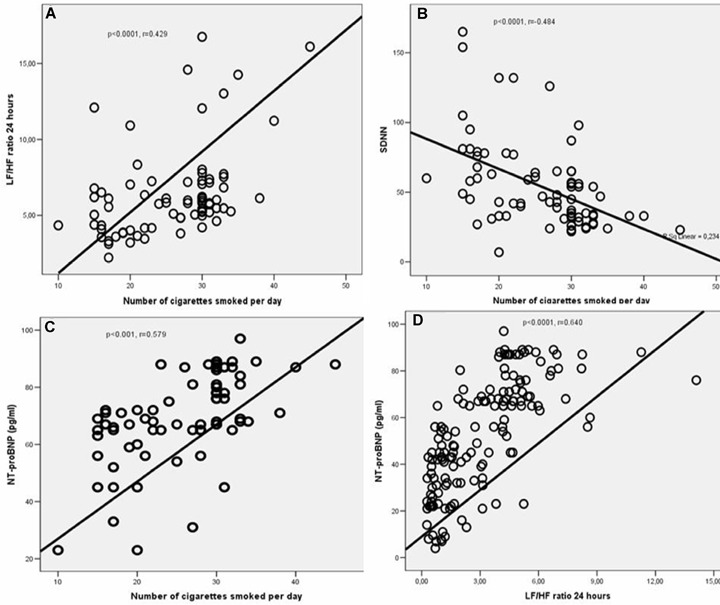
(A–D) The relationship between the number of cigarettes smoked per day and LF/HF ratio 24 hours (A), SDNN (B), and NT‐pro‐BNP (C). The correlation between LH/HF ratio 24 hours and NT‐pro‐BNP levels (D). SDNN = standard deviation of all NN intervals; LF = low frequency; HF = high frequency; NT‐pro‐BNP; = N‐Terminal pro‐B‐type Natriuretic Peptide.
NT‐pro‐BNP levels (P < 0.0001, r = 0.579) and systolic blood pressure (P = 0.01, r = 0.271).In addition, the average levels of NT‐pro‐BNP were found to be positively correlated with LF (day, night, and 24 hours), LF/HF ratio (day, night, and 24 hours) and inversely correlated with SDNN, SDANN, RMSSD, and HF (day, night, and 24 hours) (Table 3, Fig.1D). In addition, in the smoking group, it was found that the VPCs are significantly correlated with SDNN, SDANN, LF (day, night, and 24 hours), LF/HF ratio (day, night, and 24 hours), and HF day (Table 3). Moreover, it was observed that even smoking one cigarette leads to an significant increase in LF/HF ratio day by 0.18 folds (P < 0.0001, 95% confidence interval [CI], 0.09–0.27), LF/HF ratio night by 0.10 folds (P < 0.0001, 95% CI, 0.05–0.14), LF/HF ratio 24 hours by 0.13 folds (P < 0.0001, 95% CI 0.07–0.18), and NT‐pro‐BNP by 1.3 folds (P < 0001, 95% CI 0.87–1.72), number of VPCs by 11 folds (P = 0.03, 95% CI 0.8–22), and a significant decrease in SDNN by ─2.154 folds (P < 0.0001, 95% CI ─3.06 to 1.25) (Table 4).
Table 3.
The Correlation between Plasma N‐Terminal pro‐B‐Type Natriuretic Peptide, Ventricular Premature Contractions, and Heart Rate Variability Parameters in Healthy Smoker Subjects
| Variables | NT‐pro‐BNP | VPCs |
|---|---|---|
| SDNN | r =−0.451, P < 0.0001 | r =−0.387, P < 0.0001 |
| SDANN | r =−0.523, P < 0.0001 | r =−0.416, P < 0.0001 |
| RMSSD | r =−0.229, P < 0.0001 | r =−0.112, P = 0.2 |
| LF day | r = 0.572, P < 0.0001 | r = 0.447, P < 0.0001 |
| LF night | r = 0.246, P < 0.0001 | r = 0.188, P < 0.0001 |
| LF 24 hours | r = 0.568, P < 0.0001 | r = 0.465, P < 0.0001 |
| HF day | r =−0.339, P<0.0001 | r =−0.162, P = 0.04 |
| HF night | r =−0.167, P = 0.04 | r =−0.092, P = 0.2 |
| HF 24 hours | r =−0.268, P = 0.005 | r =−0.131, P = 0.1 |
| LF/HF day | r = 0.623, P < 0.0001 | r = 0.394, P < 0.0001 |
| LF/HF night | r = 0.542, P < 0.0001 | r = 0.349, P<0.0001 |
| LF/HF 24 hours | r = 0.640, P < 0.0001 | r = 0.443, P < 0.0001 |
SDNN = standard deviation of all NN intervals; SDANN = standard deviation of the 5‐minute mean RR intervals; RMSSD = root mean square of successive differences; LF = low frequency; HF = high frequency; VPCs = ventricular premature contractions; NT‐pro‐BNP = N‐Terminal pro‐B‐type natriuretic peptide (pg/mL).
Table 4.
Effect of a Single Cigarette Smoking on Heart Rate Variability, Plasma N‐Terminal pro‐B‐type Natriuretic Peptide, Ventricular Premature Contractions, and Blood Pressure in Healthy Subjects
| Variables | B | SE | Beta | t | P |
|---|---|---|---|---|---|
| Systolic BP | 0.250 | 0.098 | 0.285 | 2.541 | 0.01 |
| Diastolic BP | 0.211 | 0.082 | 0.288 | 2.567 | 0.01 |
| VPCs | 11.16 | 5.215 | 0.243 | 2.141 | 0.03 |
| NT‐pro‐BNP | 1.298 | 0.214 | 0.579 | 6.072 | <0.0001 |
| SDNN | −2.154 | 0.456 | −0.484 | −4.726 | <0.0001 |
| LF day | 43.4 | 6.65 | 0.607 | 6.525 | <0.0001 |
| LF night | 35.7 | 5.47 | 0.607 | 6.519 | <0.0001 |
| LF 24 hours | 39.8 | 6.043 | 0.611 | 6.589 | <0.0001 |
| LF/HF day | 0.182 | 0.045 | 0.429 | 4.056 | <0.0001 |
| LF/HF night | 0.099 | 0.023 | 0.450 | 4.307 | <0.0001 |
| LF/HF 24 hours | 0.126 | 0.028 | 0.465 | 4.486 | <0.0001 |
SDNN = standard deviation of all NN intervals; LF = low frequency; HF = high frequency; SE = Standard error; BP = Blood pressure; VPCs = ventricular premature contractions; NT‐pro‐BNP; = N‐Terminal pro‐B‐type natriuretic peptide (pg/mL).
Linear logistic regression analysis revealed that the number of cigarettes smoked per day independently affects NT‐pro‐BNP levels (P < 0.001, 95% CI 0.16–0.31) and SDNN (P < 0.0001, 95% CI ─0.13 to 0.10) in the smoker subjects.
DISCUSSION
This study has produced the following outcomes: 1 cigarette smoking causes an increase in LF (day, night, 24 hours), LH/HF ratio (day, night, and 24 hours), blood pressure, the number of PVCs, and plasma pro‐BNP levels, while a decrease in SDNN, SDANN, RMSSD, and HF (day, night, and 24 hour). 2 The number of cigarettes smoked per day (number/day) were positively correlated with LF (day, night, 24 hours), LH/HF ratio (day, night, and 24 hours), PVCs, and NT‐pro‐BNP and inversely correlated with SDNN. 3 In addition, it was observed that increasing NT‐pro‐BNP was correlated with an increase in LF, LH/HF ratio, while this was correlated with a decrease in SDNN, SDANN, RMSSD, and HF in healthy smoker subjects.
The assessment of HRV is based on the analysis of consecutive sinus rhythm RR intervals and may provide quantitative information about the modulation of cardiac sympathetic and parasympathetic nerve activities. Heart rate variability can be measured in a number of ways but techniques of conventional time‐domain (statistical and geometrical) and frequency‐domain measurements (power‐spectral density) remain predominantly utilized. 6 Significantly altered HRV can be found not only in cardiac diseases but also in a wide variety of pathophysiologic disorders characterized by neurohumoral activation. 7 The instantaneous RR interval depends on the continuous interplay between vagal and sympathetic efferent activity to the SA node and the intrinsic heart rate. 17 Increased sympathetic activity or decreased vagal modulation of cardiac function assessed by HRV analysis has been associated with an increased risk of coronary heart disease and mortality 18 and angiographic progression of coronary atherosclerosis, 19 as well as arrhythmia and sudden cardiac death. 20
Cigarette smoking is one of the major modifiable risk factors for cardiovascular disease. In addition to the harmful effects of chronic use, there are also harmful effects in the acute period. It possibly has an effect on various parts in the neurocardiovascular regulation system, which includes the afferent and efferent division of the autonomic nervous system and the central nervous system. 21 Most of the acute effects of smoking on neurocardiovascular regulation can be explained as the effects of nicotine. 22 The mechanisms of the effects of nicotine involve both stimulation and blocking of the autonomic ganglia, liberation of adrenomedullary epinephrine, stimulation of carotid body chemoreceptors and aortic baroreceptors, and direct action in the central nervous system. 21 , 23 , 24 A sympathetic stimulatory effect of nicotine has been demonstrated in centrally denervated, incubated cardiac tissue. 25 Also, smoking may itself impair arterial baroreflex function, 9 , 26 by reducing arterial distensibility acutely. 27 In addition, Cryer et al. 28 and Hill et al. 29 have demonstrated that plasma catecholamine levels increase within 1 minute after smoking or an infusion of nicotine. Thus, the increased LF, LF/HF, VPCs and decreased SDNN, SDANN, and HF in our findings, suggesting the sympathetic activation during cigarette smoking, may be the result of liberation of catecholamines or impaired baroreflex function. In agreement with our findings, Grassi et al. 5 have reported that the short‐term changes in LF/HF and HF after smoking may be dependent on an increased release and/or a reduced clearance of catecholamines at the neuroeffector junctions. Recently, Barutcu et al. 9 have showed that smoking leads to an increase in LF/HF ratio and a decrease in SDNN and RMSSD in healthy subjects. Similarly, Kobayashi et al. 13 have reported that LF/HF ratio significantly increases immediately after smoking in taxi drivers. Also, our results showed that there was no correlation between the duration of smoking and changes in HRV parameters, while they indicated a significant correlation between the number of cigarettes smoked per day and LF, HF, and SDNN, suggesting that cigarette smoking causes acute effects on sympathovagal control. Similarly, acute effects of smoking have also been reported in smoking cessation intervention, 30 , 31 with a rapid increase in HRV appearing as quickly as 1 day after smoking cessation.
Our findings revealed that systolic blood pressure, diastolic blood pressure, and mean heart rate were higher in smoker than in nonsmokers subjects. They indicated that the effects of cigarette smoking on blood pressure and heart rate are in agreement with the previous studies. 5 , 11 , 32 , 33 The hemodynamic changes in smoking group are thought to be as a result of nicotine‐dependent stimulation of the sympathetic control and inactivation of the vagal cardiovascular control, 32 , 33 which are consistent with our findings. Similarly, Narkiewicz et al. 11 have reported that cigarette smoking causes an increase in blood pressure and heart rate due to an increase in sympathetic outflow to skin and muscle blood vessels.
Natriuretic peptides are synthesized and secreted by the myocardium as a response to increased atrial and ventricular tension. 34 Therefore, circulating levels of these peptides are increased both in patients with left ventricular (LV) hypertrophy and in patients with LV dysfunction. 35 However, cigarette smoking has been shown to impair left ventricular diastolic function, to cause temporary rises in blood pressure and heart rate, to impair microvascular function, and aortic elastic properties. 36 , 37 , 38 In this study, we found that pro‐BNP levels were higher in the smoker subjects than the nonsmoker subjects. Thus, we speculated that increased NT‐pro‐BNP levels in smoking group may be attributed to diastolic dysfunction 39 or increased sympathetic nervous activity. But the effects of hormonal factors on autonomic nervous system functions are under debate. Atrial natriuretic peptide (ANP) exerts a sympathoinhibitory action in heart failure in normal men. 40 , 41 Brunner et al. 42 demonstrated a sympathoinhibitory effect of BNP at concentrations within the physiologic range but systemic and cardiac sympathetic nervous activity equates to baseline values with high‐dose BNP. Although there was a significant correlation between the average levels of NT‐pro‐BNP and HRV parameters (reflecting the increased sympathetic activity and decreased parasympathetic tone) in our study, it was difficult to say which is the result or cause. But to our knowledge, our study is the first to document the relationships between NT‐pro‐BNP levels and HRV parameters in healthy smoker subjects.
As a result, smoking causes a significant increase in sympathetic activity, impairs sympathovagal balance, and decreases the heart rate variability in healthy subjects. And even smoking one cigarette brings about overt sympathetic excitation and impairs sympathovagal balance. In addition, smoking results in an increase in NT‐pro‐BNP levels. The changes in adrenergic nervous system and NT‐pro‐BNP levels are well correlated. These findings could contribute to the higher rate of cardiovascular events in smokers. There are other mechanisms by which cigarette smoking increases cardiovascular events in smokers.
REFERENCES
- 1. Hallstrom AP, Cobb LA, Ray R. Smoking as a risk factor for recurrence of sudden cardiac arrest. N Engl J Med 1986;314:271–275. [DOI] [PubMed] [Google Scholar]
- 2. Celermajer DS, Sorensen KE, Georgeakopoulos D, et al Cigarette smoking is associated with dose‐related and potentially reversible impairment of endothelium‐dependent dilation in healthy young adults. Circulation 1993;88:2149–2155. [DOI] [PubMed] [Google Scholar]
- 3. Winniford MD, Weelan KR, Kremers MS, et al Smoking‐induced coronary vasoconstriction in patients with atherosclerotic coronary artery disease: Evidence for adrenergically mediated alterations in coronary tone. Circulation 1986;73:662–667. [DOI] [PubMed] [Google Scholar]
- 4. Moliterno DJ, Willard JE, Lange RA, et al Coronary‐artery vasoconstriction induced by cocaine, cigarette smoking, or both. N Engl J Med 1994;330:454–459. [DOI] [PubMed] [Google Scholar]
- 5. Grassi G, Seravalle G, Calhoun DA, et al Mechanisms responsible for sympathetic activation by cigarette smoking in humans. Circulation 1994;90:248–253. [DOI] [PubMed] [Google Scholar]
- 6. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology . Heart rate variability: Standards of measurement, physiological interpretation and clinical use. Circulation 1996;93:1043–1065. [PubMed] [Google Scholar]
- 7. Lopera GA, Huikuri HV, Makikallio TH, et al Is abnormal heart rate variability a specific feature of congestive heart failure? Am J Cardiol 2001;87:1211–1213. [DOI] [PubMed] [Google Scholar]
- 8. Bilchick KC, Fetics B, Djoukeng R, et al Prognostic value of heart rate variability in chronic congestive heart failure (Veterans Affairs' Survival Trial of Antiarrhyhmic Therapy in Congestive Heart Failure). Am J Cardiol 2002;90:24–28. [DOI] [PubMed] [Google Scholar]
- 9. Barutcu I, Esen AM, Kaya D, et al Cigarette smoking and heart rate variability: Dynamic influence of parasympathetic and sympathetic maneuvers. A.N.E. 2005;10:324–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hayano J, Yamada M, Sakakibara Y, et al Short‐and long‐term effects of cigarette smoking on heart rate variability. Am J Cardiol 1990;65:84–88. [DOI] [PubMed] [Google Scholar]
- 11. Narkiewicz K, Van de Borne PJH, Hausberg M, et al Cigarette smoking increases sympathetic outflow in humans. Circulation 1998;98:528–534. [DOI] [PubMed] [Google Scholar]
- 12. Hausberg M, Mark Al, Winniford MD, et al Sympathetic and vascular effects of short‐term passive smoke exposure in healthy nonsmokers. Circulation 1997;96:282–287. [PubMed] [Google Scholar]
- 13. Kobayashi F, Watanabe T, Akamatsu Y, et al Acute effects of cigarette smoking on the heart rate variability of taxi drivers during work. Scand J Work Environ Health 2005;31:360–366. [DOI] [PubMed] [Google Scholar]
- 14. Maisel AS, Mc Cullough PA. Cardiac natriuretic peptides: A proteomic window to cardiac function and clinical management. Rev Cardiovasc Med 2003;4 (Suppl. 4):S3–S12. [PubMed] [Google Scholar]
- 15. Lubien E, DeMaria A, Krishnaswamy P, et al Utility of B‐natriuretic peptide in detecting diastolic dysfunction. Comparison with Doppler velocity recordings. Circulation 2002;105:595–601. [DOI] [PubMed] [Google Scholar]
- 16. Pagani M, Lombardi F, Guzzetti S, et al Power spectral analysis of heart rate and arterial pressure variabilities as a marker of sympatho‐vagal interaction in man and conscious dog. Circ Res 1986;59:178–193. [DOI] [PubMed] [Google Scholar]
- 17. Malliani A, Pagani M, Lombardi F, et al Advances in Research Series: Cardiovascular neural regulation explored in the frequency domain. Circulation 1991;84:482–492. [DOI] [PubMed] [Google Scholar]
- 18. Dekker JM, Crow RS, Folsom AR, et al Low heart rate variability in a 2‐minute rhythm strip predicts risk of coronary heart disease and mortality from several causes: The ARIC Study. Circulation 2000;102:1239–1244. [DOI] [PubMed] [Google Scholar]
- 19. Huikuri HV, Jokinen V, Syvanne M, et al Heart rate variability and progression of coronary atherosclerosis. Arterioscler Thromb Vasc Biol 1999;19:1979–1985. [DOI] [PubMed] [Google Scholar]
- 20. Wilhelm FH, Grossman P, Roth WT. Assessment of heart rate variability under non‐stationary conditions: Complex demodulation vs. spectral analysis. Psychophysiology 1997;34 (Suppl. 1):S96. [Google Scholar]
- 21. Robertson D, Tseng CJ, Appalsamy M. Smoking and mechanisms of cardiovascular control. Am Heart J 1988;115:258–263. [DOI] [PubMed] [Google Scholar]
- 22. Aronow WS, Dendinger J, Rokaw SN. Heart rate and carbon monoxide level after smoking high‐, low‐, and non‐nicotine cigarettes. A study in male patients with angina pectoris. Ann Intern Med 1971;74:697–702. [DOI] [PubMed] [Google Scholar]
- 23. Nadeau RA, James TN. Effects of nicotine on heart rate studied by direct perfusion of sinus node. Am J Physiol 1967;212:911–916. [DOI] [PubMed] [Google Scholar]
- 24. Mandel WJ, Laks M, Hayakawa H, et al Cardiovascular effects of nicotine in the conscious dog. Modification by changes in autonomic tone. Am J Cardiol 1973;32:947–955. [DOI] [PubMed] [Google Scholar]
- 25. Krüger C, Haunstetter A, Gerber S, et al Nicotine‐induced exocytotic norepinephrine release in guinea‐pig heart, human atrium and bovine adrenal chromaffin cells: Modulation by single components of ischaemia. J Mol Cell Cardiol 1995;27:1491–1506. [DOI] [PubMed] [Google Scholar]
- 26. Manica G, Groppelli A, Di Rienzo M, et al Smoking impairs baroreflex sensitivity in humans. [DOI] [PubMed]
- 27. Failla M, Grappiolo A, Carugo S, et al Effects of cigarette smoking on carotid and arterial distensibility. J Hypertens 1997;15:1659–1664. [DOI] [PubMed] [Google Scholar]
- 28. Cryer PE, Haymond MW, Santiago JV, et al Norepinephrine release and adrenergic mediation of smoking‐associated hemodynamic and metabolic events. N Engl J Med 1976;295:573–577. [DOI] [PubMed] [Google Scholar]
- 29. Hill P, Wynder EL. Smoking and cardiovascular disease. Effect of nicotine on the serum epinephrine and corticoids. Am Heart J 1974;87:491–496. [DOI] [PubMed] [Google Scholar]
- 30. Minami J, Ishimitsu T, Matsuoka H. Effects of smoking cessation on blood pressure and heart rate variability in habitual smokers. Hypertension 1999;33 (1 Pt 2):586–590. [DOI] [PubMed] [Google Scholar]
- 31. Yotsukura M, Koide Y, Fujii K, et al Heart rate variability during the first month of smoking cessation. Am Heart J 1998;135 (6 Pt 1):1004–1009. [DOI] [PubMed] [Google Scholar]
- 32. Ragueneau I, Michaud P, Demolis JL, et al Effects of cigarette smoking on short‐term variability of blood pressure in smoking and non smoking healthy volunteers. Fundam Clin Pharmacol 1999;13:501–507. [DOI] [PubMed] [Google Scholar]
- 33. Groppelli A, Giorgi D, Omboni S, et al Persistent blood pressure increase induced by heavy smoking. J Hypertens 1992;10:495–499. [DOI] [PubMed] [Google Scholar]
- 34. Mair J, Hammerer‐Lercher A, Puschendorf B. The impact of cardiac natriuretic peptide determination on the diagnosis and management of heart failure. Clin Physiol 1998;18:185–201. [DOI] [PubMed] [Google Scholar]
- 35. Groenning BA, Nilsson JC, Sondergaard L, et al Detection of left ventricular enlargement and impaired systolic function with plasma N‐terminal pro brain natriuretic peptide concentrations. Am Heart J 2002;143:923–929. [DOI] [PubMed] [Google Scholar]
- 36. Alam M, Samad BA, Wardell J, et al Acute effects of smoking on diastolic function in healthy participants: Studies by conventional Doppler echocardiography and Doppler tissue imaging. J Am Soc Echocardiogr 2002;15:1232–1237. [DOI] [PubMed] [Google Scholar]
- 37. Stork T, Danne O, Muller R, et al Effect of smoking on relaxation and filling behavior of the left ventricle in healthy probands. An echocardiography study Med Klein. (Munich) 1991;15;86:173–179. [PubMed] [Google Scholar]
- 38. Kim HK, Bae JW, Chung JW, et al Differences in immediate effects of smoking on left ventricular diastolic function between healthy volunteers and patients with type 2 diabetes mellitus. J Am Soc Echocardiogr 2005;18:320–325. [DOI] [PubMed] [Google Scholar]
- 39. Arteaga E, Araujo AQ, Buck P, et al Plasma amino‐terminal pro‐B‐type natriuretic peptide quantification in hypertrophic cardiomyopathy. Am Heart J 2005;150:1228–1232. [DOI] [PubMed] [Google Scholar]
- 40. Abramson BL, Ando S, Notarius CF, et al Effect of atrial natriuretic peptide on muscle sympathetic activity and its reflex control in human heart failure. Circulation 1999;99(14):1810–1815. [DOI] [PubMed] [Google Scholar]
- 41. Butler GC, Senn BL, Floras JS. Influence of atrial natriuretic factor on heart rate variability in normal men. Am J Physiol 1994;267(2 Pt 2):H500–H505. [DOI] [PubMed] [Google Scholar]
- 42. Brunner‐La Rocca HP, Kaye DM, et al Effects of intravenous brain natriuretic peptide on regional sympathetic activity in patients with chronic heart failure as compared with healthy control subjects. J Am Coll Cardiol 2001;37(5):1221–1227. [DOI] [PubMed] [Google Scholar]


