Abstract
Objective:
Hyperglycemia leads to increase advanced glycation end products (AGEs) in patients with type 1 and type 2 diabetes. Subsequently, formation of AGEs can cause increased plantar fascial thickness (PFT), an imaging feature of plantar fasciitis (PF). This study evaluates the prevalence of PF in a contemporary cohort of type 1 diabetes and type 2 diabetes patients managed according to current standards, compared to patients without diabetes.
Research design and methods:
This is a five-year prevalence study in a large tertiary health system (approximately 535,000 patients/visits/year) with a single electronic medical record (EMR), applying a cohort discovery tool and database screen (Data Direct) with use of ICD-9 and ICD-10 codes. All patients with a PF diagnosis between 01/01/2011 and 01/01/2016 were included and divided into 3 groups: type 1 diabetes (7148 patients), type 2 diabetes (61,632 patients), and no diabetes (653,659 patients). Prevalence rates were calculated, accounting for other risk factors including BMI and gender using Fisher’s exact test.
Results:
The overall prevalence of PF in the entire study population was 0.85%. Prevalence rates were higher in patients with diabetes, particularly with type 2 diabetes (42% and 64% higher compared with patients with type 1 diabetes and no diabetes respectively). Individually, PF rates were 0.92% in type 1 diabetes and 1.31% in type 2 diabetes compared with 0.80% in patients with no diabetes (Type 1 vs. no diabetes p = 0.26; Type 2 vs. no diabetes p ≪ 0.0001; Type 1 vs. Type 2 diabetes p = 0.0054). Females in all groups had higher prevalence of PF than males (p ≪ 0.0001 for all), with those patients with diabetes having higher prevalence rates than those without diabetes. Patients with higher BMI levels (BMI ≥ 30 kg/m2) were also more likely to have PF in all categories except males with type 1 diabetes (p = 0.40).
Conclusions:
In this large contemporary population managed in a tertiary health system, prevalence rates of PF were substantially higher in patients with diabetes compared with no diabetes, particularly in type 2 diabetes. Female gender and higher BMI were also associated with higher prevalence of PF in this cohort.
Keywords: Plantar fasciitis, Type 1 diabetes mellitus, Type 2 diabetes mellitus, Secondary diabetic complications, Advanced glycation end product
1. Introduction
Plantar fasciitis (PF) is reported to be the most common cause of plantar heel pain and is one of the most commonly seen conditions by foot and ankle specialists.1–4 It has been reported that approximately 10% of the population in the United States develop PF in their lifetime5,6 and this condition accounts for ≫1 million outpatient visits annually.4–6 Nahin’s recent survey data revealed that the overall prevalence of PF was 0.85% and a higher prevalence was seen in women (1.19%) versus men (0.47%), those aged 45–64 (1.33%) versus 18–44 (0.53%), and in those with BMI ≥ 25 kg/m2 (1.48%) versus BMI ≪ 25 kg/m2 (0.29%).7 PF has been reported in both the non-active and active patient population4 and presents bilaterally in about one third of patients.8 The incidence of PF is classically seen in adults, between 40 and 60 years of age with variable reporting on gender predilection.4 The characteristic complaint is pain on standing after a period of rest and is primarily located at the plantar medial tubercle of the heel.9 Associated significant intrinsic risk factors may include increased age, obesity, biomechanical dysfunction, acquired systemic disease, ankle equinus, excessive pronation, arch collapse, and/or gene variants.4,9,10 Significant extrinsic risk factors include repetitive microtrauma, prolonged weight bearing, shoe gear, increase in physical demands, sleeping posture, and/or sport participation.4,10 Schneider et al. has described the anatomy and histologic properties of the plantar fascia in detail.4 It has been reported that asymptomatic, “normal”, plantar fascial thickness (PFT) is 2.3–4.3 mm, with an average of approximately 3.4 mm.11–19 Ultrasound evaluation studies have shown that patients with PF exhibit a thickening of their plantar fascia, compared to normal asymptomatic patients.11,20 Symptomatic PFT in patients with active PF measures approximately 4 mm or greater.11,14,21
In 2017 it was reported that 30.3 million Americans (9.4% of the United States population) had diabetes; 23.1 million people were diagnosed and 7.2 million people were undiagnosed.22 Hyperglycemia is one of the main driving risk factors involved in the pathogenesis of diabetes complications,23–26 through multiple pathways, including increased protein glycation and a gradual build-up of advanced glycation end products (AGEs) in all complications-prone tissues.27 Musculoskeletal complications of DM are the most common endocrine arthropathies.28 Frozen shoulder, rotator cuff tears, Dupuytren’s contracture, trigger finger, and cheiroarthropathy are among the most common conditions in the upper extremity, while Achilles tendon tightness (i.e. equinus), heel spurs, and increased PFT are the most common musculoskeletal conditions in the lower extremity.3,29,30
To our knowledge, the prevalence of PF in patients with type 1 diabetes and type 2 diabetes, or the association between PFT and the development of symptomatic PF in these patients has not yet been well-studied. The goal of this study was to evaluate the PF prevalence rates and risk factors in a large contemporary cohort of adult patients with type 1 diabetes and type 2 diabetes followed according to current standards of care in a tertiary health system over a 5-year period compared to no diabetes, using a cohort discovery tool.
2. Materials and methods
2.1. Study design
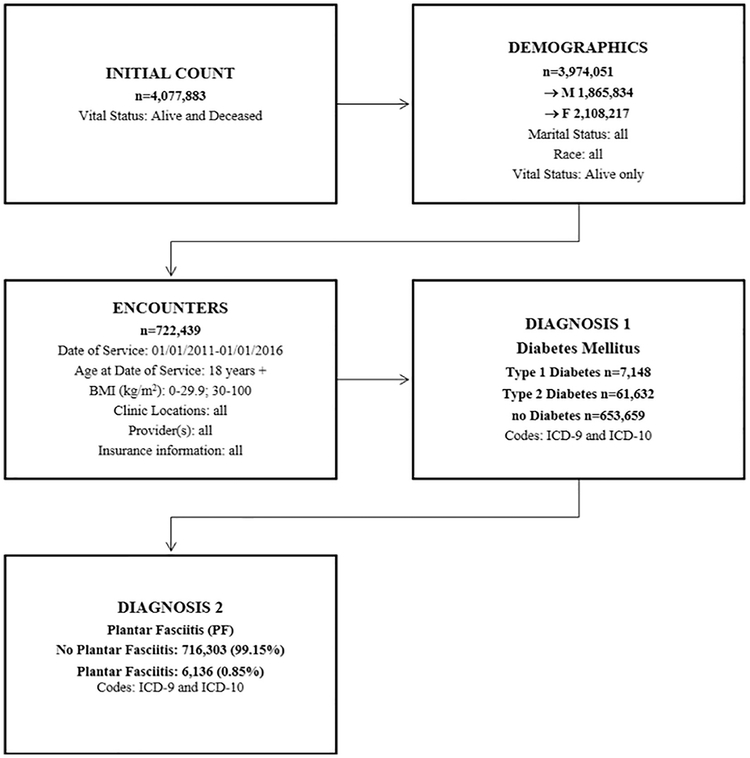
This study is a five-year prevalence study in a large tertiary health system (approximately 535,000 patients/visits/year) with a single electronic medical record (EMR) applying a cohort discovery tool and database (Data Direct) with use of ICD-9 and ICD-10 codes. Data Direct is a software developed by the University of Michigan Medical School Office of Research specifically developed to mine the entire health system database at our institution (University of Michigan Health System). This self-serve tool directly mines entire information from the EMR, including diagnoses, encounters, clinic visits, clinic locations, insurance information, demographics, vital status, and laboratory tests for all Michigan Medicine facilities (https://datadirect.med.umich.edu).31 Fig. 1 shows the study design in flow chart format.
Fig. 1.
Study design flow chart.
2.2. Setting
A large tertiary health system with a single electronic medical record (EMR) across all outpatient clinics, providing care to approximately 4 million patients available in the Data Direct database. Patients with PF are currently seen mainly across 6 specialty centers including the Comprehensive Diabetes Center Clinic of the Division of Metabolism, Endocrinology, and Diabetes (MEND), that houses a high volume podiatry service with 6 board certified podiatrists who specialize in the management of diabetic foot complications and amputation prevention.32
2.3. Participants
All alive adult patients age 18 and above presented in the Data Direct system from 01/01/2011 through 01/01/2016 were included. Inclusion criteria comprised all genders, races, marital status, locations, providers, insurances. Patients who had history of smoking, alcohol use, illegal drug, and were sexually active were included. Patients who were deceased were excluded from this study. All patients with a PF diagnosis meeting eligibility that were included in this study were divided into 3 groups: Type 1 diabetes (7148 patients), type 2 diabetes (61,632 patients), and no diabetes (653,659 patients).
2.4. Variables
The counts and rates of PF were determined from ICD-9 and ICD-10 codes. With the use of these codes, we were able to identify patients with PF and type 1 diabetes, PF and type 2 diabetes, and PF and no diabetes. We also evaluated the subsets by gender (male or female) and BMI (0–29.9 kg/m2 or 30–100 kg/m2).
2.5. Statistical methods
Prevalence rates were calculated and Fisher’s exact test was used to test association risk factor and prevalence of PF in the overall population. Cochran-Mantel-Haenszel Statistics was used for testing association between diabetes and prevalence of PF adjusting for BMI and gender effect. Fisher’s exact test was also used to test association between diabetes and prevalence of PF and within stratified sub populations by gender and BMI. Similar analysis was used to study gender and BMI effect one at a time within each of the three diabetes groups stratified by the other factor. All statistical analysis was completed using SPSS statistical software, version 22 (SPSS Inc., Chicago, IL) and SAS software (Copyright © 2002–2012 SAS Institute Inc., Cary, NC, USA). For all analysis, p-value ≤ 0.05 was considered statistically significant.
3. Results
There were 4,077,883 patients available for analysis in the Data Direct cohort tool. After eliminating deceased patients, there were 3,974,051 patients available for analysis (1,865,834 male; 2,108,217 female). When applying the study date range specifications, age restriction (18+) and BMI subsets (0–29.9 kg/m2; 30–100 kg/m2) there were 722,439 patients available for analysis (313,409 male; 409,030 female). After entering ICD-9 and ICD-10 codes for type 1 diabetes, 7148 patients were available (3595 male; 3553 female) and after entering ICD-9 and ICD-10 codes for type 2 diabetes, 61,632 patients were available (31,495 male; 30,137 female). The remaining 653,659 patients did not have diabetes (278,319 male; 375,340 female). The ICD-9 and ICD-10 codes for PF were then added to evaluate the number of patients with type 1, type 2 and no diabetes with PF (type 1 diabetes and PF 66 patients; type 2 diabetes and PF 809 patients; no diabetes and PF 5261 patients).
The overall prevalence of PF at our institution during the study period was 0.85%. The prevalence of PF in patients with type 1 diabetes, type 2 diabetes, and no diabetes was 0.92%, 1.31%, and 0.80%, respectively. Patients with type 2 diabetes had significantly higher rate of PF than patients with type 1 diabetes (p ≪ 0.0054) and no diabetes (p ≪ 0.0001). Difference between type 1 diabetes and no diabetes group was not statistically significant (Table 1).
Table 1.
Plantar fasciitis prevalence.
| Diabetes | PF | Count | Percent | p-Value |
|---|---|---|---|---|
| Type 1 | No plantar fasciitis | 7,082 | 99.08% | |
| Plantar fasciitis [PF] | 66 | 0.92% | p ≪ 0.0001 for the overall test | |
| Type 2 | No plantar fasciitis | 60,823 | 98.69% | Type 2 vs. No diabetes: p ≪ 0.0001 |
| Plantar fasciitis [PF] | 809 | 1.31% | Type 1 vs. No diabetes: p = 0.26 | |
| No diabetes | No plantar fasciitis | 648,398 | 99.20% | Type 2 vs. Type 1: p = 0.0054 |
| Plantar fasciitis [PF] | 5261 | 0.80% |
After adjusting for the effect of BMI and gender, the results remained similar. Within each of the three groups, females had significantly higher risk of PF compared to males (p ≪ 0.0001) (Table 2), with those patients with diabetes having higher prevalence rates than those without diabetes. Stratified by diabetes status and gender, those patients (males and females) in the high BMI group had a statistically higher prevalence of PF than in the low BMI group in all categories except males with type 1 diabetes (Table 3).
Table 2.
Effect of gender on prevalence of plantar fasciitis
| Effect of gender on prevalence of PF | |||||
|---|---|---|---|---|---|
| Diabetes | Gender | Prevalence % (95% CI) | Odds ratio F vs. M | Relative risk F vs. M | p-Value (Fisher’s exact test) |
| Type 1 | F | 1.11 (0.98,1.26) | 1.60 (1.31,1.97) | 1.60 (1.30,1.96) | ≪0.0001 |
| M | 0.7 (0.59,0.82) | ||||
| Type 2 | F | 1.54 (1.49,1.6) | 1.44(1.36,1.52) | 1.43 (1.35, 1.51) | ≪0.0001 |
| M | 1.08 (1.03,1.13) | ||||
| No diabetes | F | 0.9 (0.89,0.92) | 1.35 (1.31,1.40) | 1.35 (1.31, 1.39) | ≪0.0001 |
| M | 0.67 (0.65,0.69) | ||||
Table 3.
Effect of obesity (high BMI) on prevalence of planar fasciitis by gender.
| Effect of obesity (high BMI) on prevalence of PF by gender | ||||||
|---|---|---|---|---|---|---|
| Gender | Diabetes | BMI (kg/m2) | Prevalence % (95% CI) | Odds ratio BMI 30–100 vs. 0–29.9 | Relative risk BMI 30–100 vs. 0–29.9 | p-Value (Fisher’s exact test) |
| F | Type 1 | BMI 0–29.9 | 0.95 (0.79,1.15) | 1.37 (1.06,1.77) | 1.37 (1.06,1.76) | 0.018 |
| BMI 30–100 | 1.3 (1.09,1.55) | |||||
| Type 2 | BMI 0–29.9 | 0.96 (0.89,1.04) | 1.98 (1.81,2.16) | 1.96 (1.8,2.14) | ≪0.0001 | |
| BMI 30–100 | 1.89 (1.81,1.97) | |||||
| No diabetes | BMI 0–29.9 | 0.67 (0.65,0.69) | 2.1 (2.02,2.18) | 2.08 (2,2.16) | ≪0.0001 | |
| BMI 30–100 | 1.4 (1.36,1.44) | |||||
| M | Type 1 | BMI 0–29.9 | 0.65 (0.52,0.81) | 1.16 (0.84,1.6) | 1.16 (0.84,1.6) | 0.40 |
| BMI 30–100 | 0.76 (0.59,0.96) | |||||
| Type 2 | BMI 0–29.9 | 0.74 (0.68,0.8) | 1.83 (1.66,2.01) | 1.82 (1.66,2) | ≪0.0001 | |
| BMI 30–100 | 1.34(1.27,1.41) | |||||
| No diabetes | BMI 0–29.9 | 0.55 (0.53,0.57) | 1.71 (1.62,1.81) | 1.71 (1.62,1.8) | ≪0.0001 | |
| BMI 30–100 | 0.93 (0.89,0.97) | |||||
Overall, we found the prevalence of PF in patients with type 2 diabetes was 42% and 64% higher than both patients with type 1 diabetes and without diabetes, respectively. High BMI impacted the prevalence of PF throughout all groups (both male and female) with the exception of males with type 1 diabetes.
4. Discussion
This study evaluated the prevalence of PF in the type 1 diabetes and type 2 diabetes patient population when compared to patients without diabetes in a large cohort followed according to current standards of care in a tertiary health system. We found the prevalence of PF in patients with type 2 diabetes was 42% and 64% higher than both patients with type 1 diabetes and without diabetes, respectively. Overall, female gender and high BMI impacted the prevalence of PF throughout all groups with the exception of males with type 1 diabetes.
The overall prevalence of PF in our study was 0.85%, which is similar to the results demonstrated in recently published work by Nahin et al.7 Our study further suggests that patients with type 2 diabetes have a higher prevalence of PF than patients with type 1 diabetes and patients without diabetes. A potential mechanism that may explain such findings are related to AGE formation. Hyperglycemia is an important driver of increased AGE formation in patients with diabetes,27 and AGE formation was reported to cause increased plantar fascial thickness, a recognized imaging feature of PF.11,20,29 AGEs form on intracellular and extracellular proteins, lipids, nucleic acids and possess complex structures that generate protein fluorescence and cross-linking.27 Protein glycation and AGEs are accompanied by increased free radical activity that contributes towards the bio-molecular damage in diabetes.27 In patients with diabetes, the Achilles tendon is thickened by the same mechanism as the plantar fascia (i.e. AGEs).
Wrobel et al. displayed the relationship that Achilles tendon contracture (equinus) has on impacting peak forefoot pressures in patients with diabetes.33 Equinus may predispose patients to increased pressures which can lead to ulceration and poor wound healing. There is also additional evidence suggesting a significant association between vascular complications and the development of musculoskeletal manifestations of diabetes mellitus,34 which could complicate wound healing further.
The findings we report here in various subgroups bear additional attention, particularly that female gender and high BMI increase the risk of PF. The role of BMI influence on the thickness of the Achilles tendon and plantar fascia has been well established in the literature.4,35–39 Abate et al. found that plantar fascia and Achilles tendon thickness is increased in the early stages of type 2 diabetes and that BMI is related more to the plantar fascia than to Achilles tendon thickness. As thickness and stiffness of the structures increases, the more severe the overall alteration of the foot loading pattern can be. This is also a risk factor that may contribute to diabetic foot ulcer development.29,35,40
This study is not without limitations. First, this is a retrospective study by design using administrative data and ICD-9 and ICD-10 codes. It was the assumption that if a patient is linked to a specific ICD-9 or ICD-10 code in Data Direct then the diagnosis is accurate. It should be noted that the ICD-9 and ICD-10 codes for PF are the same as for a plantar fibroma (i.e. plantar fascial fibromatosis) which could also lead to inaccurate matching of diagnosis. It is known, however, that PF is a much more common diagnosis than a plantar fibroma. Allen et al. reported 69 instances of plantar fibromatosis over a 45 year period and Pickren et al. reported that the general population incidence would be lower than 1.75 per 100,000.41,42 In addition, there could potentially be a lack of accuracy in the diagnosis of plantar fasciitis by medical professionals who were not trained in the foot and ankle, possibly missing other common differential diagnoses for heel pain (i.e. tarsal tunnel syndrome, infracalcaneal bursitis, etc.). Our study also did not stratify based on different age levels, diabetic complications or glycemic control (i.e. Hemoglobin A1c levels) given the limitation of the Data Direct software. Last, it is highly possible that subjects with undiagnosed type 1 diabetes or type 2 diabetes may also have been included in the no diabetes population. Last, it should be mentioned that there has been no imaging reviewed from the patients included in this study to confirm if patients with PF have signs of PFT.
Further research is needed to specifically evaluate the link between AGE build up and PF in the patients with diabetes. It would be important to evaluate Hemoglobin A1c levels with PF diagnosis development and response to treatment. Also, it would be interesting to examine the temporal relationship between microvascular complications and PFT. The prevalence of PF concurrently with ankle equinus in patients with diabetes is unknown as well as the relationship between microvascular complications and PFT and equinus. Appropriate imaging (i.e. ultrasound, MRI) could also be reviewed and/or performed on these patients to evaluate for a correlation of increased incidence of PF and increased PFT.
5. Conclusions
We found the prevalence of PF in patients with type 2 diabetes was 42% and 64% higher than both patients with type 1 diabetes and without diabetes, respectively. Overall, female gender and high BMI impacted the prevalence of PF throughout all groups with the exception of males with type 1 diabetes.
Acknowledgments
Sari J. Priesand DPM researched the data, organized and wrote the manuscript, and compiled all of the references.
Brian M. Schmidt DPM, assisted with data collection and reviewed/edited the manuscript.
Lynn Ang MD reviewed/edited the manuscript.
James S. Wrobel DPM, MS assisted with organization of the manuscript and reviewed/edited the manuscript.
Michael Munson DPM reviewed/edited the manuscript.
Wen Ye PhD performed the statistical analysis and research design & methods section of the paper, compiled the tables in the results section, and reviewed/edited the manuscript.
Rodica Pop-Busui MD, PhD provided inspiration and direction for the research in this study and reviewed/edited the manuscript. She is currently an associate editor of Journal of Diabetes and Its Complications.
Footnotes
Conflict of interest statement: Dr. Busui is currently an associate editor of Journal of Diabetes and Its Complications. Aside from this, there are no conflicts of interest.
References
- 1.Riddle DL, Pulisic M, Pidcoe P, Johnson RE. Risk factors for plantar fasciitis: a matched case-control study. J Bone Joint Surg Am 2003;85-A:872–7. [DOI] [PubMed] [Google Scholar]
- 2.Singh D, Angel J, Bentley G, Trevino SG. Fortnightly review. Plantar fasciitis. BMJ 1997;315:172–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moroney PJ, O’Neill BJ, Khan-Bhambro K, O’Flanagan SJ, Keogh P, Kenny PJ. The conundrum of calcaneal spurs: do they matter? Foot Ankle Spec 2014;7:95–101. [DOI] [PubMed] [Google Scholar]
- 4.Schneider HP, Baca JM, Carpenter BB, Dayton PD, Fleischer AE, Sachs BD. American College of Foot and Ankle Surgeons clinical consensus statement: diagnosis and treatment of adult acquired infracalcaneal heel pain. J Foot Ankle Surg 2018;57:370–81. [DOI] [PubMed] [Google Scholar]
- 5.Cole BJ, Schumacher HR Jr. Injectable corticosteroids in modern practice. J Am Acad Orthop Surg 2005;13:37–46. [DOI] [PubMed] [Google Scholar]
- 6.Crawford F, Thomson C. Interventions for treating plantar heel pain. Cochrane Database Syst Rev 2003;3, CD000416. [DOI] [PubMed] [Google Scholar]
- 7.Nahin RL. Prevalence and pharmaceutical treatment of plantar fasciitis in United States adults. J Pain 2018;19:885–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berbrayer D, Fredericson M. Update on evidence-based treatments for plantar fasciopathy. PM R 2014;6:159–69. [DOI] [PubMed] [Google Scholar]
- 9.Thomas JL, Christensen JC, Kravitz SR, Mendicino RW, Schuberth JM, Vanore JV, et al. The diagnosis and treatment of heel pain: a clinical practice guideline-revision 2010. J Foot Ankle Surg 2010;49:S1–19. Suppl. [DOI] [PubMed] [Google Scholar]
- 10.Beeson P Plantar fasciopathy: revisiting the risk factors. Foot Ankle Surg 2014;20: 160–5. [DOI] [PubMed] [Google Scholar]
- 11.Mahowald S, Legge BS, Grady JF. The correlation between plantar fascia thickness and symptoms of plantar fasciitis. J Am Podiatr Med Assoc 2011;101:385–9. [DOI] [PubMed] [Google Scholar]
- 12.Akfirat M, Sen C, Gunes T. Ultrasonographic appearance of the plantar fasciitis. Clin Imaging 2003;27:353–7. [DOI] [PubMed] [Google Scholar]
- 13.Cardinal E, Chhem RK, Beauregard CG, Aubin B, Pelletier M. Plantar fasciitis: sonographic evaluation. Radiology 1996;201:257–9. [DOI] [PubMed] [Google Scholar]
- 14.Wall JR, Harkness MA, Crawford A. Ultrasound diagnosis of plantar fasciitis. Foot Ankle 1993;14:465–70. [DOI] [PubMed] [Google Scholar]
- 15.Kamel M, Kotob H. High frequency ultrasonographic findings in plantar fasciitis and assessment of local steroid injection. J Rheumatol 2000;27:2139–41. [PubMed] [Google Scholar]
- 16.Tsai WC, Chiu MF, Wang CL, Tang FT, Wong MK. Ultrasound evaluation of plantar fasciitis. Scand J Rheumatol 2000;29:255–9. [DOI] [PubMed] [Google Scholar]
- 17.Kane D, Greaney T, Shanahan M, Duffy G, Bresnihan B, Gibney R, et al. The role of ultrasonography in the diagnosis and management of idiopathic plantar fasciitis. Rheumatology (Oxford) 2001;40:1002–8. [DOI] [PubMed] [Google Scholar]
- 18.Vohra PK, Kincaid BR, Japour CJ, Sobel E. Ultrasonographic evaluation of plantar fascia bands. A retrospective study of 211 symptomatic feet. J Am Podiatr Med Assoc 2002;92:444–9. [DOI] [PubMed] [Google Scholar]
- 19.Sabir N, Demirlenk S, Yagci B, Karabulut N, Cubukcu S. Clinical utility of sonography in diagnosing plantar fasciitis. J Ultrasound Med 2005;24:1041–8. [DOI] [PubMed] [Google Scholar]
- 20.Fabrikant JM, Park TS. Plantar fasciitis (fasciosis) treatment outcome study: plantar fascia thickness measured by ultrasound and correlated with patient self-reported improvement. Foot (Edinb) 2011;21:79–83. [DOI] [PubMed] [Google Scholar]
- 21.McMillan AM, Landorf KB, Barrett JT, Menz HB, Bird AR. Diagnostic imaging for chronic plantar heel pain: a systematic review and meta-analysis. J Foot Ankle Res 2009;2:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. National Diabetes Statistics Report. 2017 Available from: https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf.
- 23.Reichard P, Nilsson BY, Rosenqvist U. The effect of long-term intensified insulin treatment on the development of microvascular complications of diabetes mellitus. N Engl J Med 1993;329:304–9. [DOI] [PubMed] [Google Scholar]
- 24.Martin CL, Albers JW, Pop-Busui R, Group DER. Neuropathy and related findings in the diabetes control and complications trial/epidemiology of diabetes interventions and complications study. Diabetes Care 2014;37:31–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Writing Team for the Diabetes. C, Complications Trial/Epidemiology of Diabetes I, Complications Research G. Sustained effect of intensive treatment of type 1 diabetes mellitus on development and progression of diabetic nephropathy: the Epidemiology of Diabetes Interventions and Complications (EDIC) study. JAMA 2003;290:2159–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Writing Team for the Diabetes. C, Complications Trial/Epidemiology of Diabetes I, Complications Research G. Effect of intensive therapy on the microvascular complications of type 1 diabetes mellitus. JAMA 2002;287:2563–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ahmed N Advanced glycation endproducts–role in pathology of diabetic complications. Diabetes Res Clin Pract 2005;67:3–21. [DOI] [PubMed] [Google Scholar]
- 28.Attar SM. Musculoskeletal manifestations in diabetic patients at a tertiary center. Libyan J Med 2012;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abate M, Schiavone C, Salini V, Andia I. Management of limited joint mobility in diabetic patients. Diabetes Metab Syndr Obes 2013;6:197–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tambalo C, Poli M, Mantovani G, Bressan F, Bambara LM. Enthesopathies and diabetes mellitus. Clin Exp Rheumatol 1995;13:161–6. [PubMed] [Google Scholar]
- 31.Schmidt BM, Wrobel JS, Munson M, Rothenberg G, Holmes CM. Podiatry impact on high-low amputation ratio characteristics: a 16-year retrospective study. Diabetes Res Clin Pract 2017;126:272–7. [DOI] [PubMed] [Google Scholar]
- 32.Munson ME, Wrobel JS, Holmes CM, Hanauer DA. Data mining for identifying novel associations and temporal relationships with Charcot foot. J Diabetes Res 2014;2014, 214353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wrobel JS, Connolly JE, Beach ML. Associations between static and functional measures of joint function in the foot and ankle. J Am Podiatr Med Assoc 2004;94:535–41. [DOI] [PubMed] [Google Scholar]
- 34.Abourazzak FE, Akasbi N, Houssaini GS, Bazouti S, Bensbaa S, Hachimi H, et al. Articular and abarticular manifestations in type 2 diabetes mellitus. Eur J Rheumatol 2014;1:132–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abate M, Schiavone C, Di Carlo L, Salini V. Achilles tendon and plantar fascia in recently diagnosed type II diabetes: role of body mass index. Clin Rheumatol 2012;31:1109–13. [DOI] [PubMed] [Google Scholar]
- 36.Rano JA, Fallat LM, Savoy-Moore RT. Correlation of heel pain with body mass index and other characteristics of heel pain. J Foot Ankle Surg 2001;40:351–6. [DOI] [PubMed] [Google Scholar]
- 37.Irving DB, Cook JL, Menz HB. Factors associated with chronic plantar heel pain: a systematic review. J Sci Med Sport 2006;9:11–22. [discussion 3–4]. [DOI] [PubMed] [Google Scholar]
- 38.Hill JJ Jr, Cutting PJ. Heel pain and body weight. Foot Ankle 1989;9:254–6. [DOI] [PubMed] [Google Scholar]
- 39.van Leeuwen KD, Rogers J, Winzenberg T, van Middelkoop M. Higher body mass index is associated with plantar fasciopathy/’plantar fasciitis’: systematic review and meta-analysis of various clinical and imaging risk factors. Br J Sports Med 2016;50:972–81. [DOI] [PubMed] [Google Scholar]
- 40.D’Ambrogi E, Giurato L, D’Agostino MA, Giacomozzi C, Macellari V, Caselli A, et al. Contribution of plantar fascia to the increased forefoot pressures in diabetic patients. Diabetes Care 2003;26:1525–9. [DOI] [PubMed] [Google Scholar]
- 41.Allen RA, Woolner LB, Ghormley RK. Soft-tissue tumors of the sole; with special reference to plantar fibromatosis. J Bone Joint Surg Am 1955;37-A:14–26. [PubMed] [Google Scholar]
- 42.Pickren JW, Smith AG, Stevenson TW Jr, Stout AP. Fibromatosis of the plantar fascia. Cancer 1951;4:846–56. [DOI] [PubMed] [Google Scholar]