Abstract
The electrocardiogram (ECG) is the most widely used imaging tool helping in diagnosis and initial management of patients presenting with symptoms compatible with acute coronary syndrome. Acute ischemia affects the configuration of the QRS complexes, the ST segments and the T waves. The ECG should be read along with the clinical assessment of the patient. ST segment elevation (and ST depression in leads V1–V3) in patients with active symptoms usually indicates acute occlusion of an epicardial artery with ongoing transmural ischemia. These patients should be triaged for emergent reperfusion therapy per current guidelines. However, many patients have ST segment elevation secondary to nonischemic causes. ST depression in leads other than V1–V3 usually are indicative of subendocardial ischemia secondary to subocclusion of the epicardial artery, distal embolization to small arteries or spasm supply/demand mismatch. ST depression may also be secondary to nonischemic etiologies, such as left ventricular hypertrophy, cardiomyopathies, etc. Knowing the clinical scenario, comparison to previous ECG and subsequent ECGs (in cases that there are changes in the quality or severity of symptoms) may add in the diagnosis and interpretation in difficult cases. This review addresses the different ECG patterns, typically seen in patients with active symptoms, after resolution of symptoms and the significance of such changes when seen in asymptomatic patients.
Keywords: noninvasive techniques—electrocardiography, Acute coronary syndrome, ischemia, ST elevation, ST depression, myocardial infarction
The electrical activity of the heart, as depicted by the surface electrocardiogram (ECG) is affected by ischemia, reperfusion and presence of necrosis or scar. Therefore, the ECG has remained the most widely utilized ancillary tool, along with focused history taking and physical examination, for the initial assessment of patients presenting with symptoms compatible with acute coronary syndromes (ACS).1, 2, 3
Acute occlusion of an epicardial coronary artery, resulting in transmural ischemia, usually leads to typical ECG changes: initially, the T waves in the leads facing the ischemic zone become positive, symmetrical and usually tall. Subsequently, ST elevation develops. In some patients, changes in the initial (transient Q waves)4 or terminal part of the QRS complex increase in the R‐wave amplitude and disappearance of the S wave can also be detected along with prolongation of the QRS width.5 In some patients with acute ischemia, ST depression rather than elevation can be seen. This is considered to represent either reciprocal changes to transmural ischemia in leads facing away from the ischemic zone, or predominantly subendocardial ischemia in cases without transmural involvement. The exact pathophysiology of ST depression is unclear. Partial occlusion of an epicardial coronary artery, distal embolization into small subendocardial arteries, vasoconstriction downstream of the epicardial artery thrombus, increased left ventricular diastolic pressure, and/or other causes that produce mismatch between blood supply to the subendocardial zone and demand, have been advocated as causative mechanisms.
Following reperfusion in ST‐elevation ACS, ST‐segment deviation tends to diminish, and inversion of the T waves develops, often along with changes in the QRS complexes (narrowing of the QRS complex, decrease in the amplitude of the R wave/increase in the S‐wave amplitude and increase in the depth or disappearance of Q waves), as well as QT interval prolongation.6
ECG interpretation is more accurate if performed at bedside, in conjunction with clinical assessment, taking into consideration the quality, severity, and duration of symptoms. This is especially true for patients with non–ST‐elevation ACS (NSTE‐ACS) that based on measurements of cardiac troponins can further be qualified as NSTE myocardial infarction (NSTEMI) or unstable angina. In these patients, symptoms may be intermittent and ECG obtained after resolution of symptoms may not show any change or only reflects the “foot prints” of the previous ischemic episode (deep T‐wave inversion [if the preceding ischemia was severe] or nonspecific ST and T changes [in cases of preceding nontransmural ischemia]). Retrospective understanding of transient ECG changes during ACS is often difficult when changing symptoms are not reliably correlated with individual serial ECGs taken during evolving events. This has led to misinterpretation of ECG patterns in the past,particularly for NSTE‐ACS.
THE ECG IN PATIENTS WITH ONGOING TYPICAL SYMPTOMS
ST elevation (Fig.1): ST elevation in patients presenting with ongoing typical symptoms is highly predictive of an occlusion of an epicardial coronary artery, resulting in transmural (i.e., both endocardial and epicardial) ischemia and ST elevation myocardial infarction (STEMI).1, 2, 3 This is especially true for patients with relatively normal baseline ECG with narrow QRS complexes (Fig.1A). However, ST elevation secondary to nonischemic etiologies is very common and the interpreter should be familiar with common patterns of nonischemic ST elevation (early repolarization, normal pattern, left ventricular hypertrophy [LVH], Brugada syndrome, acute pericarditis, Takotsubo, etc.).7 On the other hand, it should be remembered that patients with baseline nonischemic ST elevation may present with superimposed STEMI. Comparison with previous ECG may help to make the diagnosis.3 However, certain patterns of nonischemic ST elevation are highly dynamic and may depend on the heart rate (ST elevation secondary to aneurysm, pericarditis or LVH typically increases, whereas the “early repolarization” pattern usually decreases as heart rate increases) or show spontaneous fluctuations (e.g., Brugada pattern).7 Also, changes in lead positioning, improper high‐pass filter setting, QRS width and axis may affect the magnitude of ST elevation.
Figure 1.
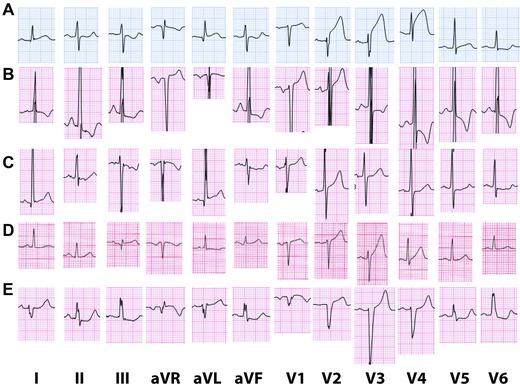
(A) Typical ECG of acute anterior STEMI. There is marked ST elevation in leads V2–V4 with mild ST elevation in leads I, aVL and V5 and reciprocal ST depression in leads III and aVF, which suggests proximal occlusion of a short left anterior descending (LAD) artery. Interestingly, the ST segments in leads V2–V3 have an upward concave pattern. (B) Typical pattern of ST elevation secondary to LVH. Criteria for biatrial enlargement are fulfilled. The QRS amplitude is high and there is QRS widening. There is ST elevation in V1–V2 (concave pattern) with ST depression and T wave inversion in leads I, II, III, aVL, V4–V6. (C) Atypical form of ST elevation secondary to LVH. The QRS amplitude is high. There is ST elevation in leads I, aVL with mild ST elevation in V1–V2. There is ST depression in the inferior leads and leads V4–V6. (D) Presenting ECG of a 64‐year‐old woman with anterior STEMI. Emergent coronary angiography demonstrated total occlusion of the LAD with collateral circulation. There is less than 0.1 mV ST elevation in leads III and V1 and less than 0.1 mV ST elevation in V2 to V4. T waves are relatively tall in V3–V4. Symptoms resolved after pPCI and repeat ECG showed resolution of ST elevation and decrease in the T wave amplitude. (E) ST elevation secondary to nonspecific intraventricular conduction delay (IVCD). The QRS complex is wide with right axis deviation. There are QS waves in leads V1–V2. There is ST elevation in leads aVL, V1–V4 and ST depression in the inferior leads and V5–V6.
Based on epidemiologic data from Scotland,3, 8 the American College of Cardiology (ACC)/ American Heart Association (AHA) and European Society of Cardiology (ESC) STEMI guidelines have recommended absolute cutoffs for the amplitude of ST elevation at the J point (in leads V2–V3: 0.2 mV in men and 0.15 mV in women, 0.1 mV in all other leads in patients without left bundle branch block or LVH).1, 2 Interestingly, both guidelines quote the Third Universal Definition of Myocardial Infarction document.3 However, the definitions in this document are slightly different for leads V2–V3: ≥0.2 mV in men 40 years or older, ≥0.25 mV in men younger than 40 years and ≥0.15 mV in women. Moreover, the restriction to patients without left bundle branch block or LVH was omitted in this document.3 Figures 1B and C are examples of ST elevation secondary to LVH. Additionally, it is unclear whether these cutoffs apply for people from other ethnicities. For example, Macfarlane et al. described a higher magnitude of “normal” ST elevation in Nigerian healthy men.9 These criteria have been adopted as rigid rules by many “quality assurance” programs. However, it should be emphasized that the clinical management should focus on treatment of transmural myocardial ischemia (that is manifested by ST elevation) and not the ST‐segment elevation per se. Many patients presenting with ST elevation above this threshold do not have acute transmural ischemia. On the other hand, at times, patients with true transmural ischemia secondary to an acute occlusion of an epicardial artery, present with ST elevation less than the recommended threshold (Fig.1D). There are several possible reasons for such a presentation: presence of collateral circulation; ischemia in remote zones that tends to diminish the injury vector in leads facing the main ischemic zone; pulmonary disease, effusion, or anasarca that decrease the amplitude of the QRST complexes, etc. Thus, not every patient with positive cardiac markers and ST elevation above the threshold has an acute occlusion of an epicardial artery, mandating emergent reperfusion (true STEMI). On the other hand, not every patient with positive cardiac markers and with ST elevation lower than the threshold should be defined as having NSTEMI, as many of them have acute occlusions of an epicardial artery. It is plausible that adjusting the magnitude of ST deviation to the total QRS amplitude could be more predictive than the current recommendation for absolute ST elevation amplitude.
While in the 2004 ACC/AHA STEMI guidelines it was stated that concave upwards pattern of ST elevation predicts nonischemic causes,10 it became apparent that this is not accurate (Fig. 1A). Athletes may have convex upwards ST elevation, especially in lead V2.11, 12 However, usually the T wave in lead V2 is negative; thus, the pattern may be mistaken for a recent STEMI, rather than acute STEMI. On the other hand, Smith et al. reported that 43% of patients (16/37) who underwent emergent primary percutaneous coronary intervention (PCI) of the left anterior descending (LAD) coronary artery had concave morphology of ST elevation in the anterior leads, concluding that this feature was unable to exclude anterior STEMI.13 In a recent article, there was large overlap of concave and nonconcave ST elevations in patients with true and false STEMI.14 Concave ST elevation was found in 24.1% of the patients with STEMI versus 51.6% in patients with nonischemic ST elevation.14 Indeed, this recommendation was omitted from the most recent guidelines.1, 2, 3
Things can become even more complex when dealing with patients presenting with atypical symptoms. Diabetics, the elderly and women with STEMI often present with symptoms other than typical chest pain.3 Pulmonary edema, shortness of breath, hypotension, back and epigastric pain, syncope, or even cardiac arrest can all be the presenting symptoms of acute myocardial infarction.3 Yet, they may be also caused by other etiologies. Activation of the catheterization laboratory, or more so, administration of thrombolytic therapy could be potentially harmful in such cases. This is especially true for patients with cardiomyopathy presenting with heart failure exacerbation, as many have baseline ST deviation secondary to bundle branch block, nonspecific intraventricular conduction delay (IVCD), preexisting aneurysm, hypertrophy, etc.7 As abovementioned, comparison with previous ECG and follow up of dynamic changes may help. However, it should be remembered that significant fluctuation in the magnitude of ST elevation can be seen that are secondary to changes in the QRS width, axis, and heart rate. Moreover, edema and effusion tend to diminish the absolute amplitude of the QRS and ST segment.15 Wide variations in lead positioning further explain why relying on comparison with previous ECGs could be at times misleading.
Currently, the ACC/AHA and ESC STEMI guidelines do not give any threshold for ST elevation for leads V1–V3 in patients with LVH 1, 2 (Fig. 1B and C), whereas the Third Universal definition of Myocardial Infarction Document does not address this issue.3 Currently, there are no recommended thresholds for ST elevation in patients with nonspecific IVCD (Fig.1E).
In addition to merely diagnosing STEMI, it is believed that the ECG can give more information concerning the extent and severity of ischemia and hence, prognosis of the patients.
It is commonly accepted that the absolute amplitude of ST segment deviation and/or extent of ST deviation (as reflected in the number of leads with threshold ST elevation) correlate with the size and/or severity of the ischemic insult. It has been suggested that the magnitude of ST elevation in the inferior leads in inferior STEMI and the number of leads with ST elevation in the precordial leads in anterior STEMI (Aldrich score) correlate with the predischarge ECG Selvester QRS score (a measure of infarct size).16 However, in patients undergoing reperfusion therapy the correlation between the Aldrich score and the size of the ischemic area at risk (assessed by pretreatment technetium Tc 99 m sestamibi scan or cardiac magnetic resonance imaging) was found to be weak.17, 18 Due to concomitant ischemia of segments that are opposite to each other, occasionally there is cancellation of the ischemic vector and attenuation of ST segment deviation in some segments. It is commonly believed that “anteroseptal” STEMI pattern (ST elevation limited to V1–V3) represents a smaller infarct that “extensive anterior” STEMI pattern (ST elevation extension to V4–V6) due to an occlusion of a short LAD coronary artery. However, often a proximal occlusion of a long wrapping LAD can result in limited “anteroseptal” STEMI pattern.19 The ischemic vector generated from the basal anterior segments and the more inferior and apical segments cancel each other and the ECG presentation is indistinguishable from that of limited small infarction due to an occlusion of a short LAD.19 An occlusion of the LAD before the first diagonal branch causes ST elevation in I and aVL with reciprocal ST depression in the inferior leads, if the LAD is short. However, in cases with proximal occlusion of a wrapping long LAD, the ischemic vectors of the inferior segments and lateral segments tend to attenuate ST deviation in the limb leads.20 On the other hand, small apical infarction due to a distal occlusion of a wrapping LAD often manifests as ST elevation in both the inferior and precordial leads.20
Other possible explanations for attenuation of ST elevation is preconditioning by either ischemia or pharmacological agents 21 or gradual reduction in the magnitude of ST elevation over time, even without reperfusion.22
It has been suggested that positive tall symmetric T waves in the leads with ST elevation signify a more “acute” phase of the process with a greater potential of salvage with reperfusion. This sign has been incorporated into a theoretical Anderson‐Wilkins acuteness score that includes also the presence of Q waves in the leads with ST elevation.23 However, the independent significance of the T‐wave amplitude apart from the presence of Q waves has not been tested. A recent study has shown that the configuration of the T waves (tall vs. normal) do not predict myocardial salvage assessed by cardiac magnetic resonance in patients undergoing primary percutaneous coronary intervention (pPCI).24
Changes in the terminal portion of the QRS in leads with ST elevation (Sclarovsky‐Birnbaum grade 3 ischemia) predict poorer outcome, larger infarct size, and less myocardial salvage, especially in patients presenting relatively late (>2–3 hours after onset of symptoms).24, 25 However, all the data have been obtained in retrospective analyses of studies. Prospective studies assessing the role of incorporating the Sclarovsky‐Birnbaum score in decisions concerning transfer for pPCI versus thrombolytic therapy in patients presenting to sites without 24/7 catheterization laboratory availability are lacking.
Presence of abnormal Q waves in leads with ST elevation may signify a more advanced stage of infarction with less potential for myocardial salvage by reperfusion therapy24, 26 and poorer prognosis,27 irrespective if they present necrosis or local conduction delay.
-
(2)
ST depression: Several patterns of ST depression can be seen: horizontal, down‐sloping or up‐sloping and accompanied by tall positive, biphasic or negative T waves. Distinct patterns at different lead groups signify distinctunderlying pathologies, if recorded in patients with ongoing symptoms.
ST depression mainly in leads V1–V4. The ST segment in these leads is usually isoelectric or slightly elevated. In patients without right bundle branch block (RBBB) ST depression in leads V1–V4 should be regarded as the “mirror image” of ST elevation and a sign of STEMI equivalence.1, 2, 3 As none of the standard leads faces the inferolateral region, transmural ischemia of this zone can be detected as reciprocal ST depression in the opposing segments (V1–V4; Fig. 2A). Quoting the study by Boden et al., it is frequently stated that inferolateral (formerly called posterior) STEMI equivalent should be suspected especially in cases when ST depression is accompanied by positive tall T waves in V1–V2.28 The patients included in the study were part of the Diltiazem Reinfarction Study. This study was conducted before the acute reperfusion era and probably patients presented relatively late. A later manuscript by the same group confirmed that they addressed patients at a relatively advanced stage of their infarction (concomitant T‐wave inversion without ST elevation in the inferior leads).29 Their observation is challenged by the fact that the mirror image of the acute phase of STEMI (ST elevation with positive T wave) is expected to be ST depression with negative T waves (Fig. 2A).30 Only in the more advanced stage of infarction or after reperfusion do the T waves evolve to become positive (a mirror image of ST elevation with negative T waves in leads facing the ischemic zone (Fig. 2B).30 A frequent, intermediate finding is a biphasic T wave (initial part is negative and terminal part is positive). Thus, the diagnosis should not be limited to patients with ST depression and positive T waves. The ESC STEMI guidelines still restrict this pattern to ST depression confined to leads V1–V3 2; whereas the most recent ACC/AHA guidelines and the Third Universal Definition of Myocardial Infarction document have eliminated the restriction that ST depression should be confined to leads V1–V3.1, 3 However, it is still unclear whether we can use ST depression in leads V1–V4 to diagnose “inferolateral” STEMI equivalent in patients with complete or incomplete right bundle branch block (RBBB; Fig. 2C), as RBBB is frequently associated with ST depression in V1–V4.31, 32 Of note, in patients with isolated RBBB, it is commonly believed that the T waves are usually not symmetrical as seen in patients with inferolateral ischemia. Things are more complicated as RBBB is considered a high‐risk marker in patients with ACS.33, 34, 35
ST depression in leads V4–V6. Horizontal or downsloping ST depression confined to these leads, along with inversion of the T waves (occasionally the terminal part of the T wave is slightly positive) is considered a sign of subendocardial ischemia and is an independent predictor of one‐year mortality in patients with NSTEMI (Fig. 2C and D).36, 37, 38, 39 However, such pattern of ST depression may also be seen in patients with LVH, cardiomyopathy etc. Per current guidelines, this ECG pattern is not an indication for acute reperfusion therapy.1, 2
Diffuse ST depression with negative T waves associated with ST elevation in aVR. Diffuse ST depression with T‐wave inversion in ≥6 of the inferior and anterolateral leads with ST elevation in aVR can be a manifestation of diffuse circumferential subendocardial ischemia in patients with significant left main, left main equivalent or three vessel coronary artery disease (Fig. 3B).40 In fact ST elevation in aVR is the exact mirror‐image pattern of ST depression in I + II, that is half‐way between limb leads I and II, equivalent to a frontal plane axis of +30 degrees, where –aVR is calculated as (I + II)/2. Again, this pattern can also be seen in patients with many other conditions, including cardiomyopathy and LVH with secondary repolarization changes (Fig. 3D).41 It has been reported that in acute ischemia due to left main trunk subocclusion, ST elevation in lead aVR is greater than in lead V1, as ischemia of the inferolateral segments due to left circumflex involvement attenuates ST elevation in V1 that is secondary to proximal LAD involvement.42, 43 In patients with typical chest pain, and especially if ST changes normalize after resolution of symptoms, this pattern very likely indicates diffuse circumferential subendocardial ischemia (Fig. 3A–C).40, 44, 45 Current ACC/ AHA and ESC STEMI guidelines accept this pattern as a predictor of left main coronary artery subocclusion or multivessel disease, particularly if the patient presents with hemodynamic compromise and otherwise unremarkable ECG.1, 2 Of note, the criteria for an “otherwise unremarkable” ECG are not defined in the guidelines. The ESC guidelines do not give a clear recommendation as to whether these patients should be treated as STEMI equivalent and undergo immediate reperfusion therapy similar to patients presenting with STEMI, 2 whereas the ACC/AHA guidelines recommend reperfusion therapy (even with thrombolytic therapy) for patients with ST depression (do not specify number of leads and location) if there is ST elevation in aVR.1 ST elevation in lead aVR in patients with first NSTEMI has been associated with higher in hospital mortality and higher prevalence of left main or three‐vessel disease, despite not having higher levels of creatine‐kinase MB levels.46 Lead aVR point ∼180 degrees to the midpoints of leads I and II. As lead aVR and leads I, II, and V5–V6 point to opposite direction, ST depression in these leads is almost always accompanied by ST elevation in aVR. We believe that further data are needed before recommending thrombolytic therapy and/or pPCI to all patients with ST elevation in lead aVR. We believe that emergent coronary angiography should be considered in patients with diffuse ST depression and ST elevation in aVR, especially if seen in a patient with ongoing symptoms or hemodynamic compromise and the ECG before the event was relatively normal.32, 40
Upsloping ST depression with tall positive T waves in the anterolateral leads. Traditionally, upsloping ST depression was not considered as ECG sign of ischemia, as it is commonly seen with increased heart rates (especially during stress tests). The current Third Universal Definition of Myocardial Infarction document does not acknowledge that this pattern indicates ischemia.3 However, if seen in patients with typical symptoms and at a relatively slow heart rate, regional ischemia due to subtotal occlusion of the LAD or left circumflex coronary artery should be suspected.47, 48 Patients presenting with such an ECG pattern often progress to typical STEMI (Fig. 4). We believe that this pattern, if seen in a patient with suggestive ongoing symptoms and without tachycardia, should be considered as an indication for urgent reperfusion therapy by pPCI. The role of thrombolytic therapy in such patients has not been established, especially as clear distinction between normal tachycardia induced upsloping ST depression and true subendocardial ischemia cannot be made in many patients.
ST depression in leads I and aVL. At times, patients with inferior STEMI initially present with nondiagnostic (ie, subthreshold) ST elevation in the inferior leads, especially in patients with low‐voltage QRS complexes in these leads. At times, reciprocal ST depression in I and especially aVL may be easier to detect and should raise the suspicion of inferior STEMI.49 Repeat ECG after 10–15 minutes, as well as whenever there is a change in clinical conditions, may reveal typical ST elevation in the inferior leads in many of these patients.
-
(3)
Tall T waves without significant ST changes: As noted previously, the first ECG change seen after an occlusion of a coronary artery is that the T waves become tall and symmetrical (Fig. 5A).5 Patients may present with tall positive T waves without ST deviation or with minimal ST deviation.50 It has been suggested that persistent positive T waves without ST elevation can be caused by an occlusion of an artery with preexisting severe narrowing and well developed collateral circulation.50 Some of these patients will develop abnormal Q waves without significant ST elevation (Fig. 5B), but the majority will progress to STEMI followed by Q‐wave infarction. As the normal range of T‐wave amplitude is wide, some of these cases are diagnosed only after ST elevation developed or occasionally, after T‐wave inversion and Q waves evolve. One important differential diagnosis is hyperkalemia. Current ACC/AHA and ESC STEMI guidelines recognize hyperacute tall T waves without ST deviation as a sign of acute coronary occlusion, but do not give firm recommendation concerning emergent reperfusion therapy.1, 2 Echocardiogram for detection of regional wall motion abnormalities is recommended and if symptoms continue, immediate coronary angiography should be performed.1
T‐wave inversion. T‐wave inversion without concomitant ST depression is typically not a sign of ongoing acute ischemia (Fig. 5C). It is usually seen after reperfusion (see below).51 In a patient with ongoing symptoms, findings of isolated T‐wave inversion should probably not be interpreted as a sign of ischemia, except when there is a recently recorded ECG indicating that the depth of the T wave has decreased; before the development of “pseudonormalization” of the T waves, there may a stage with diminution of the T‐wave inversion due to myocardial ischemia. Thus, we strongly disagree with the statement of the Third Universal Definition of Myocardial Infarction document 3 that “T‐wave inversion of ≥0.1 mV in two contiguous leads with prominent R waves or R/S ration >1” is an ECG sign of acute myocardial ischemia. The pattern of T‐wave inversion without ST depression may commonly represent recent and reperfused, rather than ongoing acute ischemia. This is a situation in which absence of carefully correlated clinical information can be confounding during the interpretation of serial ECGs over the course of ACS.
-
(4)
No ST–T changes: Normal ECG or lack of changes compared to baseline ECG without symptoms in a patient with ongoing symptoms usually points against ischemia as the etiology of the symptoms. Yet, theoretically, several conditions may lead to diminution of ECG changes despite ongoing ischemia: (1) Transition from subendocardial ischemia (ST depression) to transmural ischemia (ST elevation) should have a phase of isoelectric ST.52 We are not aware of such a description in patients, although it has been detected in an animal model.53 Nevertheless, repeat ECGs several minutes later should probably show dynamic changes. (2) Ischemia of two remote segments that oppose each other could lead to mutual cancelation of the “injury vector.” This might explain lack of significant ECG changes in many patients with left circumflex occlusion. For example, ischemia of the lateral segments (supplied by the obtuse marginal branches) should cause ST elevation in leads I and aVL, whereas ischemia of the inferior segments should lead to ST elevation in the inferior leads. Theoretically, ischemia of both zones could attenuate ST deviation, as leads I and aVL face the opposite direction of leads III and aVF. Nevertheless, subtle changes could be detected in other standard or additional (V3R, V4R, V7–V9) leads. (3) Ischemia that results in tall T wave without ST deviation.50 As there are wide variations in the absolute amplitude of the T waves, relative increase in the T waves that could be detected by continuous ECG recording that begins before ischemia, cannot be appreciated by a “snapshot” ECG. (4) Small myocardial segments that are not covered by any of the 12 standard ECG leads, and (5) preexisting ECG changes that “hide” possible ischemic changes, such as “pseudonormalization” of the T waves in patients with baseline negative T waves. In cases where there is still clinical suspicion of active ischemia despite equivocal ECG changes, it is recommended to use additional leads, repeat the ECG several minutes apart and order an echocardiogram for detection of regional wall motion abnormalities. Many patients have minor ST deviation than do not fulfill the current threshold; yet, many of them have total occlusion of the culprit coronary artery with good collateral circulation.54 The ACC/AHA and ESC guidelines recommend emergent coronary angiography despite lack of qualifying ECG changes if doubt still exists regarding the possibility of acute myocardial infarction.1, 2
Figure 2.
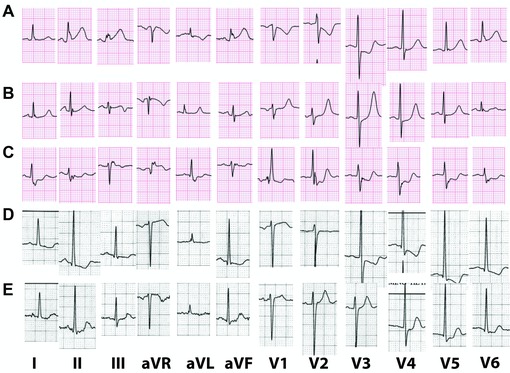
(A) Presenting ECG of a 70‐year‐old patient with STEMI equivalent due to an occlusion of the left circumflex artery. There is mild ST elevation in the inferior leads. There is ST depression with negative T wave in leads V1–V3. (B) Three hours after pPCI, the ECG shows resolution of ST elevation and inversion of the terminal portion of the T wave in lead III. There is less ST depression in V1–V3 and the T waves are now tall (mirror image of T wave inversion in leads facing the infarction). (C) Presenting ECG of a patient with acute myocardial infarction due to an occlusion of a venous graft to an obtuse marginal branch of the left circumflex. Baseline ECG shows RBBB. There is mild ST elevation in lead III and ST depression in II, V1–V6. The terminal portion of the T wave is positive. (D) An ECG of a 56‐year‐old hypertensive woman with severe chest pain. Voltage criteria for LVH are fulfilled. There is ST depression and T‐wave inversion in leads I, II, aVF, V3–V6. This pattern could represent repolarization changes secondary to LVH. However, comparison to a recent recording (E) confirms that there is acute ischemia. (E) Baseline ECG of the patient in (D) There are voltage criteria for LVH. There is mild ST depression with positive T wave in leads I, II, aVF, V4–V6 compatible with changes secondary to LVH.
Figure 3.
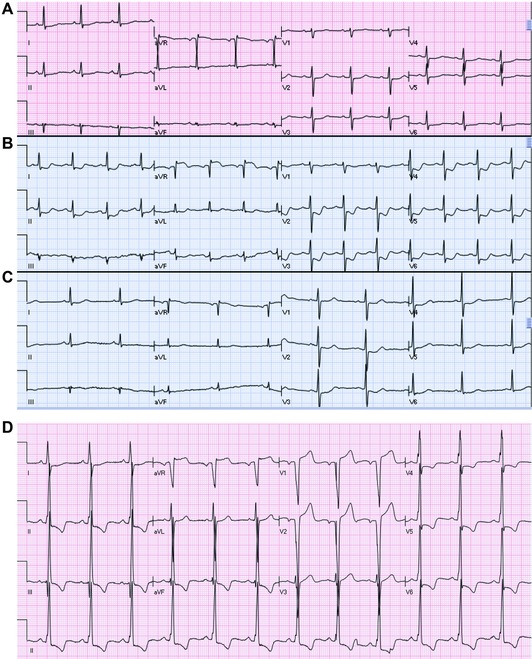
(A–C) Serial ECGs of a patient, before, during and after resolution of severe chest pain associated with shortness of breath and hypotension. The ECG before (A) and after (C) ischemia are relatively normal, showing minimal ST depression and nonspecific T changes. However, during ischemia (B), there is ST depression in leads I, II, aVL, aVF, V2–V6 with marked ST elevation in aVR. Coronary angiography revealed critical left main coronary artery stenosis. (D) An ECG of a patient with nonischemic dilated cardiomyopathy. The patient was in baseline clinical condition when the ECG was recorded. There is QRS widening and voltage criteria for LVH. There is ST elevation in leads aVR, V1–V2 with marked ST depression in leads II, III, aVF, V4–V6. This pattern is not due to acute circumferential subendocardial ischemia/left main subocclusion.
Figure 4.
(A) ECG of a 61‐year‐old female with severe chest pain. There is sinus tachycardia with ST elevation in aVR, ST depression in the inferior leads and mild upsloping ST depression in leads V3–V4 and tall T waves in V1–V3. Coronary angiography showed three vessel disease with total occlusion of the LAD. Left ventricular ejection fraction 20%. Patient underwent CABG. (B) Few days later, ECG shows QS wave in V2 with mild ST elevation in V2–V5, compatible with recent anterior STEMI. (C) An ECG of a patient with acute myocardial infarction. There is upsloping ST depression with positive T waves in leads I, II, III, aVF, V2–V6. There is ST elevation in leads aVR and V1.
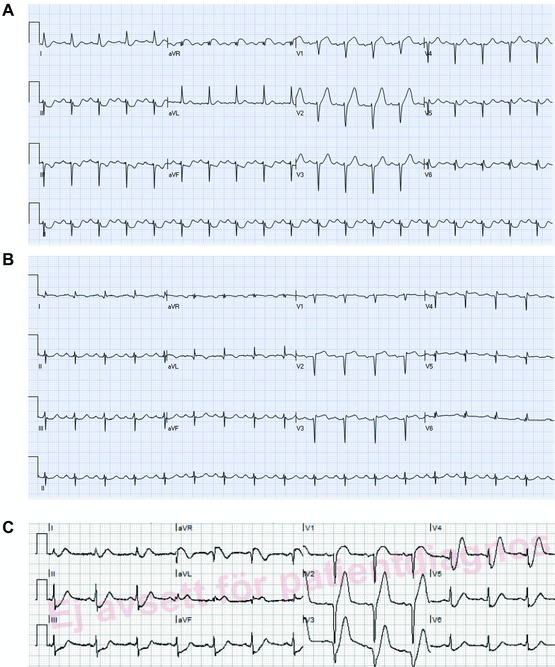
Figure 5.
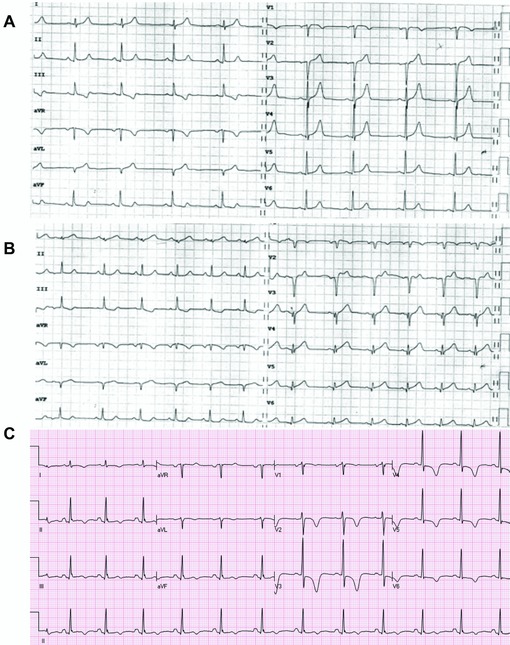
(A) Presenting ECG of a patient with chest pain. There are tall symmetric T waves in V2–V4 without significant ST elevation. (B) Predischarge ECG of the same patient. There are now Q waves in leads V2–V3 and mild ST elevation in lead V2. T wave amplitude has decreased in the precordial leads. Coronary angiography showed subocclusion of the proximal part of the LAD. (C) An ECG of a patient after resolution of symptoms. There are symmetric deep negative T waves in the precordial leads without significant ST deviation (“Wellens” sign).
THE ECG IN PATIENTS AFTER RESOLUTION OF SYMPTOMS
In patients in whom the severity of symptoms ameliorated or completely resolved, the ECG is not expected to show changes compatible with acute ischemia. Even though, changes related to “footprints” of the preceding ischemia (minor ST deviation, terminal or complete T‐wave inversion) or signs of evolved myocardial infarction (ST elevation with terminal T‐wave inversion, Q waves) can be found. This is also true for patients with critical left main or three vessel disease, in whom if the ECG is recorded when symptoms subside, changes may not be detected at all (Fig. 3A and C).40, 44 This issue is especially relevant for patients with NSTE‐ACS. Many of these patients present after symptoms have resolved and the presenting ECG is therefore, relatively unremarkable.
ST elevation: In the absence of symptoms, ST elevation may be secondary to nonischemic causes or reflects residual changes of the previous ischemia, including aneurysm.7 In many patients, if ECG is recorded earlier, while the patient is having symptoms, (partial) ST resolution can be appreciated. Yet, the current ACC/AHA and the ESC STEMI guidelines do not address the issue of reperfused STEMI.1, 2 According to the current guidelines, if ST elevation above the threshold is noted and symptoms onset is within the preceding 12 hours, patients should referred for emergency reperfusion therapy, irrespective if symptoms are ongoing or resolved.1, 2 On the other hand, if the onset of symptoms was >12 hours before presentation and the symptoms resolved, emergency reperfusion therapy is no longer indicated by the ACC/AHA guidelines despite the presence of persistent ST elevation,1 whereas such a restriction is not mentioned in the ESC guidelines (Class IIb indication).2 Yet, the benefits of emergent reperfusion therapy in patients with spontaneous reperfusion and the ability of the ECG to detect reperfusion upon presentation have not been demonstrated. A recent study suggested that T‐wave inversion in the leads with ST elevation in the initial ECG of patients with anterior STEMI, referred for pPCI, predicts reperfusion (TIMI flow grade 2–3) before intervention.55 Currently, neither the ACC/AHA nor the ESC guidelines address the issue of T‐wave configuration in the leads with ST elevation.1, 2 Persistent ST elevation 3 months or more after STEMI is traditionally considered to be a sign of aneurysm.
ST depression: In the absence of symptoms, ST depression may reflect residual effects of the preceding ischemia. However, in many cases it is caused by chronic preexisting conditions: LVH with secondary repolarization changes, cardiomyopathy, etc. (Fig. 3D).41 Often, several different causes can contribute to ST depression in the same patient resulting in a mixed pattern. Comparison to previous ECG tracings may help. However, as previously noted, factors other than ischemia may affect the magnitude and extent of ST depression (heart rate, QRS width, QRS axis, medications, lead positioning, filter setting, etc.).
Isolated T‐wave inversion: In the ACS setting, T‐wave inversion without concomitant ST depression is a sign of reperfusion of prior active ischemia, not of ongoing active ischemia itself.37, 40, 56, 57 This is to be distinguished from the isolated chronic localized T waves that may persist after resolution of an acute event. The extent and magnitude of T‐wave inversion may assist in the estimation of the extent and severity of the preceding acute ischemic insult. An example of such pattern is the “Wellens” sign (Fig. 5C). Deep T‐wave inversion in the precordial leads with insignificant ST deviation after resolution of symptoms is indicative of a tight proximal LAD lesion.51 Without aggressive treatment, (re)occlusion may occur with transformation to acute STEMI. Thus, this pattern should be considered as a marker of a high risk, though the patient does not have active ongoing ischemia.40, 57 In other cases, T‐wave inversion may be less prominent or biphasic T waves with inversion of the terminal portion of the T waves can be seen as a sign of reperfusion. Although there is no indication for emergent reperfusion therapy to prevent ongoing necrosis, this ECG evidence of reperfusion points toward the presence of a culprit coronary lesion that may progress and lead to reischemia/ reinfarction. It is plausible that the degree and depth of T‐wave inversion reflect the severity and extent of the previous ischemia; thus, deep negative T waves could be a sign of more extensive preceding transmural ischemia. However, this issue has not been adequately studied.
Presence of Q wave in the ECG of acute phase of ACS: In addition to the changes reflecting acute ischemia and reperfusion, the ECG gives information concerning the presence of preexisting coronary heart disease and hence, myocardial reserves. Q waves in leads without acute changes indicate prior myocardial infarction/ scar. Such patients could have low myocardial reserves and even a relatively small infarction may lead to cardiogenic shock and heart failure, as for example, inferior STEMI in a patient with an old extensive anterior infarction.58 Additional markers of advanced diffuse disease are QRS widening with intraventricular conduction abnormalities, LVH with repolarization changes and criteria for atrial enlargement.
REFERENCES
- 1. O'Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA Guideline for the management of ST‐elevation myocardial infarction: A report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 2013;127:e362–e425. [DOI] [PubMed] [Google Scholar]
- 2. Steg PG, James SK, Atar D, et al. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST‐segment elevation. Eur Heart J 2012;33:2569–2619. [DOI] [PubMed] [Google Scholar]
- 3. Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. Circulation 2012;126:2020–2035. [DOI] [PubMed] [Google Scholar]
- 4. Barold SS, Falkoff MD, Ong LS, Heinle RA. Significance of transient electrocardiographic Q waves in coronary artery disease. Cardiol Clin 1987;5:367–380. [PubMed] [Google Scholar]
- 5. Sclarovsky S, Mager A, Kusniec J, et al. Electrocardiographic classification of acute myocardial ischemia. Isr J Med Sci 1990;26:525–231. [PubMed] [Google Scholar]
- 6. Atar S, Barbagelata A, Birnbaum Y. Electrocardiographic markers of reperfusion in ST‐elevation myocardial infarction. Cardiol Clin 2006;24:367–376, viii. [DOI] [PubMed] [Google Scholar]
- 7. Huang HD, Birnbaum Y. ST elevation: Differentiation between ST elevation myocardial infarction and nonischemic ST elevation. J Electrocardiol 2011;44:494 e1–e12. [DOI] [PubMed] [Google Scholar]
- 8. Macfarlane PW. Age, sex, and the ST amplitude in health and disease. J Electrocardiol 2001;34(Suppl):235–241. [DOI] [PubMed] [Google Scholar]
- 9. Katibi I, Clark EN, Devine B, Lloyd SM, Macfarlane PW. Normal limits of the electrocardiogram in Nigerians. J Electrocardiol 2013;46:289–295. [DOI] [PubMed] [Google Scholar]
- 10. Antman EM, Anbe DT, Armstrong PW, et al. ACC/AHA guidelines for the management of patients with ST‐elevation myocardial infarction; a report of the American college of cardiology/American heart Association task force on practice guidelines (Committee to Revise the 1999 Guidelines for the Management of patients with acute myocardial infarction). J Am Coll Cardiol 2004;44:E1–E211. [DOI] [PubMed] [Google Scholar]
- 11. Bayés de Luna A. Clinical Electrocardiography. Hoboken, NJ: Wiley Blackwell, 2012. [Google Scholar]
- 12. Serra‐Grima R, Estorch M, Carrio I, Subirana M, Berna L, Prat T. Marked ventricular repolarization abnormalities in highly trained athletes’ electrocardiograms: Clinical and prognostic implications. J Am Coll Cardiol 2000;36:1310–1316. [DOI] [PubMed] [Google Scholar]
- 13. Smith SW. Upwardly concave ST segment morphology is common in acute left anterior descending coronary occlusion. J Emerg Med 2006;31:69–77. [DOI] [PubMed] [Google Scholar]
- 14. Chung SL, Lei MH, Chen CC, Hsu YC, Yang CC. Characteristics and prognosis in patients with false‐positive ST‐elevation myocardial infarction in the ED. Am J Emerg Med 2013;31:825–829. [DOI] [PubMed] [Google Scholar]
- 15. Madias JE. The impact of changing oedematous states on the QRS duration: Implications for cardiac resynchronization therapy and implantable cardioverter/defibrillator implantation. Europace 2005;7:158–164. [DOI] [PubMed] [Google Scholar]
- 16. Clemmensen P, Grande P, Aldrich HR, Wagner GS. Evaluation of formulas for estimating the final size of acute myocardial infarcts from quantitative ST‐segment elevation on the initial standard 12‐lead ECG. J Electrocardiol 1991;24:77–83. [DOI] [PubMed] [Google Scholar]
- 17. Christian TF, Gibbons RJ, Clements IP, Berger PB, Selvester RH, Wagner GS. Estimates of myocardium at risk and collateral flow in acute myocardial infarction using electrocardiographic indexes with comparison to radionuclide and angiographic measures. J Am Coll Cardiol 1995;26:388–393. [DOI] [PubMed] [Google Scholar]
- 18. Korver FW, Hassell M, Smulders MW, Bekkers SC, Gorgels AP. Correlating both Aldrich and Hellemond score with cardiac magnetic resonance imaging endocardial surface area calculations in the estimation of the area at risk. Electrocardiography scores and endocardial surface area calculations: Do they correlate? J Electrocardiol 2013;46:229–234. [DOI] [PubMed] [Google Scholar]
- 19. Huang HD, Tran V, Jneid H, Wilson JM, Birnbaum Y. Comparison of angiographic findings in patients with acute anteroseptal versus anterior wall ST‐elevation myocardial infarction. Am J Cardiol 2011;107:827–832. [DOI] [PubMed] [Google Scholar]
- 20. Atar S, Birnbaum Y. Ischemia‐induced ST‐segment elevation: Classification, prognosis, and therapy. J Electrocardiol 2005;38:1–7. [DOI] [PubMed] [Google Scholar]
- 21. Birnbaum Y, Hale SL, Kloner RA. Progressive decrease in the ST segment elevation during ischemic preconditioning: Is it related to recruitment of collateral vessels? J Mol Cell Cardiol 1996;28:1493–1499. [DOI] [PubMed] [Google Scholar]
- 22. Heng MK, Singh BN, Norris RM, John MB, Elliot R. Relationship between epicardial ST‐segment elevation and myocardial ischemic damage after experimental coronary artery occlusion in dogs. J Clin Invest 1976;58:1317–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Heden B, Ripa R, Persson E, et al. A modified Anderson‐Wilkins electrocardiographic acuteness score for anterior or inferior myocardial infarction. Am Heart J 2003;146:797–803. [DOI] [PubMed] [Google Scholar]
- 24. Schoos MM, Lonborg J, Vejlstrup N, et al. A novel prehospital electrocardiogram score predicts myocardial salvage in patients with ST‐segment elevation myocardial infarction evaluated by cardiac magnetic resonance. Cardiology 2013;126:97–106. [DOI] [PubMed] [Google Scholar]
- 25. Birnbaum GD, Birnbaum I, Birnbaum Y. Twenty years of ECG grading of the severity of ischemia. J Electrocardiol 2014:47:546–555. [DOI] [PubMed] [Google Scholar]
- 26. Eskola MJ, Holmvang L, Nikus KC, et al. The electrocardiographic window of opportunity to treat vs. the different evolving stages of ST‐elevation myocardial infarction: Correlation with therapeutic approach, coronary anatomy, and outcome in the DANAMI‐2 trial. Eur Heart J 2007;28:2985–2991. [DOI] [PubMed] [Google Scholar]
- 27. Birnbaum Y, Chetrit A, Sclarovsky S, et al. Abnormal Q waves on the admission electrocardiogram of patients with first acute myocardial infarction: Prognostic implications. Clin Cardiol 1997;20:477–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Boden WE, Kleiger RE, Gibson RS, et al. Electrocardiographic evolution of posterior acute myocardial infarction: Importance of early precordial ST‐segment depression. Am J Cardiol 1987;59:782–787. [DOI] [PubMed] [Google Scholar]
- 29. Mamby SA, Bradley AB, Boden WE. Early precordial ST‐segment depression due to isolated acute right or left circumflex coronary artery occlusion. Am J Cardiol 1987;60:726–728. [DOI] [PubMed] [Google Scholar]
- 30. Porter A, Vaturi M, Adler Y, et al. Are there differences among patients with inferior acute myocardial infarction with ST depression in leads V2 and V3 and positive versus negative T waves in these leads on admission? Cardiology 1998;90:295–298. [DOI] [PubMed] [Google Scholar]
- 31. Bayes de Luna A, Wagner G, Birnbaum Y, et al. A new terminology for left ventricular walls and location of myocardial infarcts that present Q wave based on the standard of cardiac magnetic resonance imaging: A statement for healthcare professionals from a committee appointed by the international society for holter and noninvasive electrocardiography. Circulation 2006;114:1755–1760. [DOI] [PubMed] [Google Scholar]
- 32. Birnbaum Y, Wilson JM, Fiol M, Bayés de Luna A, Eskola M, Nikus K. ECG diagnosis and classification of acute coronary syndromes. Ann Noninvasive Electrocardiol 2014;19:4–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Horton CL, Brady WJ. Right bundle‐branch block in acute coronary syndrome: Diagnostic and therapeutic implications for the emergency physician. Am J Emerg Med 2009;27:1130–1141. [DOI] [PubMed] [Google Scholar]
- 34. Nielsen KM, Faergeman O, Larsen ML, Foldspang A. How can we identify low‐ and high‐risk patients among unselected patients with possible acute coronary syndrome? Am J Emerg Med 2007;25:23–31. [DOI] [PubMed] [Google Scholar]
- 35. Saltups A, Bett N, McLean KH. Prognostic factors in right bundle‐branch block complicating acute myocardial infarction. Aust N Z J Med 1973;3:25–30. [DOI] [PubMed] [Google Scholar]
- 36. Atar S, Fu Y, Wagner GS, Rosanio S, Barbagelata A, Birnbaum Y. Usefulness of ST depression with T‐wave inversion in leads V(4) to V(6) for predicting one‐year mortality in non‐ST‐elevation acute coronary syndrome (from the Electrocardiographic Analysis of the Global Use of Strategies to Open Occluded Coronary Arteries IIB Trial). Am J Cardiol 2007;99:934–938. [DOI] [PubMed] [Google Scholar]
- 37. Nikus KC, Eskola MJ, Virtanen VK, et al. ST‐depression with negative T waves in leads V4–V5–a marker of severe coronary artery disease in non‐ST elevation acute coronary syndrome: A prospective study of Angina at rest, with troponin, clinical, electrocardiographic, and angiographic correlation. Ann Noninvasive Electrocardiol 2004;9:207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hasdai D, Yeshurun M, Birnbaum Y, Sclarovsky S. Inferior wall acute myocardial infarction with one‐lead ST‐segment elevation: Electrocardiographic distinction between a benign and a malignant clinical course. Coron Artery Dis 1995;6:875–881. [PubMed] [Google Scholar]
- 39. Barrabes JA, Figueras J, Moure C, Cortadellas J, Soler‐Soler J. Prognostic significance of ST segment depression in lateral leads I, aVL, V5 and V6 on the admission electrocardiogram in patients with a first acute myocardial infarction without ST segment elevation. J Am Coll Cardiol 2000;35:1813–1819. [DOI] [PubMed] [Google Scholar]
- 40. Nikus K, Pahlm O, Wagner G, et al. Electrocardiographic classification of acute coronary syndromes: A review by a committee of the International Society for Holter and Non‐Invasive Electrocardiology. J Electrocardiol 2010;43:91–103. [DOI] [PubMed] [Google Scholar]
- 41. Knotts RJ, Wilson JM, Kim E, Huang HD, Birnbaum Y. Diffuse ST depression with ST elevation in aVR: Is this pattern specific for global ischemia due to left main coronary artery disease? J Electrocardiol 2013;46:240–248. [DOI] [PubMed] [Google Scholar]
- 42. Gorgels AP, Engelen DJ, Wellens HJ. Lead aVR, a mostly ignored but very valuable lead in clinical electrocardiography. J Am Coll Cardiol 2001;38:1355–1356. [DOI] [PubMed] [Google Scholar]
- 43. Yamaji H, Iwasaki K, Kusachi S, et al. Prediction of acute left main coronary artery obstruction by 12‐lead electrocardiography. ST segment elevation in lead aVR with less ST segment elevation in lead V(1). J Am Coll Cardiol 2001;38:1348–1354. [DOI] [PubMed] [Google Scholar]
- 44. Atie J, Brugada P, Brugada J, et al. Clinical presentation and prognosis of left main coronary artery disease in the 1980s. Eur Heart J 1991;12:495–502. [DOI] [PubMed] [Google Scholar]
- 45. Gorgels AP, Vos MA, Mulleneers R, de Zwaan C, Bar FW, Wellens HJ. Value of the electrocardiogram in diagnosing the number of severely narrowed coronary arteries in rest angina pectoris. Am J Cardiol 1993;72:999–1003. [DOI] [PubMed] [Google Scholar]
- 46. Barrabes JA, Figueras J, Moure C, Cortadellas J, Soler‐Soler J. Prognostic value of lead aVR in patients with a first non‐ST‐segment elevation acute myocardial infarction. Circulation 2003;108:814–819. [DOI] [PubMed] [Google Scholar]
- 47. de Winter RJ, Verouden NJ, Wellens HJ, Wilde AA. A new ECG sign of proximal LAD occlusion. N Engl J Med 2008;359:2071–2073. [DOI] [PubMed] [Google Scholar]
- 48. Verouden NJ, Koch KT, Peters RJ, et al. Persistent precordial “hyperacute” T‐waves signify proximal left anterior descending artery occlusion. Heart 2009;95:1701–1706. [DOI] [PubMed] [Google Scholar]
- 49. Birnbaum Y, Sclarovsky S, Mager A, Strasberg B, Rechavia E. ST segment depression in a VL: A sensitive marker for acute inferior myocardial infarction. Eur Heart J 1993;14:4–7. [DOI] [PubMed] [Google Scholar]
- 50. Sagie A, Sclarovsky S, Strasberg B, et al. Acute anterior wall myocardial infarction presenting with positive T waves and without ST segment shift. Electrocardiographic features and angiographic correlation. Chest 1989;95:1211–1215. [DOI] [PubMed] [Google Scholar]
- 51. de Zwaan C, Bar FW, Wellens HJ. Characteristic electrocardiographic pattern indicating a critical stenosis high in left anterior descending coronary artery in patients admitted because of impending myocardial infarction. Am Heart J 1982;103:730–736. [DOI] [PubMed] [Google Scholar]
- 52. Gorgels AP. ST‐elevation and non‐ST‐elevation acute coronary syndromes: Should the guidelines be changed? J Electrocardiol 2013;46:318–323. [DOI] [PubMed] [Google Scholar]
- 53. Birnbaum Y, Zhou S, Wagner GS. New considerations of ST segment “elevation” and “depression” and accompanying T wave configuration in acute coronary syndromes. J Electrocardiol 2011;44:1–6. [DOI] [PubMed] [Google Scholar]
- 54. Figueras J, Ferreira‐Gonzalez I, Rizzo M, et al. High incidence of TIMI flow 0 to I in patients with ST‐elevation myocardial infarction without electrocardiographic lytic criteria. Am Heart J 2009;158:1011–1017. [DOI] [PubMed] [Google Scholar]
- 55. Alsaab A, Hira RS, Alam M, Elayda M, Wilson JM, Birnbaum Y. Usefulness of T wave inversion in leads with ST elevation on the presenting electrocardiogram to predict spontaneous reperfusion in patients with anterior ST elevation acute myocardial infarction. Am J Cardiol 2014;113:270–274. [DOI] [PubMed] [Google Scholar]
- 56. Hayden GE, Brady WJ, Perron AD, Somers MP, Mattu A. Electrocardiographic T‐wave inversion: Differential diagnosis in the chest pain patient. Am J Emerg Med 2002;20:252–262. [DOI] [PubMed] [Google Scholar]
- 57. Birnbaum Y, Bayes de Luna A, Fiol M, et al. Common pitfalls in the interpretation of electrocardiograms from patients with acute coronary syndromes with narrow QRS: A consensus report. J Electrocardiol 2012;45:463–475. [DOI] [PubMed] [Google Scholar]
- 58. Moshkovitz Y, Sclarovsky S, Behar S, Reicher‐Reiss H, Kaplinsky E, Goldbourt U. Infarct site‐related mortality in patients with recurrent myocardial infarction. SPRINT Study Group Am J Med 1993;94:388–394. [DOI] [PubMed] [Google Scholar]


