Abstract
Increasing awareness of atrial fibrillation (AF) and its impact on public health revives interest in identification of noninvasive markers of predisposition to AF and ECG‐based risk stratification. P‐wave duration is generally accepted as the most reliable noninvasive marker of atrial conduction, and its prolongation has been associated with history of AF. However, patients with paroxysmal AF without structural heart disease may not have any impressive P‐wave prolongation, thus suggesting that global conduction slowing is not an obligatory requirement for development of AF. P‐wave morphology is therefore drawing increasing attention as it reflects the three‐dimensional course of atrial depolarization propagation and detects local conduction disturbances. The factors that determine P‐wave appearance include (1) the origin of the sinus rhythm that defines right atrial depolarization vector, (2) localization of left atrial breakthrough that defines left atrial depolarization vector, and (3) the shape and size of atrial chambers. However, it is often difficult to distinguish whether P‐wave abnormalities are caused by atrial enlargement or interatrial conduction delay. Recent advances in endocardial mapping technologies have linked certain P‐wave morphologies with interatrial conduction patterns and the function of major interatrial conduction routes. The value of P‐wave morphology extends beyond cardiac arrhythmias associated with atrial conduction delay and can be used for prediction of clinical outcome of a wide range of cardiovascular disorders, including ischemic heart disease and congestive heart failure.
Keywords: atrial fibrillation, atrial arrhythmias, basic, noninvasive techniques, electrocardiography, clinical
Increasing awareness of atrial fibrillation (AF) and its impact on public health revives the interest in identifying noninvasive markers of predisposition to this arrhythmia and ECG‐based risk stratification. As pathophysiological mechanisms of AF largely depend on the degree of structural atrial remodeling and are directly linked to the extent of fibrosis in atrial walls, 1 studies of the association between the atrial substrate, abnormal atrial depolarization in surface ECG and atrial arrhythmias become a rapidly expanding research field.
P‐wave duration is generally accepted as the most reliable noninvasive marker of atrial conduction, and its prolongation has been associated with a history of AF, 2 development of arrhythmia after bypass surgery, 3 and deterioration of paroxysmal AF into a permanent form of arrhythmia. 4 Recently, association between the P‐wave duration and long‐term AF risk was shown in an elderly community‐based cohort of the Framingham Heart Study. 5 However, P‐wave prolongation per se may be regarded only as a crude marker of atrial conduction slowing. In several reports, patients with lone paroxysmal AF failed to demonstrate any impressive P‐wave prolongation. 6 , 7 Neither in patients with congestive heart failure was P‐wave duration predictive of new‐onset AF. 8 Global conduction slowing is therefore not an obligatory requirement for the development of AF.
Under these circumstances, P‐wave morphology is drawing increasing attention due to its inherent ability to reflect the three‐dimensional course of atrial depolarization propagation and detect local conduction disturbances with or without impact on total time of atrial depolarization measured as P‐wave duration.
WHAT IS NORMAL?
Due to the sinus node being adjacent to the superior caval vein opening in the right atrium, the main vector of atrial depolarization is pointed downward and leftward in the frontal plain corresponding to a mean frontal plane P‐wave axis of approximately 60 degrees. Consequently, atrial activation projects in the frontal plane as positive or upright P waves in leads I, II and aVF, with some variability observed in leads aVL and III depending on atrial geometry and exact orientation of the depolarization vector. 9
Despite relative stability of sinus P‐wave vector direction in the frontal plane, P‐wave morphology in the sagittal plane or in the right precordial leads is more variable, and P wave can appear as a unimodal positive or a biphasic wave with terminal negative deflection. This so‐called negative P‐wave terminal force (PTF) 10 can be characterized by different "depth" or amplitude measured in millimetres (mm) and duration. Sometimes the terminal portion of the P wave appears nearly isoelectric or, depending on amplitude resolution, has very low negative or positive amplitude, thus suggesting the left atrial activation vector projected perpendicularly to the V1 axis. Understandably, measurable P‐wave duration in this lead would be quite short, corresponding to the right atrial activation only.
In the absolute terms, PTF that does not exceed 0.1 mV in its negative amplitude is considered normal; 9 however, as early as 1978 it was reported that abnormal PTF was not uncommon in healthy middle‐aged men, and its prevalence increased after exercise. 11 In the study of age‐related P‐wave morphology features in ostensibly healthy adults representing different age groups, biphasic P waves in the sagittal plane were seen in a minority of individuals under 50 years of age, 12 and also present in school‐age children as reported by Dilaveris et al. 13 In individuals older than 50 years, the biphasic P waves were observed in 70%–80% of subjects. 12
However, the precision of the PTF measurement is based on the assumption that P‐wave signal returns to baseline at the end of atrial depolarization. In reality, electrical silence in the heart does not occur until the end of ventricular repolarization (and even then the If channels slowly reduce the membrane voltage of sinoatrial node pacemaker cells approaching the depolarization threshold). Atrial repolarization is the natural phase of the atrial cycle, expressed on surface ECG as an atrial repolarization wave (Ta wave), which is a low‐amplitude and low‐frequency deflection two to three times longer than the P wave itself. 14 , 15 , 16 Understanding of atrial repolarization expression on ECG is particularly important when signal‐averaged high‐resolution ECG techniques are used for P‐wave analysis. Superimposition of the Ta wave on the P wave may distort the P‐wave terminal component. Even though differences in amplitude and frequency content of depolarization and repolarization waves make them quite distinct ECG entities (Fig. 1), the exact definition of P‐wave end is more complicated. Conventional ECG equipment handles this by applying noise filtration and baseline drift correction algorithms that eliminate low‐amplitude deflections in the PQ interval, including the Ta wave. The neglected ECG component can, however, contain information about atrial repolarization that is relevant in the context of AF. 17
Figure 1.
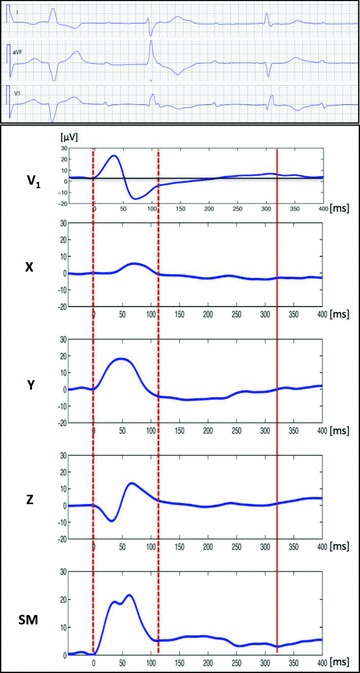
Signal‐averaged orthogonal unfiltered PTa‐wave morphology in a patient with complete atrio‐ventricular block. Note the low‐amplitude and low‐frequency Ta repolarization wave that follows P wave (dotted lines), best seen in lead Y as well as in the spatial magnitude (SM) vector. While P‐wave onset is a very distinct event, the end of the P wave may be obscured by the superimposed Ta wave that affects the morphology of the P‐wave terminal part. Solid line indicates the end of the Ta wave. The upper panel represents ECG from standard leads I, aVF and V1 from the same patient.
DETERMINANTS OF P‐WAVE MORPHOLOGY
Since P‐wave morphology reflects the projection of the depolarization vector on the ECG lead axes in three‐dimentional space and covers both right and left atrial activation, it largely depends on: (1) the origin of the [sinus] rhythm that defines the right atrial depolarization vector, (2) left atrial breakthrough that defines the left atrial depolarization vector, and (3) shape and size of atrial chambers that affect the time required for completion of the depolarization process, as well as the course of depolarization wave propagation. In addition, structural abnormalities in atrial walls may contribute to conduction slowing and prolongation of P‐waves.
Sinus Rhythm Origin
Contrary to the common perception based on schematic and often simplified illustrations of the sinus node being a relatively small structure located at the superior caval vein opening in the right atrium, the most recent anatomical reconstruction indicates that the compact sinus node may reach 20 mm in length and extend downward in the projection of the terminal groove. 18 These findings are supported by clinical experience of catheter ablation of inappropriate sinus tachycardia in the sinus node region. 19
However, functional studies indicate that the right atrial pacemaker complex in humans may encompass a considerably larger area—as large as 7.5 cm × 1.5 cm as shown by Bouneau et al. 20 Using epicardial mapping during open‐chest surgery for Wolff‐Parkinson‐White syndrome, the same group has reported considerable interindividual variability with regard to the location of the earliest activation recorded in this region. The activation can originate anywhere—from the most inferior part of the right atrial pacemaker complex at the level of mid‐septal region to the most superior part at the junction with right atrial appendage. 20 , 21 The variable location of the atrial pacemaker can cause variability of the initial right atrial activation vector and upward or biphasic P‐wave appearance in the inferior leads (Fig. 2). 20 , 22
Figure 2.
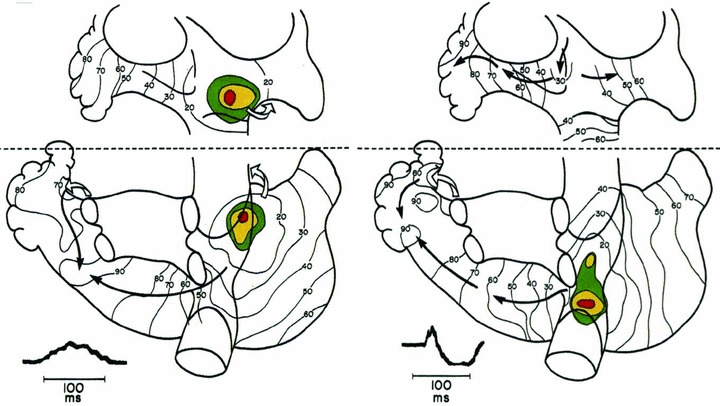
Variability in sinus rhythm origin recorded using epicardial mapping in subjects undergoing open‐chest surgery for Wolff‐Parkinson‐White syndrome (adapted from Boineau et al. 20 with permission). Isochronal maps illustrate the propagation of atrial depolarization via either Bachmann's bundle anteriorly (left panel) or via the inferior route beneath the inferior pulmonary veins (right panel). Examples of P waves from lead aVF illustrate the impact of sinus rhythm origin and propagation route on P‐wave morphology.
Intraindividual beat‐to‐beat variability of P waves has also been reported. At the dawn of computer‐assisted signal‐averaged ECG analysis, Brody et al. described the presence of secondary and tertiary P‐wave morphologies at rest in as many as one‐third of apparently healthy subjects. 23 Boineau et al. (in the study cited above) reported the possibility of a spontaneous P‐wave morphology change caused by a shift in the site of the sinus depolarization wave origin. 20 Whether variability of sinus rhythm origin is due to the actual switch from one group of pacemaking cells within the sinus node to another, or whether variability is caused by a different route of propagation within and out of the sinus node via distinct exit pathways is a matter of debate that was recently reviewed. 22 Animal experiments performed nearly a hundred years ago by Meek and Eyster 24 documented that vagal stimulation resulted in slowing of the heart rate and also shifted the impulse initiation site. More recently, the association between heart rate acceleration and the earliest activation site shift to superior/anterior regions of the right atrial pacemaker complex has been documented in independent experimental studies. 25 , 26 These find‐ings are in agreement with P‐wave morphology change in healthy subjects during exercise described either as an increase in negative PTF in lead V1 11 or increase in maximal posterior and inferior component in orthogonal Frank leads. 27
Our group has studied the effect of atropine on P‐wave morphology in patients without structural heart disease 28 and found that despite relative stability of P‐wave gross morphology, expressed as positive, negative of biphasic P‐wave configuration in the orthogonal leads, heart rate increase was associated with a reduction of initial positive component and more prominent terminal negative P‐wave component in lead V1 (Fig. 3A and 3B), consistent with earlier findings. 11 Notably, the only patient who had negative P wave in V1 at baseline consistent with backward propagation of atrial depolarization wave from a pacemaker site located anteriorly to the Bachmann's bundle did not show any change in P‐wave morphology after atropine administration (Fig. 3C). This observation indicates that even though some anterior/superior shift of sinus rhythm driver could have occurred, it could not affect the sequence of atrial activation via intra‐ and interatrial pathways. Negative P waves in lead V1 may be observed during atrial pacing in patients with the atrial lead placed in the right atrial appendage, and during ectopic atrial tachycardia originating from the right atrial appendage. 29
Figure 3.
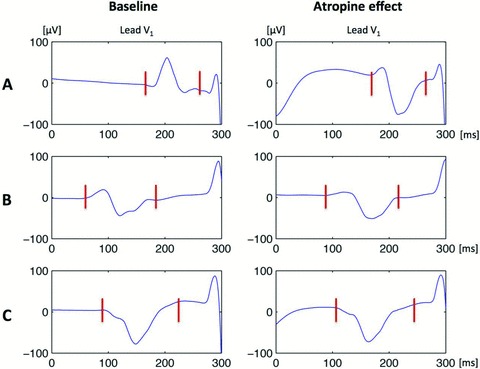
P‐wave morphology change in lead V1 following i.v. atropine administration. Note the increase in amplitude of the negative terminal component seen in patients A (upright P wave at baseline) and B (biphasic P wave at baseline). Patient C who had negative P waves at baseline did not show any notable P‐wave morphology change despite heart rate increase in response to atropine administration.
Left Atrial Breakthrough
The localization of the depolarization wave breakthrough site in the left atrium that becomes a starting point for left atrial activation is another factor that, to a large extent, defines the left atrial depolarization vector and shapes the terminal part of the P wave. While Bachmann's bundle is considered the most important interatrial conduction pathway, the depolarization breakthrough to the left atrium may occur in the posterior‐inferior region of the atrial septum in up to one‐third of patients, as reported using noncontact mapping. 30 , 31 Using electro‐anatomical mapping in the largest reported patient series to date, we found that, remarkably in agreement with the above‐cited studies, left atrial breakthrough occurred outside the Bachmann bundle via posterior or inferior interatrial connections in one‐third of patients. 32 In 14 of 50 patients in our cohort, more than one breakthrough was observed.
What makes the depolarization wave cross the atrial septum boundary at a certain location is not entirely clear at this time. Contributing factors may include the reported high interindividual variability in the structure and location of interatrial pathways, 33 as well as their conduction properties. Relative proximity of the sinus rhythm origin to the superior (Bachmann's bundle) or posterior/inferior interatrial routes may also affect the route “chosen” for interatrial conduction and, hence, the site of the left atrial breakthrough (Fig. 2). Regardless of the cause, the route used for transseptal conduction determines the morphology of the P‐wave terminal part. 34
Using electroanatomical mapping of the left atrium during sinus rhythm, our group proposed an explanation of the P‐wave morphology origin in subjects without structural heart disease. 34 The upright P wave in right precordial leads appears to be associated with conduction not only via Bachmann's bundle, but also via other interatrial connections in the vicinity of the right pulmonary veins on the back side of the heart, thus resulting in posterior‐to‐anterior propagation of the sinus beat in both right and left atria (Fig. 4 upper panel). Biphasic P waves in these leads, on the other hand, are associated with conduction via the Bachmann's bundle resulting only in the anterior‐to‐posterior direction of left atrial activation, i.e., the terminal part of the P wave (Fig. 4 lower panel).
Figure 4.
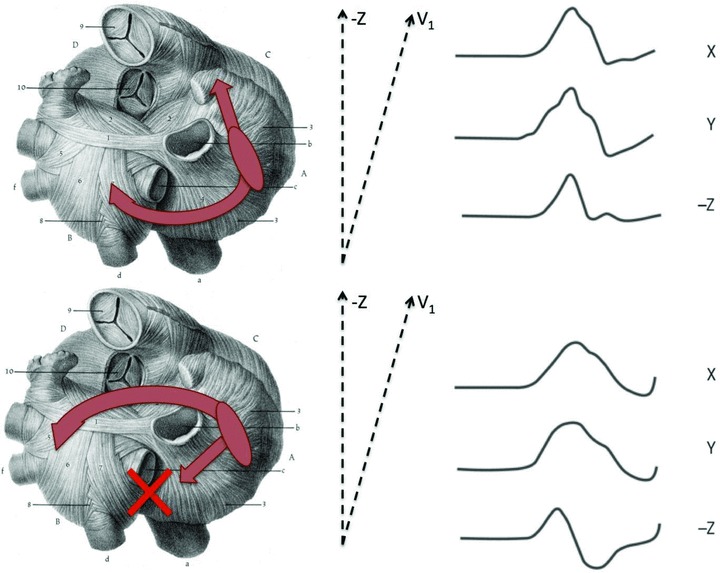
Schematic illustration of mechanisms underlying the development of biphasic P waves in the sagittal plane. Interatrial conduction over posterior interatrial connections (with or without participation of Bachmann's bundle) result in posterior‐to‐anterior propagation of excitation in the left atrium, leading to positive or isoelectric P waves in the sagittal plane (upper panel, Type 1 P‐wave morphology). Interatrial conduction over Bachmann's bundle only, without contribution from posterior fibers, results in anterior‐to‐posterior activation of the left atrium and biphasic P waves in the same leads (lower panel, Type 2 morphology).
The Shape and Geometry of Atrial Chambers
In the mid‐1960s, when Morris et al. first described negative PTF in patients with mitral and aortic valve disease, 10 PTF was regarded as an ECG‐manifestation of left atrial enlargement. However, several years later this concept was challenged by observations made by Josephson et al., who suggested that biphasic P waves in the right precordial leads may represent an interatrial conduction defect, not necessarily linked to left atrial enlargement. 35 While abnormal PTF may indeed be a sensitive sign of left atrial dilatation, its specificity is low, as recently shown in studies that utilized conventional echocardiography 36 or cardiac magnetic resonance imaging (MRI). 37 In a more recent report, PTF was not associated with left atrial volume assessed with computed tomography. 38
The exact mechanism underlying the abnormal negative PTF in patients with left atrial dilatation is currently not fully clarified. Recently, we showed that atrial dilatation in patients with atrial fibrillation is associated with fibro‐fatty replacement of the atrial myocardium that equally affects the atrial walls and the major conduction routes. 1 In this setting, thickness of interatrial pathways may be directly linked to the pathways’ ability to maintain conduction despite myocyte loss. Posterior interatrial fibers that are likely to represent the substrate for left atrial activation breakthrough in the vicinity of fossa ovalis observed in a minority of patients with AF history are generally thinner and potentially more vulnerable than Bachmann's bundle. 33 , 39 It may therefore be suggested that in the majority of elderly patients or patients with structural atrial remodeling, the Bachmann's bundle remains functional when posterior connections become replaced by fibrotic tissue. The left atrial breakthrough corresponding to conduction over the Bachmann's bundle is located on the anterior and superior region of the atrial septum, thus leading to the backward propagation of the atrial depolarization wave across the left atrium 32 and the pronounced negative PTF or Type 2 P‐wave morphology. 34 More advanced fibrosis may interrupt conduction over the Bachmann's bundle and result in so‐called advanced interatrial block with retrograde left atrial activation via the inferior interatrial pathway near the coronary sinus ostium described by Bayes de Luna et al. 40
CLINICAL IMPLICATIONS OF P‐WAVE MORPHOLOGY ASSESSMENT
Understanding the Mechanisms Underlying AF
The association between electrocardiographic manifestation of advanced interatrial block with retrograde left atrial activation (biphasic P waves in inferior leads) and atrial tachyarrhythmias has been proposed by Bayes de Luna et al. in the mid‐1980s, 40 and was later confirmed by others. 4 , 41 The presence of biphasic P waves in the right precordial leads, on the other hand, is a much more common finding that was associated with the presence of paroxysmal atrial fibrillation in as early as 1967 by Robitaile et al. 42 In the orthogonal lead system, the same phenomenon is observed as biphasic P waves in lead Z (Type 2, Figs. 1 and 4, lower panel). Using a signal‐averaging approach and automated P‐wave morphology classification, we have shown that Type 2 P waves were significantly associated with the presence of paroxysmal atrial fibrillation in patients under 40 years of age without structural heart disease, 43 thus suggesting that alterations in interatrial conduction may well be the cause rather than the consequence of atrial fibrillation. This concept held true in the population of heart failure patients enrolled in the Multicenter Automatic Defibrillator Implantation Trial II (MADIT‐II). Using the same methodology of P‐wave assessment, we found significant differences in the distribution of P‐wave morphology classes between patients who developed new‐onset AF during follow‐up and those who did not. 8 Notably, P‐wave duration was not predictive of AF development in this cohort.
Abnormal PTF is suggested as an indicator of atrial structural remodeling shown to be predictive of AF relapse following electrical cardioversion. 44 The clinical value of P‐wave morphology in the context of catheter ablation for AF is less well established. Janin et al. reported loss of P‐wave terminal negative component in lead V1, as well as reduction of P‐wave duration following circumferential pulmonary vein isolation. 45 Although the prognostic value of these findings is yet to be determined, procedure‐related modification of P‐wave appearance may indeed be a marker of completeness of ablation lines that exclude a considerable amount of the atrial myocardium surrounding pulmonary vein ostia from excitation.
P‐wave Morphology as Predictor of Clinical Outcome
P‐wave morphology appears to bear a prognostic value for prediction of clinical outcome beyond the atrial fibrillation context.
In patients with acute myocardial infarction, Mehta et al. studied the association between abnormal negative PTF in V1 and angiographic, hemodynamic, and echocardiographic correlates, and found PTF to be highly suggestive of dyskinetic or aneurismal areas with significantly increased left ventricular end‐diastolic pressure, creatine kinase‐MB and decreased ejection fraction. 46 These pathophysiological associations may likely explain the difference in long‐term outcome associated with abnormal PTF in patients discharged after ST‐elevation myocardial infarction. 47 , 48
In the MADIT‐II cohort, atypical P‐wave morphology (i.e., P waves that did not belong to any of the two most common morphology classes (Type 1 or Type 2, see Fig. 3)) was independently associated with heart failure‐related death in a multivariate analysis. 49 The exact mechanism underlying this association is currently unknown, but it may be suggested that more advanced heart failure leading to left atrial overload and dilatation inevitably deteriorates atrial conductive properties and may cause abnormal atrial activation reflected with corresponding changes in P‐wave morphology. Although no data on left atrial size were systematically collected in MADIT II, the markedly reduced left ventricular ejection fraction in this patient cohort suggests that left atrial enlargement may have contributed to the abnormal P‐wave morphology observed in nearly one‐fourth of patients.
CONCLUSIONS
In conclusion, P‐wave morphology during sinus rhythm is the result of complex interplay of anatomical, electrophysiological, and geometrical factors whose exact impact on P‐wave appearance is often difficult to delineate. However, regardless of the exact mechanism underlying P‐wave morphology, the value of morphology analysis extends beyond the atrial arrhythmias context in which P‐wave morphology may be used as a marker of conduction defect associated with arrhythmia mechanisms. Recent studies suggest that P‐wave morphology should be considered as an ECG‐based predictor of clinical outcome in patients with common cardiovascular conditions such as congestive heart failure and ischemic heart disease.
Disclosure: None.
REFERENCES
- 1. Platonov PG, Mitrofanova LB, Orshanskaya V, et al Structural abnormalities in atrial walls are associated with presence and persistency of atrial fibrillation but not with age. J Am Coll Cardiol 2011;58:2225–2232. [DOI] [PubMed] [Google Scholar]
- 2. Stafford PJ, Turner I, Vincent R. Quantitative analysis of signal‐averaged P waves in idiopathic paroxysmal atrial fibrillation. Am J Cardiol 1991;68:751–755. [DOI] [PubMed] [Google Scholar]
- 3. Steinberg JS, Zelenkofske S, Wong SC, et al Value of the P‐wave signal‐averaged ECG for predicting atrial fibrillation after cardiac surgery. Circulation 1993;88:2618–2622. [DOI] [PubMed] [Google Scholar]
- 4. Yasushi A, Fukutami M, Yamada T, et al Prediction of transition to chronic atrial fibrillation in patients with paroxysmal atrial fibrillation by signal‐averaged electrocardiography. Prospective study. Circulation 1997;96:2612–2616. [DOI] [PubMed] [Google Scholar]
- 5. Magnani JW, Johnson VM, Sullivan LM, et al P wave duration and risk of longitudinal atrial fibrillation in persons ≥ 60 years old (from the Framingham Heart Study). Am J Cardiol 2011;107:917–921 e911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jurkko R, Vaananen H, Mantynen V, et al High‐resolution signal‐averaged analysis of atrial electromagnetic characteristics in patients with paroxysmal lone atrial fibrillation. Ann Noninvasive Electrocardiol 2008;13:378–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nemirovsky D, Hutter R, Gomes JA. The electrical substrate of vagal atrial fibrillation as assessed by the signal‐averaged electrocardiogram of the P wave. Pacing Clin Electrophysiol 2008;31:308–313. [DOI] [PubMed] [Google Scholar]
- 8. Holmqvist F, Platonov PG, Carlson J, et al Altered interatrial conduction detected in MADIT II patients bound to develop atrial fibrillation. Ann Noninvasive Electrocardiol 2009;14:268–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bonow RO, Mann DL, Zipes DP, et al Braunwald's Heart Disease. A Textbook of Cardiovascular Medicine. New York , NY , USA , W.B. Saunders Company, 2011. [Google Scholar]
- 10. Morris JJ Jr, Estes EH Jr, Whalen RE, et al P‐wave analysis in valvular heart disease. Circulation 1964;29:242–252. [DOI] [PubMed] [Google Scholar]
- 11. Forfang K, Erikssen J. Significance of P wave terminal force in presumably healthy middle‐aged men. Am Heart J 1978;96:739–743. [DOI] [PubMed] [Google Scholar]
- 12. Havmoller R, Carlson J, Holmqvist F, et al Age‐related changes in P wave morphology in healthy subjects. BMC Cardiovasc Disord 2007;7:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dilaveris P, Raftopoulos L, Giannopoulos G, et al Prevalence of interatrial block in healthy school‐aged children: Definition by p‐wave duration or morphological analysis. Ann Noninvasive Electrocardiol 2010;15:17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sprague HB, White PD. Clinical observations on the T wave of the auricle appearing in the human electrocardiogram. J Clin Invest 1925;1:389–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Debbas NM, Jackson SH, de Jonghe D, et al Human atrial repolarization: Effects of sinus rate, pacing and drugs on the surface electrocardiogram. J Am Coll Cardiol 1999;33:358–365. [DOI] [PubMed] [Google Scholar]
- 16. Holmqvist F, Carlson J, Platonov PG. Detailed ECG analysis of atrial repolarization in humans. Ann Noninvasive Electrocardiol 2009;14:13–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Holmqvist F, Carlson J, Waktare JE, Platonov PG. Noninvasive evidence of shortened atrial refractoriness during sinus rhythm in patients with paroxysmal atrial fibrillation. Pacing Clin Electrophysiol 2009;32:302–307. [DOI] [PubMed] [Google Scholar]
- 18. Sanchez‐Quintana D, Cabrera JA, Farre J, et al Sinus node revisited in the era of electroanatomical mapping and catheter ablation. Heart 2005;91:189–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lee RJ, Kalman JM, Fitzpatrick AP, et al Radiofrequency catheter modification of the sinus node for “inappropriate” sinus tachycardia. Circulation 1995;92:2919–2928. [DOI] [PubMed] [Google Scholar]
- 20. Boineau JP, Canavan TE, Schuessler RB, et al Demonstration of a widely distributed atrial pacemaker complex in the human heart. Circulation 1988;77:1221–1237. [DOI] [PubMed] [Google Scholar]
- 21. Schuessler RB, Boineau JP, Bromberg BI. Origin of the sinus impulse. J Cardiovasc Electrophysiol 1996;7:263–274. [DOI] [PubMed] [Google Scholar]
- 22. Monfredi O, Dobrzynski H, Mondal T, et al The anatomy and physiology of the sinoatrial node – a contemporary review. Pacing Clini Electrophysiol 2010;33:1392–1406. [DOI] [PubMed] [Google Scholar]
- 23. Brody DA, Woolsey MD, Arzbaecher RC. Application of computer techniques to the detection and analysis of spontaneous P‐wave variations. Circulation 1967;36:359–371. [DOI] [PubMed] [Google Scholar]
- 24. Meek WJ, Eyster JAE. Experiments on the origin and propagation of the impulse in the heart. IV. The effect of vagal stimulation and of cooling the location of the pacemaker within the sino‐auricular node. Am J Physiol 1914;34:368–383. [Google Scholar]
- 25. Jones SB, Euler DE, Hardie E, et al Comparison of SA nodal and subsidiary atrial pacemaker function and location in the dog. Am J Physiol 1978;234:H471–476. [DOI] [PubMed] [Google Scholar]
- 26. Boineau JP, Schuessler RB, Roeske WR, et al Quantitative relation between sites of atrial impulse origin and cycle length. Am J Physiol 1983;245:H781–789. [DOI] [PubMed] [Google Scholar]
- 27. Yokota M, Noda S, Koide M, et al Analysis of the exercise‐induced orthogonal P wave changes in normal subjects and patients with coronary artery disease. Jpn Heart J 1986;27:443–460. [DOI] [PubMed] [Google Scholar]
- 28. Bakos Z, Medvedev MM, Carlson J, et al Stability of P wave morphology and duration during atropine challenge. Europace 2011;13:P474. [Google Scholar]
- 29. Suenari K, Chang SL, Lin YJ, et al Unusual ECG pattern of right atrial appendage atrial tachycardia in one patient with right pneumonectomy. Pacing Clin Electrophysiol 2010;33:e46–48. [DOI] [PubMed] [Google Scholar]
- 30. Betts TR, Roberts PR, Morgan JM. High‐density mapping of left atrial endocardial activation during sinus rhythm and coronary sinus pacing in patients with paroxysmal atrial fibrillation. J Cardiovasc Electrophysiol 2004;15:1111–1117. [DOI] [PubMed] [Google Scholar]
- 31. Markides V, Schilling RJ, Ho SY, et al Characterization of left atrial activation in the intact human heart. Circulation 2003;107:733–739. [DOI] [PubMed] [Google Scholar]
- 32. Tapanainen JM, Jurkko R, Holmqvist F, et al Interatrial right‐to‐left conduction in patients with paroxysmal atrial fibrillation. J Interv Card Electrophysiol 2009;25:117–122. [DOI] [PubMed] [Google Scholar]
- 33. Platonov PG, Mitrofanova L, Ivanov V, et al Substrates for intra‐atrial and interatrial conduction in the atrial septum: Anatomical study on 84 human hearts. Heart Rhythm 2008;5:1189–1195. [DOI] [PubMed] [Google Scholar]
- 34. Holmqvist F, Husser D, Tapanainen JM, et al Interatrial conduction can be accurately determined using standard 12‐lead electrocardiography: Validation of p‐wave morphology using electroanatomic mapping in man. Heart Rhythm 2008;5:413–418. [DOI] [PubMed] [Google Scholar]
- 35. Josephson ME, Kastor JA, Morganroth J. Electrocardiographic left atrial enlargement. Electrophysiologic, echocardiographic and hemodynamic correlates. Am J Cardiol 1977;39:967–971. [DOI] [PubMed] [Google Scholar]
- 36. Birkbeck JP, Wilson DB, Hall MA, et al P‐wave morphology correlation with left atrial volumes assessed by 2‐dimensional echocardiography. J Electrocardiol 2006;39:225–229. [DOI] [PubMed] [Google Scholar]
- 37. Tsao CW, Josephson ME, Hauser TH, et al Accuracy of electrocardiographic criteria for atrial enlargement: Validation with cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2008;10:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Truong QA, Charipar EM, Ptaszek LM, et al Usefulness of electrocardiographic parameters as compared with computed tomography measures of left atrial volume enlargement: From the romicat trial. J Electrocardiol 2011;44:257–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Platonov PG, Mitrofanova LB, Chireikin LV, et al Morphology of inter‐atrial conduction routes in patients with atrial fibrillation. Europace 2002;4:183–192. [DOI] [PubMed] [Google Scholar]
- 40. Bayes de Luna A, Fort de Ribot R, Trilla E, et al Electrocardiographic and vectorcardiographic study of interatrial conduction disturbances with left atrial retrograde activation. J Electrocardiol 1985;18:1–13. [DOI] [PubMed] [Google Scholar]
- 41. Agarwal YK, Aronow WS, Levy JA, et al Association of interatrial block with development of atrial fibrillation. Am J Cardiol 2003;91:882. [DOI] [PubMed] [Google Scholar]
- 42. Robitaille GA, Phillips JH. An analysis of the P wave in patients with transient benign atrial fibrillation. Dis Chest 1967;52:806–812. [DOI] [PubMed] [Google Scholar]
- 43. Holmqvist F, Olesen MS, Tveit A, et al Abnormal atrial activation in young patients with lone atrial fibrillation. Europace 2011;13:188–192. [DOI] [PubMed] [Google Scholar]
- 44. Martin Garcia A, Jimenez‐Candil J, Hernandez J, et al P wave morphology and recurrence after cardioversion of lone atrial fibrillation. Rev Esp Cardiol 2012;65(3):289–290. [DOI] [PubMed] [Google Scholar]
- 45. Janin S, Wojcik M, Kuniss M, et al Pulmonary vein antrum isolation and terminal part of the P wave. Pacing Clin Electrophysiol 2010;33:784–789. [DOI] [PubMed] [Google Scholar]
- 46. Mehta A, Jain AC, Mehta MC, et al Left atrial abnormality in acute myocardial infarction. Am J Cardiol 1997;79:807–811. [DOI] [PubMed] [Google Scholar]
- 47. Kentala E, Sarna S. Sudden death and factors related to long‐term prognosis following acute myocardial infarction. Scand J Rehabil Med 1976;8:27–32. [PubMed] [Google Scholar]
- 48. Pohjola S, Siltanen P, Romo M. The prognostic value of the P wave morphology in the discharge ECG in a 5‐year follow‐up study after myocardial infarction. Am Heart J 1979;98:32–38. [DOI] [PubMed] [Google Scholar]
- 49. Holmqvist F, Platonov PG, McNitt S, et al Abnormal P‐wave morphology is a predictor of atrial fibrillation development and cardiac death in MADIT‐II patients. Ann Noninvasive Electrocardiol 2010;15:63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]


