Abstract
Objective: P wave dispersion (PD) is considered to reflect the heterogeneous conduction in atria. We investigated whether there was a correlation between the left ventricular (LV) relaxation and PD.
Method and Results: Fifty‐three hypertensive patients ≤60 years old were divided into two groups: Group A, 27 patients, aged 54 ± 5 years with the impaired LV relaxation and Group B, 26 patients, aged 51 ± 8 years with normal LV relaxation. The P wave durations were measured in all 12 leads of ECG and PD was defined as the difference between maximum and minimum P wave duration (Pmax−Pmin). Mitral inflow velocities (E and A), E deceleration time (DT), isovolumic relaxation time (IVRT), left atrial and ventricular diameters, and wall thickness of LV were obtained by echocardiography. Clinical characteristics of both groups were comparable. The wall thickness of LV, Pmax, and left atrial dimension were not different in both groups. A velocity was higher (P < 0.001), but E velocity (P = 0.03) and E/A ratio (P < 0.001) were lower in group A than in group B. IVRT and DT were also significantly longer in group A. PD was significantly higher in group A compared to group B (51 ± 9 vs 41 ± 11 ms, P = 0.01). This difference resulted from the Pmin (61 ± 10 vs 67 ± 9 ms, P = 0.03, respectively). Multivariate analysis revealed a significant correlation between PD and A velocity (r = 0.46, P = 0.01), E/A ratio (r =−0.53, P = 0.001), DT (r = 0.65, P < 0.001), and IVRT (r = 0.73, P < 0.001).
Conclusion: This study suggests that impaired LV relaxation contributes to the heterogeneous atrial conduction in hypertensive patients.
Keywords: P wave dispersion, diastolic function, hypertension
Hypertension is a frequently encountered pathology in cases after 30 years of age and results in formidable complications. It has been cited as the causative factor for paroxysmal atrial fibrillation (AF) in 7–10% of cases over the three decades in a series of 1212 patients with AF. 1 , 2 Intraventricular high pressure results in deterioration of both systolic and diastolic function of the left ventricle (LV). It can also affect the morphology, size, and function of the left atrium. 3 , 4 These changes in the left atrium (LA) contribute to the discontinuous anisotropic propagation of sinus impulses and heterogeneous atrial conduction. 5 Heterogeneous conduction is also a risk factor for atrial fibrillation (AF) in hypertensive patients. 6 , 7 , 8 It has been recently evaluated with a simple ECG index and the P wave dispersion (PD), which is defined as the difference between maximum and minimum P wave durations 9 and proposed as being useful for the prediction of paroxysmal AF. 10
Current evidence suggests that the risk of paroxysmal AF is increased in hypertensive patients with the prolongation of the maximum duration and PD. 6 A previous study demonstrated that atrial dilatation and depression of LA mechanical functions secondary to left ventricular dysfunction were related to the onset of AF in hypertensive patients. 11
The purpose of this study was to investigate whether there is a relationship between PD and the impaired LV relaxation detected by the Doppler technique in hypertensive patients with sinus rhythm.
METHODS
Patients
Fifty‐three consecutive hypertensive patients ≤60 years of age were included in the study. All patients were receiving angiotensin‐converting enzyme inhibitors, angiotensin II receptor blockers, or diuretics for hypertension. After echocardiographic examination, the patients were divided into two groups: Group A consisted of 27 patients (mean age = 54.5 ± 5.2 years) with impaired LV relaxation and group B included 26 patients (mean age = 51.1 ± 8.2 years) with normal LV relaxation indexes (Table 1).
Table 1.
Clinical and Echocardiographical Characteristics of the Hypertensive Patients with (Group A) and without (Group B) the Impaired Left Ventricular Relaxation
| Group A (n = 27) | Group B (n = 26) | P value | |
|---|---|---|---|
| Mean age (year) | 54.5 ± 5.2 | 51.1 ± 8.2 | 0.09 |
| Male/Female | 11/16 | 10/16 | 0.87 |
| Duration of hypertension (year) | 5.3 ± 4.7 | 4.5 ± 3.6 | 0.49 |
| Diabetes mellitus Drugs | 3 (11%) | 2 (7.6%) | 0.67 |
| ACE inhibitors | 23 (85.2%) | 22 (84.6%) | 0.89 |
| Others (ARB and diuretics) | 4 (14.8%) | 4 (15.4%) | 0.93 |
| IVS (mm) | 11.9 ± 1.4 | 11.6 ± 0.9 | 0.34 |
| PW (mm) | 11.2 ± 1.2 | 10.9 ± 0.9 | 0.24 |
| LA dimension (mm) | 36.2 ± 4.5 | 35.3 ± 3.1 | 0.41 |
| Atrial ejection fraction (%) | 44 ± 11 | 48 ± 9 | 0.15 |
ACE: angiotensin converting enzyme, ARB: angiotensin II reseptor blocker, IVS: interventricular septum, PW: posterior wall, LA: left atrium.
Exclusion criteria were as follows: persistent or permanent AF, bundle branch block, preexcitation syndrome, LV systolic dysfunction, being on antiarrhythmic drug therapy, and known structural heart diseases (valvular, congenital, or coronary heart disease, cardiomyopathy, pericarditis), except for hypertension. Most of the patients were receiving angiotensin‐converting enzyme inhibitors or its combined form with diuretics for hypertension. Diabetes mellitus, hyperlipidemia, and duration of hypertension were also recorded (Table 1).
Electrocardiographic (ECG) Analyses
A 12‐lead surface ECG at a paper speed of 50 mm/s and 0.2 mV/mm standardization, was obtained from all patients in the supine position. The P wave durations were calculated in all 12 leads of ECG and they were manually measured by the use of a magnifying glass and a scale graduated in milliseconds, and by a blinded investigator with no knowledge of the status of patients. 12 The onset of the P wave was defined as the point of the first visible upward deflection of the trace from the bottom of the baseline for the positive waves and as the point of the first downward deflection from the top of the baseline for negative waves. The return to the baseline of the bottom of the trace in positive waves and of the top of the trace in negative waves was considered to be the end of the P wave. 6 , 7 , 8 , 9 , 10 The maximum and minimum durations of the P wave (Pmax, Pmin) were measured in all of the 12‐leads. The difference between Pmax and Pmin was considered as PD (PD = Pmax−Pmin) in each of the ECGs. 6 , 7 , 8 , 9 , 10 The intraobserver reproducibility of the Pmax, Pmin, and PD was 4 ± 3 ms, 3 ± 2 ms and 1 ± 3 ms in 20 patients, respectively.
Echocardiographic Examination
Transthoracic echocardiography was performed in the left lateral decubitus position. The LA and LV dimensions, and the thickness of the ventricular septum and posterior wall were measured by the M‐mode technique according to the recommendations of the American Society of Echocardiography. 13 Maximum and minimum LA volumes were calculated from the apical four‐chamber view using the area‐length method. Atrial ejection fraction (AEF) also was calculated from the formula, AEF = (maximum LA volume−minimum LA volume)/maximum LA volume. 7 Pulsed Doppler recordings of the diastolic mitral inflow velocities of the E wave, A wave (m/sec), and the E/A ratio were obtained by placing the sample volume over the tips of the mitral valve leaflets in the apical four‐chamber view. The mitral E deceleration time (DT) also was measured. 14 Isovolumic relaxation time (IVRT) also was measured by pulsed Doppler echocardiography in the apical five‐chamber view as previously described. 14 Diastolic mitral flow velocities, DT, and IVRT were obtained on the basis of the mean of at least three consecutive cycles at the end of normal expiration. The impaired LV relaxation was defined as having an E/A ratio of <1, DT > 200 ms, and IVRT > 110 ms, all together. 14 Intraobserver variability was, respectively, 8 ± 6 ms for DT and 3 ± 2 ms for IVRT in 20 patients.
Statistical Analyses
Data were presented as the mean ± SD. Statistical analyses were performed using the Student t‐test for continuous variables and chi‐square test for categorical variables. The influence of various factors such as age, duration of hypertension, LA dimension, AEF, thickness of the ventricular septum and posterior wall on Pmax, Pmin, and PD measurements were investigated by multivariate analysis. A P value <0.05 was considered significant.
RESULTS
There were no significant differences in age, gender, duration of hypertension, LA dimension, AEF, septum, and posterior wall thickness of LV between groups A and B (Table 1).
Mitral E and A velocities were 0.8 ± 0.1 and 0.9 ± 0.2 m/s in group A; 0.9 ± 0.2 and 0.7 ± 0.1 ms in group B, respectively. Mitral A velocity was significantly higher (P < 0.001), while E velocity and E/A ratio was lower in group A (P = 0.03 and P < 0.001, respectively). The mean durations of DT and IVRT were significantly longer in group A than in group B (P < 0.001, Table 2).
Table 2.
P Wave Durations and Diastolic Doppler Indexes in Hypertensive Patients with (Group A) and without (Group B) the Impaired Left Ventricular Relaxation
| Variables | Group A (n = 27) | Group B (n = 26) | P value |
|---|---|---|---|
| E velocity (m/s) | 0.8 ± 0.1 | 0.9 ± 0.2 | 0.03 |
| A velocity (m/s) | 0.9 ± 0.2 | 0.7 ± 0.1 | <0.001* |
| E/A ratio | 0.8 ± 0.3 | 1.2 ± 0.2 | <0.001* |
| Deceleration time (ms) | 254.2 ± 20.3 | 168.7 ± 27.1 | <0.001* |
| Isovolumic relaxation time (ms) | 128.8 ± 5.2 | 99.1 ± 4.7 | <0.001* |
| Pmax (ms) | 116.6 ± 6.7 | 115.3 ± 5.2 | 0.43 |
| Pmin (ms) | 61.5 ± 10.9 | 67.2 ± 8.8 | 0.03* |
| P wave dispersion (ms) | 51.5 ± 9.4 | 41.2 ± 10.6 | 0.01 |
*:Correlated with P wave dispersion, P < 0.05 significant.
P wave dispersion was found to be significantly higher in group A than in group B (51.5 ± 9.4 vs 41.2 ± 10.6 ms, P = 0.01, Table 2). However, Pmin was shorter in group A than in group B (61.5 ± 10.3 vs 67.2 ± 8.8 ms, P = 0.03). A significant correlation was observed between Pmin and PD (r =−0.73, P < 0.001). The duration of Pmax in both groups was comparable (P = 0.43, Table 2) (Fig. 1).
Figure 1.
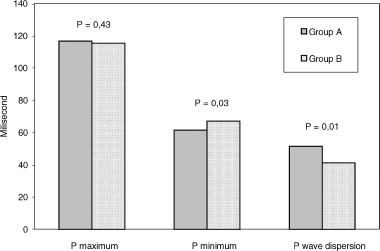
P wave measurements and P wave dispersion in hypertensive patients with impaired left ventricular relaxation (Group A) and with no impaired left ventricular relaxation (Group B).
By multivariate analysis, A velocity (r = 0.46, P = 0.01), E/A ratio (r =−0.53, P = 0.001, r = 0.65, P = 0.001), and IVRT (r = 0.73, P = 0.001) were detected to be significantly associated with PD.
DISCUSSION
To our knowledge, this is the first trial to investigate whether there is a relationship between PD and LV diastolic function in hypertensive patients' sinus rhythm. Hypertension leads to the impairment of LV relaxation and even the reduction in LV compliance has been reported as the earliest and most consistent abnormality in the repercussions of hypertension on the heart. 15 This impairment contributes toward the increase in dimension of LA and/or atrial hypertrophy. These morphological changes in LA can be responsible for various atrial arrhythmias. 3 , 7
In a recent study, 6 it was shown that hypertensive patients who had a history of AF, had a reduced mitral A velocity compared with those who remained in sinus rhythm. A wave velocity was a predictive factor for the onset of AF independent of age. This finding may be a reflection of an early deterioration in LA systolic function associated with hypertension. There was a significant correlation between the A velocity and LA dimension but not LV hypertrophy. In addition, no significant correlation was detected between PD and LA dimension, LV mass, A velocity, or E/A ratio. However, a slight correlation existed between Pmax and LA dimension, and between LV mass and E/A ratio. Furthermore, another study showed that A wave velocity was improved or normalized after surgical reduction of the LA dimension for long‐term AF. 16 In this study, however, we also found that hypertensive patients with impaired LV relaxation had an increased A velocity but reduced E velocity and E/A ratio compared to those with normal LV functions. AEF, ventricular septum, and posterior wall thickness were similar in both groups.
AF is one of the most common arrhythmias 1 and results from the deteriorated atrial conduction that will manifest as lengthening of the P wave duration recorded on the ECG. 5 , 6 , 7 , 8 , 9 , 10 P wave duration and PD have been shown to be useful for the detection of patients at risk of paroxysmal AF. 6 , 7 , 8 , 9 , 10 Dilaveris et al. 9 reported that PD was much longer in patients with paroxysmal AF than in controls (49 ± 15 vs 28 ± 7 ms, P < 0.05). Using a cutpoint of 40 ms for PD, they suggested that the patients with a history of AF could be separated from healthy controls with a sensitivity of 83% and a specificity of 85%. Weber et al. 17 also found a significant difference in PD between the patients who developed AF after coronary artery bypass surgery and those who did not (49 ± 12 vs 41 ± 12 ms, P < 0.05).
In hypertensive patients, Dilaveris et al. 8 showed that PD was significantly higher in patients with a history of AF than in those with sinus rhythm (52 ± 19 vs 41 ± 15 ms, P = 0.001). Pmean and Pmin were significantly lower in patients with a history of AF than in those without it (P < 0.001). Pmean, Pmin, and PD were found to be significant predictors of paroxysmal AF in univariate analysis, but only Pmin remained a predictor of AF in the multivariate analysis. They concluded that hypertensive patients at risk for AF could be detected by P wave analysis while in sinus rhythm.
In the current study, PD and Pmean were significantly longer and Pmin was shorter in patients with impaired LV relaxation than in those with normal diastolic function. In multivariate analysis, we found a significant correlation between PD and mitral inflow velocities, E/A ratio, DT, or IVRT. However, we did not detect a significant correlation between PD and LV wall thicknesses, LA dimension, AEF, or the duration of hypertension.
It has been postulated that there are two kinds of relations between AF and LA morphological structure and function. First, the increase in the left atrial dimension may depress the homogeneous conduction in atria, and contribute to the development AF. 18 , 19 Second, AF itself may damage the atrial function and result in atrial dilatation. 20 , 21 Barbier et al. 8 found that the hypertensive patients with paroxysmal AF had significantly larger LA compared to those with no history of AF. On the other hand, Tukek et al. 7 reported no significant difference in LA dimension between hypertensive patients with and without a history of AF. In our study, we found no significant difference in LA dimension and AEF between the patients with and without the delayed LV relaxation.
Study Limitations
There are some limitations of this study. First, there is no consensus about the cut‐off value of PD that differentiates the patients who have a history of AF from healthy subjects. Secondly, IVRT was measured by using the Doppler technique. So, interobserver and intraobserver variability in the measurements of IVRT and DT measured by this technique may be relatively high. In order to minimize this variability, these variables were measured by one investigator with no knowledge of the status of the patients. Thirdly, LV relaxation is affected by aging. Aging itself can also result in loss of myocardial fibers and an increase in fatty metamorphosis and connective tissue in the atria. 22 These changes may be the cause of the inhomogeneous electrophysiological properties of the atria rather than the delayed LV relaxation.
In conclusion, this study shows that there is a correlation between PD on surface ECG and LV relaxation indexes in hypertensive patients. The impaired LV relaxation may contribute to the heterogeneous atrial conduction in these patients. However, the underlying electrophysiological mechanism is unclear for this longer PD. It is likely that the increase of PD in hypertensive patients may represent a pathological process in atrium that creates an environment for AF in the future.
REFERENCES
- 1. Kaunnel WB, Wolf PA, Benjamin EJ, et al Prevalence, incidence, prognosis and predisposing condition for atrial fibrillation: Population‐based estimates. Am J Cardiol 1998;82: 2N–9N. [DOI] [PubMed] [Google Scholar]
- 2. Godtfredson J. Atrial fibrillation; etiology, course and prognosis: A follow‐up study of 1212 cases In Kulbertus HE, Olsson SB, Schlepper M. (eds.) Atrial fibrillation. AB Hässle , Mâlndal , Sweden , 1982, pp. 134–145. [Google Scholar]
- 3. Rizzo V, Maio FD, Campbell SV, et al Left ventricular function, cardiac dysrhythmias, atrial activation and volumes in nondipper hypertensive individuals with left ventricular hypertrophy. Am Heart J 2000;139(3):529–536. [DOI] [PubMed] [Google Scholar]
- 4. Matsuzaki M, Tamitani M, Toma Y, et al Mechanism of augmented left atrial pump function in myocardial infarction and essential hypertension evaluated by left atrial pressure‐dimension relation. Am Heart J 1991;67(13):1121–1126. [DOI] [PubMed] [Google Scholar]
- 5. Spach MS, Dolber PC. Relating extracelullar potentials and their derivatives to anisotropic propagation at a microscopic level in human cardiac muscle. Evidence for electrical uncoupling of side to side fiber connection with increased age. Circ Res 1986;58: 356–371. [DOI] [PubMed] [Google Scholar]
- 6. Ciaroni S, Cuenoud L, Bloch A. Clinical study to investigative the predictive parameters for the onset of atrial fibrillation in patients with essential hypertension. Am Heart J 2000;139: 814–819. [DOI] [PubMed] [Google Scholar]
- 7. Tukek T, Akkaya V, Atilgan D, et al Changes in P wave dispersion, left atrial size and function in hypertensive patients with paroxysmal atrial fibrillation. Arch Turkish Soc Cardiol 2000;28: 538–542. [Google Scholar]
- 8. Dilaveris PE, Gialafos EJ, Chrissos D, et al Detection of hypertensive patients at risk for paroxysmal atrial fibrillation during sinus rhythm by computer assisted P wave analysis. J Hypertension 1999;17(10):1463–1470. [DOI] [PubMed] [Google Scholar]
- 9. Dilaveris P, Gialofos EJ, Sideris SK, et al Simple electrocardiographic markers for the prediction of paroxysmal idiopathic atrial fibrillation. Am Heart J 1998;135(5):733–738. [DOI] [PubMed] [Google Scholar]
- 10. Aydemir K, Özer N, Atalar E, et al P wave dispersion on 12‐lead electrocardiography in patients with paroxysmal AF. Pacing Clin Electrophysiol 2000: 23(7); 1109–1112. [DOI] [PubMed] [Google Scholar]
- 11. Barbier P, Aliot G, Guazi M. Left atrial function and left ventricular filling in hypertensive patients with paroxysmal atrial fibrillation. J Am Coll Cardiol 1994;24: 165–170. [DOI] [PubMed] [Google Scholar]
- 12. Dilaveris P, Patchvarov V, Gialofos J, et al Comparison of different methods for manual P wave duration measurement in 12‐lead electrocardiograms. Pacing Clin Electrophysiol 1999;22: 1532–1538. [DOI] [PubMed] [Google Scholar]
- 13. Sahn DJ, De Maria A, Kisslo J, et al Recommendations regarding quantitation in M‐mode electrocardiographic measurements. Circulation 1978;58(6):1072–1083. [DOI] [PubMed] [Google Scholar]
- 14. Cohen GI, Pietrolungo JF, Thomas JD, et al A practical guide to assessment of ventricular diastolic function using Doppler echocardiography. J Am Coll Cardiol 1996;27: 1753–1760. [DOI] [PubMed] [Google Scholar]
- 15. Lutas EM, Devereux RB, Reis G, et al Increased cardiac performance in mild essential hypertension: Left ventricular mechanics. Hypertension 1985;7(6):979–988. [DOI] [PubMed] [Google Scholar]
- 16. Albirini A, Scalia GM, Murray RD, et al Left and right atrial transport function after the maze procedure for AF: An echocardiographic Doppler flow‐up study. J Am Soc Echocardiogr 1997;10(9):927–945. [DOI] [PubMed] [Google Scholar]
- 17. Weber UK, Osswald S, Huber M, et al Selective versus non‐selective antiarrhythmic approach for prevention of AF after coronary surgery: Is there a need for pre‐operative risk stratification. Eur Heart J 1998;19(5):794–800. [DOI] [PubMed] [Google Scholar]
- 18. Henry WL, Morganroth J, Pearlman AS, et al Relation between echocardiographically determined left atrial size and AF. Circulatiion 1976;53(2):273–279. [DOI] [PubMed] [Google Scholar]
- 19. Takahashi N, Imataka K, Seki A, et al Left atrial enlargement in patients with paroxysmal AF. Jpn Heart J 1982;23: 677–683. [DOI] [PubMed] [Google Scholar]
- 20. Peterson P, Kastrup J, Brinch K, et al Relation between left atrial dimension and duration of AF: Am J Cardiol 1987;60: 382–384. [DOI] [PubMed] [Google Scholar]
- 21. Sanfilippo AJ, Abascal VM, Sheehan M, et al Atrial enlargement as a consequence of AF. A prospective echocardiographic study. Circulation 1990: 82(3); 792–797. [DOI] [PubMed] [Google Scholar]
- 22. Prystowsky EN, Katz A. Atrial Fibrillation In Topol EJ, Califf R, Isher JM. (eds.): Textbook of Cardiovascular Medicine. Philadelphia/New York , Lipincott‐Raven Publishers, 1998, pp. 1661–1694. [Google Scholar]


