Abstract
Magnesium is a divalent cation that fills essential roles as regulator and cofactor in a variety of biological pathways, and maintenance of magnesium balance is vital to human health. The kidney, in concert with the intestine, has an important role in maintaining magnesium homeostasis. Although micropuncture and microperfusion studies in the mammalian nephron have shone a light on magnesium handling in the various nephron segments, much of what we know about the protein mediators of magnesium handling in the kidney have come from more recent genetic studies. In the proximal tubule and thick ascending limb, magnesium reabsorption is believed to occur primarily through the paracellular shunt pathway, which ultimately depends on the electrochemical gradients set up by active sodium reabsorption. In the distal convoluted tubule, magnesium transport is transcellular, although magnesium reabsorption also appears to be related to active sodium reabsorption in this segment. In addition, evidence suggests that magnesium transport is highly regulated, although a specific hormonal regulator of extracellular magnesium has yet to be identified.
Keywords: magnesium, homeostasis, nephrons, claudins, renal reabsorption
II. Whole body magnesium homeostasis
In its roles as both a direct allosteric modulator and a cofactor in complex with ATP, magnesium is essential for the normal function of a multitude of proteins, including a number of enzymes involved in glycolysis and oxidative phosphorylation 1, 2. Magnesium also plays a direct role in the stabilization of nucleotides, and both intracellular and extracellular magnesium concentrations have been shown to modulate the activity of ion channels 3-5. Accordingly, perturbations of magnesium balance are associated with systemic signs and symptoms. In particular, magnesium disturbances are associated with tetany, muscle fasciculations, and weakness, especially at serum levels less than 1.2 mg/dL 6. In addition to disruption of the aforementioned processes, symptoms of hypomagnesemia may be related to hypoparathyroidism and hypocalcemia that arise secondarily from reduced parathyroid hormone (PTH) secretion 7.
Under normal physiologic conditions, the serum magnesium is held relatively constant between 1.6-2.3 mg/dL 8. The overwhelming majority (approximately 99%) of magnesium in the body is either stored in bone or within cells 8. Bone contains about half of total body magnesium, and bone magnesium seems to correlate well with serum magnesium across species, including man 9. While magnesium is much higher in cells than serum, the free magnesium ion concentrations are very similar due to buffering by proteins and ATP and sequestration in organelles such as mitochondria 10, 11. Due to the relatively negative intracellular environment, magnesium entrance into the cell is passive but extrusion of magnesium out of the cell occurs against an electric gradient 10. Consequentially, transcellular magnesium transport is likely to be active.
Magnesium balance is achieved by the concerted actions of the intestine and kidneys. Indeed, net kidney excretion of magnesium has a linear relationship to net intestinal absorption across a wide range of magnesium intakes 12, 13. A normal daily magnesium intake averages around 300 mg, of which approximately 50% is absorbed by the intestine 14. Net intestinal magnesium absorption follows a curvilinear rise as dietary magnesium intake increases, revealing a hyperbolic, saturable increase in absorption predominating at lower lumen magnesium concentrations and a linear, nonsaturable rise which dominates with normal magnesium intake 12. These two separate processes likely correspond to active and passive transport, respectively.
Recent segmental analysis of intestinal magnesium transport in mice suggests that, in contrast to calcium reabsorption, the majority of active magnesium absorption occurs in the later intestinal segments 15. In these segments, mRNA expression of the magnesium channel, melastatin-related transient receptor potential cation channel 6 (TRPM6), is highest in humans and mice 15. Homozygous loss-of-function mutations in the magnesium channel TRPM6 cause the magnesium wasting disorder hypomagnesemia with secondary hypocalcemia (HSH), a disorder in which intestinal malabsorption of magnesium occurs alongside a kidney magnesium leak 16, 17. Thus, TRPM6 is likely the major mediator of active magnesium transport in the distal colon. The role of TRPM6 in kidney magnesium handling will be reviewed further in the sections below.
The paracellular transport properties of a given epithelial layer are determined by the electrochemical gradients as well as the protein composition of the most apical structure of the junctional complex, the tight junction. Namely, the claudin family of tetraspan proteins form barrier or charge- and size-selective pores at the tight junction, the expression of which varies between tissue and cell type (for an extensive review, see the study by Hou and colleagues 18). The small intestine has a lower transepithelial resistance (TER) and higher permeability to sodium over chloride (PNa/PCl) than the colon, corresponding to the site of highest expression of cation-selective claudin isoforms such as claudins -12 and -15 19-22. Notably, the transepithelial potential of the small intestine is lumen negative, suggesting that paracellular magnesium transport is primarily driven by its concentration gradient 23, 24. Magnesium absorption in the proximal intestine has a linear relationship with luminal magnesium concentration and exceeds that of the distal intestine at higher magnesium concentrations 15. Together, these data suggest that the bulk of paracellular magnesium absorption occurs in the small intestine.
As intestinal magnesium absorption has a direct relationship to dietary magnesium intake, its role in the regulation of serum magnesium is not clear. The kidney rapidly responds to changes in serum magnesium, and infusion studies reveal that urine magnesium acutely increases as serum magnesium increases during a 30 minute infusion 25. This reveals a striking role for the kidney in maintaining extracellular magnesium concentrations (Figure 1). The physiology of kidney magnesium transport and its regulation are detailed in the sections below.
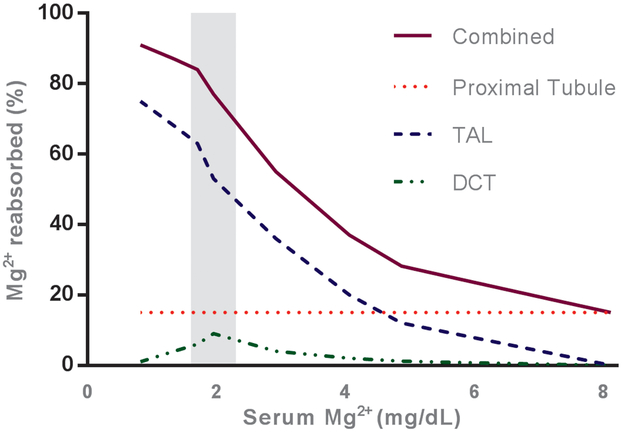
Figure 1. Relationship of plasma values to magnesium reabsorption in the kidney.
The percentage of magnesium reabsorbed by the major sites of transport in the kidney is illustrated relative to serum magnesium (increasing from left to right). As serum magnesium (and thus the amount of magnesium filtered by the kidney) increases, the percentage of filtered magnesium reabsorbed by the thick ascending limb of the Loop of Henle (TAL) and distal convoluted tubule (DCT) decreases. Compare this with the proximal tubule (PT), which reabsorbs magnesium in constant proportion to the amount filtered. The solid line represents total magnesium absorption by the kidney. The normal range of serum magnesium, 1.6-2.3 mg/dL, falls within the shaded area. Figure was adapted using data from Carney and colleagues and Wong and colleagues 89 and Wong 31.
III. Magnesium transport in the kidney
Serum magnesium circulates in both soluble and protein bound forms. The pool of magnesium that is bound by proteins in human serum averages 32% 26. The remainder of serum magnesium is in its ionized form (55%) or within soluble complexes with anions like phosphate and citrate (13%); and is ultrafilterable 8, 26. Despite a mere 1% of total body magnesium being present in the extracellular fluid, about one tenth of total body magnesium is filtered by the kidney in a 24 hour period 8.
a. Proximal tubule
The proximal tubule (PT) is the site of the majority of sodium, potassium, chloride, and calcium reabsorption in the nephron, yet only about 10-20% of filtered magnesium is reabsorbed in this segment 8, 27. The PT has a high permeability to water and small ions such as sodium and chloride. These properties allow active sodium reabsorption and its concomitant electrochemical gradients to drive isosmotic reabsorption of many solutes 8. Micropuncture studies in rats report that magnesium, far from being isosmotic, becomes concentrated to a tubular fluid to ultrafilterable (TF/UF) magnesium ratio of about 1.65 in the late PT 28, and the permeability of the PT to magnesium is so low that significant transport only occurs at a TF/UF above 1.9 29. Experimental extracellular fluid volume expansion results in the near absence of net PT magnesium transport 30. The molecular mediator of magnesium reabsorption has not yet been identified, although it is often speculated that magnesium reabsorption occurs through a paracellular shunt. This is in part because PT magnesium reabsorption has an unsaturable, linear relationship with the luminal magnesium concentration 31. Claudin-2 is a cation-selective claudin isoform that greatly increases sodium permeability (PNa) when overexpressed in kidney epithelial cells 32. Due to its high expression in the PT compared with other cation-selective isoforms 33-36, it is a reasonable candidate to mediate PT magnesium transport. Deletion of the claudin-2 gene in mice results in reduced reabsorption of water and solutes in isolated PT segments, but does not result in an increased fractional excretion of magnesium (FEMg) 37. It is certainly possible that, given the diminished role of PT magnesium reabsorption compared with calcium, a PT magnesium leak in claudin-2 knockout mice might be negated by efficient compensation in the downstream nephron. While claudin-10 is also highly expressed in the PT, there are multiple splice variants of claudin-10 with differing charge selectivity and tissue expression, and the anion-selective splice variant claudin-10a is the predominant isoform expressed in the PT of mice 38, 39. Studies in rats reveal that neonatal animals reabsorb about two-thirds of magnesium in the PT, similar to reabsorption of sodium and calcium in this segment 40. Interestingly, claudins-6 and -9 are highly expressed in the PTs of neonatal mice but not in adult mice 41-43. However, both claudin isoforms increase TER and reduce cation permeability when overexpressed in kidney epithelial cells 44. Thus, the molecular facilitators of PT magnesium reabsorption in adult and neonatal mammals remain a mystery.
b. Thick ascending limb
The thick ascending limb of the Loop of Henle (TAL) is the predominant site of magnesium reabsorption in the kidney, reabsorbing approximately 60% of filtered magnesium 28, 31. Unlike the PT, the TAL has a very low permeability to water. Electroneutral transport by the Na+-K+-2Cl− cotransporter (NKCC2) in the TAL is responsible for diluting the tubular fluid and creating the corticomedullary osmotic gradient. At the apical membrane, the electrogenic potassium channel ROMK allows the potassium transported into the cell by NKCC2 to return to the urinary space. Simultaneously, the basolateral chloride channel ClC-Kb allows reabsorbed chloride to enter the bloodstream. These processes combine to produce a large lumen-positive transepithelial voltage 45. Studies in mice suggest that the majority of magnesium and calcium reabsorption occur in the cortical TAL, while very little occurs in the medulla 46. In isolated tubule segments of cortical TAL, magnesium flux is in direct linear correlation to the transepithelial voltage 47, suggesting that magnesium is reabsorbed along the paracellular shunt pathway. The relationship between TAL sodium and magnesium reabsorption is underlined by the fact that loss of function mutations in SLC12A1 (coding for NKCC2), KCNJ1 (coding for ROMK), and CLCNKB or BSND (which code for ClC-Kb and its subunit Barttin, respectively) lead to TAL dysfunction and Bartter syndrome, characterized by magnesium and calcium wasting, polyuria, and sodium wasting 45. Importantly, unlike the previous paracellular processes discussed, paracellular magnesium permeability (PMg) in the TAL is dynamically regulated, independently of active TAL sodium reabsorption.
i. Claudin-16
Considerable progress has been made in the past two decades to elucidate the important role for claudins in human disease, beginning with the identification of mutations in the CLDN16 gene, which codes for the protein claudin-16, by Simon and colleagues in 1999 48. These mutations cause the autosomal recessive disorder familial hypomagnesemia with hypercalciuria and nephrocalcinosis (FHHNC), a severe disorder marked by magnesium and calcium wasting, nephrocalcinosis, and kidney failure in early life. Patients with FHHNC experience resolution of the electrolyte disturbances upon kidney transplantation 48. Sodium wasting does not occur in FHHNC, but magnesium and calcium excretion fails to rise upon furosemide infusion despite an appropriate rise in sodium excretion 25. While claudin-16 was initially hypothesized to directly form the magnesium pore at the tight junction, that idea has come under scrutiny with the findings of subsequent studies in vitro and in vivo. Perhaps due to differing background claudin expression between cell lines, the transport data on claudin-16 is conflicting. In LLC-PK1 porcine kidney cells, expression of claudin-16 leads to reduction of TER and a greater increase in PNa than PMg 49, whereas expression in MDCK C7 cells does not alter PNa or PCa but nearly doubles the PMg 50. Claudin-16 knockdown (KD) mice develop hypomagnesemia, nephrocalcinosis, and a two-fold increase in the kidney excretion of magnesium 51. Surprisingly, isolated TAL segments from claudin-16 KD mice have a reduced transepithelial voltage and reduced permeability to sodium, with the PMg/PNa remaining unchanged 51. This has led to the hypothesis that claudin-16 acts as a sodium channel, allowing paracellular backflux of sodium across the tight junction to increase the lumen-potential, thus increasing the driving force for paracellular magnesium reabsorption. Similar to claudin-16 KD mice, claudin-16 knockout (KO) mice also exhibit hypomagnesemia and hypercalciuria 43. Unlike claudin-16 KD mice, claudin-16 KO TAL segments showed no difference in PNa/PCl but a significant decrease in the PMg/PNa, supporting the initial hypothesis that claudin-16 forms a magnesium pore 43. Clearly, more studies are needed to determine the physiologic function of claudin-16, in vivo.
ii. Claudin-19
In 2006, mutations in CLDN19, which encodes claudin-19, were found to cause a disorder almost identical to FHHNC with the addition of colobomata of the maculae, horizontal nystagmus, myopia, and severe visual impairment 52. Claudin-16 and -19 have the same expression pattern in the kidney as both are expressed in the TAL and the distal convoluted tubule (DCT) 52. In vitro data suggests that claudin-16 and -19 interact and traffic to the tight junction together, and transport studies in LLC-PK1 cells shows the PNa/PCl in cells expressing both claudins to be much higher than either isoform individually 53. Claudin-19 KD mice phenocopy claudin-16 KD mice, with hypomagnesemia and kidney magnesium and calcium wasting. Interestingly, it was found that claudin-19 KD in mice also results in loss of claudin-16 at the TAL tight junction, and vice versa 54. This may partially explain why a number of FHHNC mutations have been found to interrupt the interaction and synergism between claudins-16 and -19, in vitro 53.
iii. Claudin-14
CLDN14 mutations that lead to loss of the encoded protein, claudin-14, cause deafness in humans due to degeneration of cochlear hair cells 55, 56. Claudin-14 increases TER and decreases PNa/PCl in kidney epithelial cells 56. Although claudin-14 was found to be expressed in the kidney, no kidney phenotype was identified initially 56. A surprising finding came in 2009, as a genome wide association study identified SNPs in CLDN14 that associate with increased risk for kidney stones 57. Whereas FHHNC is an exceedingly rare disorder, the risk variants in CLDN14 had a 75% frequency within the Icelandic population studied 57. These mutations are both synonymous and non-exonic 57. In mice, claudin-14 is largely restricted to the TAL and mRNA and protein expression have a direct relationship to dietary magnesium and calcium intake 58, 59. Gong, et al found evidence that claudin-14 interacts with claudin-16 and, in contrast to claudin-19, reduces PNa and PNa/PCl in cultured cells 58. Furthermore, claudin-14 KO mice have a striking decrease in FEMg and FECa on a high calcium diet 58, while transgenic overexpression of claudin-14 in the TAL causes FEMg and FECa to increase 60. These synonymous CLDN14 variants occur at putative microRNA binding sites, and it was supposed that they lead to an increase in function of the protein 58, 60, 61. Given this, it is perhaps surprising that CLDN14 variants that associate with kidney stones are also associated with an increase in serum magnesium, reminiscent of findings in claudin-14 KO mice 58, 62. More recent studies have shown an association of CLDN14 variants with the urine magnesium to urine calcium ratio, further enforcing the clinical relevance of claudin-14 in magnesium balance 59.
iv. Claudin-10
Finally, the cation-selective claudin-10b is highly expressed in the TAL 39. Claudin-10b and claudin-16/19 positive cells appear to be distinct populations, with claudin-16/19 cells predominating in the more magnesium permeable cortical TAL 63. In mice, conditional deletion of the claudin-10 gene in the TAL leads to nephrocalcinosis, hypermagnesemia, and a reduction in FEMg 64. While PNa is reduced, the PMg/PNa and PCa/PNa are greatly increased and the transepithelial voltage is nearly doubled 64. Interpretation of these results is complicated by numerous electrolyte disturbances, volume depletion, and an increase in the expression of claudins-16, -19, and -14 in the TAL 64. Recently, claudin-10 mutations have been discovered in humans as the cause of a rare autosomal recessive tubulopathy, which, similar to the conditional KO mice, includes magnesium retention and kidney failure65-67.
Clearly, claudins and paracellular permeability play a prominent role in TAL physiology and magnesium homeostasis (Figure 2).
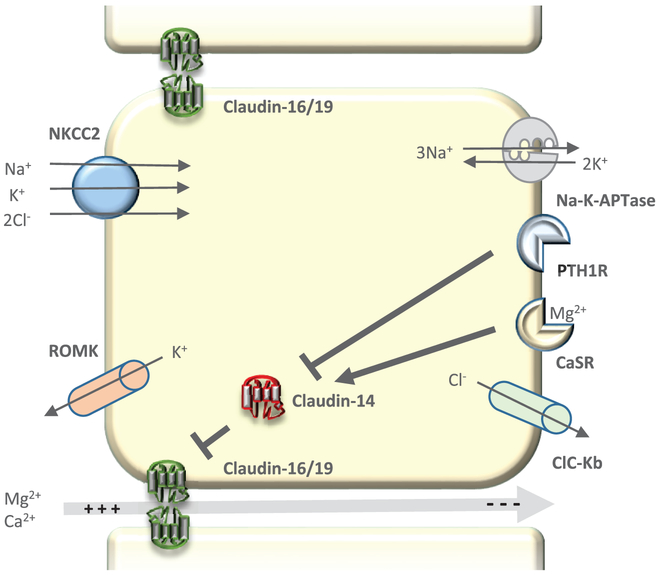
Figure 2. Magnesium transport in the Thick Ascending Limb of the Loop of Henle (TAL).
Magnesium transport in the cortical TAL primarily occurs through the paracellular shunt pathway, driven by a highly positive lumen potential. The lumen potential produced is a result of active sodium transport by NKCC2 at the apical membrane and consequent apical backflux of potassium via ROMK and basolateral chloride reabsorption via Clc-Kb. Paracellular magnesium permeability is increased by claudins-16 and -19 and decreased by claudin-14. Major pathways in the regulation of TAL magnesium transport occur through activation of basolateral receptors CaSR or PTH1R. Claudin-14 expression is decreased by activation of PTH1R and increased by activation of CaSR, thus altering magnesium permeability and reabsorption in the TAL.
c. Distal tubule
The DCT is the final site of magnesium reabsorption in the nephron, transporting about 5% of filtered magnesium 28. As mentioned above, the magnesium channel TRPM6 is mutated in patients with HSH, a disorder that includes kidney magnesium wasting 17. In the kidney, TRPM6 is located along the apical membrane of the entire DCT, colocalizing with the sodium chloride cotransporter (NCC) 68. Underlining the importance of the DCT in magnesium handling, patients with Gitelman Syndrome, caused by loss of function mutations in the gene coding for NCC, as well as NCC KO mice exhibit hypomagnesemia and kidney magnesium wasting 69. Importantly, NCC KO mice have a dramatic reduction in the protein expression of TRPM6 70. The DCT is highly impermeable to water and has a high transepithelial resistance 71, so that the majority of reabsorption in the segment occurs via the transcellular pathway. This includes magnesium, which is often present in luminal concentrations lower than intracellular free magnesium, and transcellular magnesium transport necessitates an electrical driving force. This may be provided by the voltage gated potassium channel Kv1.1, which is coexpressed with TRPM6 in the DCT 72. Mutations in the gene encoding it, KCNA1, cause autosomal dominant hypomagnesemia 72. Once within the cell, magnesium has been proposed to become buffered by a binding protein such as parvalbumin, which is found in the first part of the DCT, or calbindin-D28k, found along the DCT and connecting tubule. Mice placed on a low magnesium diet have increased expression of parvalbumin 73. Genetic deletion of parvalbumin in mice causes kidney salt wasting and increased bone mineral density, but no appreciable difference in urine magnesium, suggesting that parvalbumin is not necessary for DCT magnesium reabsorption 74. Similarly, calbindin D-28k KO mice do not have an increase in serum or urine magnesium 75.
On the basolateral side of DCT cells, magnesium must be actively extruded against its electrochemical gradient. Cyclin M2 is a basolateral protein which is upregulated in mice in response to magnesium depletion 76. Pathogenic mutations in the cyclin M2 gene CNNM2 lead to dominant hypomagnesemia, while common variants in the gene are associated with serum magnesium values 76, 77. While expression of cyclin M2 in X. laevis oocytes suggest it acts as a divalent cation transporter 78, studies in Hek293 cells point to a role as a magnesium sensing sodium channel 76. Thus, the protein mediator of basolateral magnesium extrusion remains unknown. Also at the basolateral side is the inward rectifier potassium channel Kir4.1, which causes a Gitelman-like syndrome in humans when its gene, KCNJ10, is disrupted 79. Kir4.1 is likely to be involved in recycling potassium for maintenance of the Na-K-ATPase and normal DCT function 79 (Figure 3).
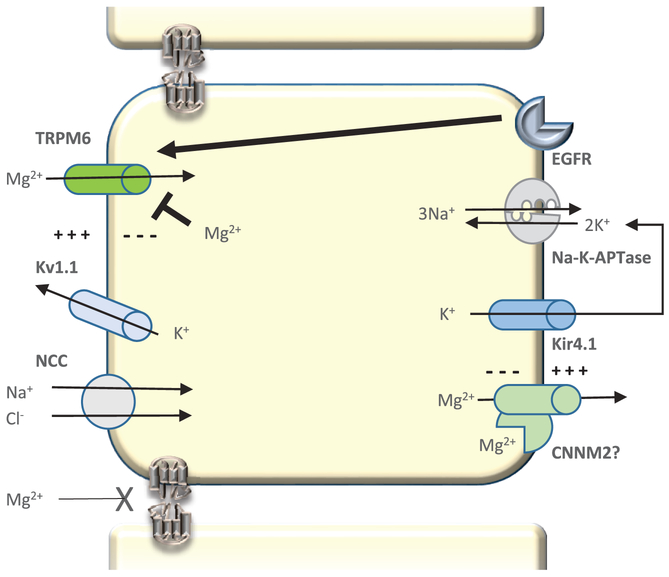
Figure 3. Magnesium transport in the Distal Convoluted Tubule (DCT).
Magnesium transport in the DCT is an active transcellular process. Polarization of the apical membrane by the voltage gated potassium channel Kv1.1 provides the driving force for magnesium to enter the cell via the magnesium channel TRPM6. The molecular mediator of magnesium extrusion at the basolateral membrane remains unknown. Regulation of DCT magnesium transport seems to occur in part by the direct action of intracellular free magnesium. EGFR activation also leads to increased active TRPM6 at the apical membrane.
While the TAL and DCT are well defined by their resident sodium transporters, there is often overlap in some of the above mediators of magnesium reabsorption. For instance, Kirk shows claudin-14 localization in the DCT of human kidney, and Corre shows the same in mice 34, 59. In the human kidney, CNNM2 is found in the TAL as well as the DCT 76. Perhaps the processes of magnesium transport in these segments have some overlap as well, but an important difference is that dysfunction of the DCT leads to hypocalciuria, in contrast to the hypercalciuria that occurs with TAL dysfunction.
IV. Regulation of kidney magnesium transport
Regulation of magnesium transport in the kidney occurs primarily in the TAL and DCT. In the TAL, both magnesium and calcium can activate the calcium-sensing receptor (CaSR) on the basolateral membrane and modulate paracellular magnesium transport 58. In line with this, the urinary excretion of calcium goes up on a high magnesium diet and down on a low magnesium diet 15. The molecular mechanisms by which this occurs have been elegantly and exhaustively shown to be through claudin-14 (Figure 2). Systemic activation of CaSR will cause a reduction of PTH and serum calcium. While claudin-14 expression is increased on a high calcium diet, it is also increased in wild type and thyroparathyroidectomized mice upon administration of the CaSR agonist, cinacalcet 80. Conversely, CaSR antagonism causes a reduction in claudin-14 expression in mice 60 and increases PCa in isolated TAL segments from rats 81. Importantly, while wild type mice acutely respond to CaSR activation or antagonism by increasing or decreasing FEMg (and FECa), respectively, claudin-14 KO showed no response to either treatment 60. The CLDN14 3’-UTR is predicted to interact with microRNAs that decrease in expression in mice receiving a high calcium diet or cultured primary cells exposed to high extracellular calcium 58. These same microRNAs are negatively regulated by CaSR 60. In addition to CaSR, PTH has been found to regulate claudin-14 expression. Cortical TALs of rats respond to PTH by increasing the transport of magnesium 82. Recent work has shown that mice with conditional deletion of the PTH receptor PTH1R in the distal tubule exhibit hypercalciuria and a large increase in claudin-14 expression 83 (Figure 2).
In the DCT, TRPM6 is inhibited directly by intracellular magnesium 68, and its mRNA and protein expression decrease in mice on a high magnesium diet 84(Figure 3). In addition to TRPM6, a number of genes are regulated in response to magnesium levels 73. PTH has also been shown to stimulate magnesium reabsorption in the DCT, but it does not alter TRPM6 expression and the mechanism remains unclear 84. Several Gitelman-like hypomagnesemia syndromes occur in this segment from mutations in regulatory genes- HNF1β, a transcription factor, and FXYDY2, one of its downstream targets 85. Another genetic hypomagnesemia is caused by mutations in EGF, coding for the epidermal growth factor (EGF) 86. Activation of the EGF receptor increases the activation and surface expression of TRPM6 86, and patients on anti-EGFR monoclonal antibody therapy have a much higher risk of developing hypomagnesemia 87 (Figure 3).
In stark contrast to calcium handling, the role of 1,25(OH)2 vitamin D in magnesium homeostasis appears to be minimal at physiologic concentrations 88. Recently, it was found that 14 days of pharmacologic treatment with vitamin D increased serum magnesium and kidney magnesium excretion in mice 15. These changes were not due to increases in intestinal magnesium absorption or changes in mRNA expression of kidney TRPM6 15, and so questions remain regarding the role of vitamin D in magnesium balance.
V. Clinical summary.
Magnesium is an important mineral for human health and levels are kept constant primarily by the gastrointestinal tract and kidneys
The proximal tubule reabsorbs 10-20% of filtered magnesium
The thick ascending limb is responsible for the majority of kidney magnesium reabsorption, and regulation of this transport occurs in large part by modulation of tight junction permeability
The distal convoluted tubule reabsorbs 5% of magnesium through a transcellular pathway that is disrupted in a number of tubulopathies
Acknowledgments
Grants: Supported in part by NIH grant F30DK109605.
Footnotes
Financial disclosures: None
References
- 1.Wolf FI, Trapani V. Cell (patho)physiology of magnesium. Clin Sci (Lond). 2008;114(1):27–35. doi: 10.1042/CS20070129. PubMed PMID: 18047467. [DOI] [PubMed] [Google Scholar]
- 2.Garfinkel L, Garfinkel D. Calculation of free-Mg2+ concentration in adenosine 5'-triphosphate containing solutions in vitro and in vivo. Biochemistry. 1984;23(15):3547–52. PubMed PMID: 6331847. [DOI] [PubMed] [Google Scholar]
- 3.Vandenberg CA. Inward rectification of a potassium channel in cardiac ventricular cells depends on internal magnesium ions. Proc Natl Acad Sci U S A. 1987;84(8):2560–4. PubMed PMID: 2436236; PMCID: PMC304694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lansman JB, Hess P, Tsien RW. Blockade of current through single calcium channels by Cd2+, Mg2+, and Ca2+. Voltage and concentration dependence of calcium entry into the pore. J Gen Physiol. 1986;88(3):321–47. PubMed PMID: 2428920; PMCID: PMC2228830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Quigley GJ, Teeter MM, Rich A. Structural analysis of spermine and magnesium ion binding to yeast phenylalanine transfer RNA. Proc Natl Acad Sci U S A. 1978;75(1):64–8. PubMed PMID: 343112; PMCID: PMC411184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Topf JM, Murray PT. Hypomagnesemia and hypermagnesemia. Rev Endocr Metab Disord. 2003;4(2):195–206. PubMed PMID: 12766548. [DOI] [PubMed] [Google Scholar]
- 7.Anast CS, Mohs JM, Kaplan SL, Burns TW. Evidence for parathyroid failure in magnesium deficiency. Science. 1972;177(4049):606–8. PubMed PMID: 5049304. [DOI] [PubMed] [Google Scholar]
- 8.Taal MW, Brenner BM, Rector FC. Brenner & Rector's the kidney. 9th ed. Philadelphia, PA: Elsevier/Saunders; 2012. [Google Scholar]
- 9.Alfrey AC, Miller NL. Bone magnesium pools in uremia. J Clin Invest. 1973;52(12):3019–27. doi: 10.1172/JCI107500. PubMed PMID: 4584344; PMCID: PMC302576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schweigel M, Martens H. Magnesium transport in the gastrointestinal tract. Front Biosci. 2000;5:D666–77. PubMed PMID: 10922297. [DOI] [PubMed] [Google Scholar]
- 11.Brierley GP, Bachmann E, Green DE. Active transport of inorganic phosphate and magnesium ions by beef heart mitochondria. Proc Natl Acad Sci U S A. 1962;48:1928–35. PubMed PMID: 14015424; PMCID: PMC221100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fine KD, Santa Ana CA, Porter JL, Fordtran JS. Intestinal absorption of magnesium from food and supplements. J Clin Invest. 1991;88(2):396–402. doi: 10.1172/JCI115317. PubMed PMID: 1864954; PMCID: PMC295344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marxhall DH, Nordin BE, Speed R. Calcium, phosphorus and magnesium requirement. Proc Nutr Soc. 1976;35(2):163–73. PubMed PMID: 972869. [DOI] [PubMed] [Google Scholar]
- 14.Standing Committee on the Scientific Evaluation of Dietary Reference Intakes FaNB, Institute of Medicine Magnesium. Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride. Washington (DC) 1997. [Google Scholar]
- 15.Lameris AL, Nevalainen PI, Reijnen D, Simons E, Eygensteyn J, Monnens L, Bindels RJM, Hoenderop JGJ. Segmental transport of Ca2+ and Mg2+ along the gastrointestinal tract. American Journal of Physiology - Gastrointestinal and Liver Physiology. 2015;308(3):G206–G16. doi: 10.1152/ajpgi.00093.2014. [DOI] [PubMed] [Google Scholar]
- 16.Chubanov V, Schlingmann KP, Waring J, Heinzinger J, Kaske S, Waldegger S, Mederos y Schnitzler M, Gudermann T. Hypomagnesemia with secondary hypocalcemia due to a missense mutation in the putative pore-forming region of TRPM6. J Biol Chem. 2007;282(10):7656–67. doi: 10.1074/jbc.M611117200. PubMed PMID: 17197439. [DOI] [PubMed] [Google Scholar]
- 17.Schlingmann KP, Sassen MC, Weber S, Pechmann U, Kusch K, Pelken L, Lotan D, Syrrou M, Prebble JJ, Cole DE, Metzger DL, Rahman S, Tajima T, Shu SG, Waldegger S, Seyberth HW, Konrad M. Novel TRPM6 mutations in 21 families with primary hypomagnesemia and secondary hypocalcemia. J Am Soc Nephrol. 2005;16(10):3061–9. doi: 10.1681/ASN.2004110989. PubMed PMID: 16107578. [DOI] [PubMed] [Google Scholar]
- 18.Hou J, Rajagopal M, Yu AS. Claudins and the kidney. Annu Rev Physiol. 2013;75:479–501. doi: 10.1146/annurev-physiol-030212-183705. PubMed PMID: 23140368; PMCID: PMC3759403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Powell DW. Barrier function of epithelia. Am J Physiol. 1981;241(4):G275–88. PubMed PMID: 7032321. [DOI] [PubMed] [Google Scholar]
- 20.Fujita H, Chiba H, Yokozaki H, Sakai N, Sugimoto K, Wada T, Kojima T, Yamashita T, Sawada N. Differential expression and subcellular localization of claudin-7, -8, -12, -13, and -15 along the mouse intestine. J Histochem Cytochem. 2006;54(8):933–44. doi: 10.1369/jhc.6A6944.2006. PubMed PMID: 16651389. [DOI] [PubMed] [Google Scholar]
- 21.Van Itallie CM, Fanning AS, Anderson JM. Reversal of charge selectivity in cation or anion-selective epithelial lines by expression of different claudins. Am J Physiol Renal Physiol. 2003;285(6):F1078–84. doi: 10.1152/ajprenal.00116.2003. PubMed PMID: 13129853. [DOI] [PubMed] [Google Scholar]
- 22.Fujita H, Sugimoto K, Inatomi S, Maeda T, Osanai M, Uchiyama Y, Yamamoto Y, Wada T, Kojima T, Yokozaki H, Yamashita T, Kato S, Sawada N, Chiba H. Tight junction proteins claudin-2 and -12 are critical for vitamin D-dependent Ca2+ absorption between enterocytes. Mol Biol Cell. 2008;19(5):1912–21. doi: 10.1091/mbc.E07-09-0973. PubMed PMID: 18287530; PMCID: 2366872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fordtran JS, Rector FC Jr., Carter NW. The mechanisms of sodium absorption in the human small intestine. J Clin Invest. 1968;47(4):884–900. doi: 10.1172/JCI105781. PubMed PMID: 5641624; PMCID: PMC297237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Larsson MH, Simren M, Thomas EA, Bornstein JC, Lindstrom E, Sjovall H. Elevated motility-related transmucosal potential difference in the upper small intestine in the irritable bowel syndrome. Neurogastroenterol Motil. 2007;19(10):812–20. doi: 10.1111/j.1365-2982.2007.00941.x. PubMed PMID: 17883433. [DOI] [PubMed] [Google Scholar]
- 25.Blanchard A, Jeunemaitre X, Coudol P, Dechaux M, Froissart M, May A, Demontis R, Fournier A, Paillard M, Houillier P. Paracellin-1 is critical for magnesium and calcium reabsorption in the human thick ascending limb of Henle. Kidney Int. 2001;59(6):2206–15. doi: 10.1046/j.1523-1755.2001.00736.x. PubMed PMID: 11380823. [DOI] [PubMed] [Google Scholar]
- 26.Walser M Ion association. VI. Interactions between calcium, magnesium, inorganic phosphate, citrate and protein in normal human plasma. J Clin Invest. 1961;40:723–30. doi: 10.1172/JCI104306. PubMed PMID: 13782899; PMCID: PMC373170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Le Grimellec C, Roinel N, Morel F. Simultaneous Mg, Ca, P,K,Na and Cl analysis in rat tubular fluid. II. During acute Mg plasma loading. Pflugers Arch. 1973;340(3):197–210. PubMed PMID: 4736757. [DOI] [PubMed] [Google Scholar]
- 28.Brunette MG, Vigneault N, Carriere S. Micropuncture study of magnesium transport along the nephron in the young rat. Am J Physiol. 1974;227(4):891–6. PubMed PMID: 4429138. [DOI] [PubMed] [Google Scholar]
- 29.Le Grimellec C Micropuncture study along the proximal convoluted tubule. Electrolyte reabsorption in first convolutions. Pflugers Arch. 1975;354(2):133–50. PubMed PMID: 1167675. [DOI] [PubMed] [Google Scholar]
- 30.Poujeol P, Chabardes D, Roinel N, De Rouffignac C. Influence of extracellular fluid volume expansion on magnesium, calcium and phosphate handling along the rat nephron. Pflugers Arch. 1976;365(2-3):203–11. PubMed PMID: 988560. [DOI] [PubMed] [Google Scholar]
- 31.Wong NL, Dirks JH, Quamme GA. Tubular reabsorptive capacity for magnesium in the dog kidney. Am J Physiol. 1983;244(1):F78–83. PubMed PMID: 6849387. [DOI] [PubMed] [Google Scholar]
- 32.Yu AS, Cheng MH, Angelow S, Gunzel D, Kanzawa SA, Schneeberger EE, Fromm M, Coalson RD. Molecular basis for cation selectivity in claudin-2-based paracellular pores: identification of an electrostatic interaction site. J Gen Physiol. 2009;133(1):111–27. doi: 10.1085/jgp.200810154. PubMed PMID: 19114638; PMCID: 2606938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee JW, Chou CL, Knepper MA. Deep Sequencing in Microdissected Renal Tubules Identifies Nephron Segment-Specific Transcriptomes. J Am Soc Nephrol. 2015. doi: 10.1681/ASN.2014111067. PubMed PMID: 25817355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kirk A, Campbell S, Bass P, Mason J, Collins J. Differential expression of claudin tight junction proteins in the human cortical nephron. Nephrol Dial Transplant. 2010;25(7):2107–19. doi: 10.1093/ndt/gfq006. PubMed PMID: 20124215; PMCID: 2891746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Enck AH, Berger UV, Yu AS. Claudin-2 is selectively expressed in proximal nephron in mouse kidney. Am J Physiol Renal Physiol. 2001;281(5):F966–74. PubMed PMID: 11592954. [DOI] [PubMed] [Google Scholar]
- 36.Kiuchi-Saishin Y, Gotoh S, Furuse M, Takasuga A, Tano Y, Tsukita S. Differential expression patterns of claudins, tight junction membrane proteins, in mouse nephron segments. J Am Soc Nephrol. 2002;13(4):875–86. PubMed PMID: 11912246. [DOI] [PubMed] [Google Scholar]
- 37.Muto S, Hata M, Taniguchi J, Tsuruoka S, Moriwaki K, Saitou M, Furuse K, Sasaki H, Fujimura A, Imai M, Kusano E, Tsukita S, Furuse M. Claudin-2-deficient mice are defective in the leaky and cation-selective paracellular permeability properties of renal proximal tubules. Proc Natl Acad Sci U S A. 2010;107(17):8011–6. doi: 10.1073/pnas.0912901107. PubMed PMID: 20385797; PMCID: 2867900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Van Itallie CM, Rogan S, Yu A, Vidal LS, Holmes J, Anderson JM. Two splice variants of claudin-10 in the kidney create paracellular pores with different ion selectivities. Am J Physiol Renal Physiol. 2006;291(6):F1288–99. doi: 10.1152/ajprenal.00138.2006. PubMed PMID: 16804102. [DOI] [PubMed] [Google Scholar]
- 39.Gunzel D, Stuiver M, Kausalya PJ, Haisch L, Krug SM, Rosenthal R, Meij IC, Hunziker W, Fromm M, Muller D. Claudin-10 exists in six alternatively spliced isoforms that exhibit distinct localization and function. J Cell Sci. 2009;122(Pt 10):1507–17. doi: 10.1242/jcs.040113. PubMed PMID: 19383724. [DOI] [PubMed] [Google Scholar]
- 40.Lelievre-Pegorier M, Merlet-Benichou C, Roinel N, de Rouffignac C. Developmental pattern of water and electrolyte transport in rat superficial nephrons. Am J Physiol. 1983;245(1):F15–21. PubMed PMID: 6869534. [DOI] [PubMed] [Google Scholar]
- 41.Abuazza G, Becker A, Williams SS, Chakravarty S, Truong HT, Lin F, Baum M. Claudins 6, 9, and 13 are developmentally expressed renal tight junction proteins. Am J Physiol Renal Physiol. 2006;291(6):F1132–41. doi: 10.1152/ajprenal.00063.2006. PubMed PMID: 16774906; PMCID: PMC4131871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schwab K, Patterson LT, Aronow BJ, Luckas R, Liang HC, Potter SS. A catalogue of gene expression in the developing kidney. Kidney Int. 2003;64(5):1588–604. doi: 10.1046/j1523-1755.2003.00276.x. PubMed PMID: 14531791. [DOI] [PubMed] [Google Scholar]
- 43.Will C, Breiderhoff T, Thumfart J, Stuiver M, Kopplin K, Sommer K, Gunzel D, Querfeld U, Meij IC, Shan Q, Bleich M, Willnow TE, Muller D. Targeted deletion of murine Cldn16 identifies extra- and intrarenal compensatory mechanisms of Ca2+ and Mg2+ wasting. Am J Physiol Renal Physiol. 2010;298(5):F1152–61. doi: 10.1152/ajprenal.00499.2009. PubMed PMID: 20147368. [DOI] [PubMed] [Google Scholar]
- 44.Sas D, Hu M, Moe OW, Baum M. Effect of claudins 6 and 9 on paracellular permeability in MDCK II cells. Am J Physiol Regul Integr Comp Physiol. 2008;295(5):R1713–9. doi: 10.1152/ajpregu.90596.2008. PubMed PMID: 18784328; PMCID: PMC2584851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Seyberth HW, Schlingmann KP. Bartter- and Gitelman-like syndromes: salt-losing tubulopathies with loop or DCT defects. Pediatr Nephrol. 2011;26(10):1789–802. doi: 10.1007/s00467-011-1871-4. PubMed PMID: 21503667; PMCID: PMC3163795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bailly C, Imbert-Teboul M, Roinel N, Amiel C. Isoproterenol increases Ca, Mg, and NaCl reabsorption in mouse thick ascending limb. Am J Physiol. 1990;258(5 Pt 2):F1224–31. PubMed PMID: 2337151. [DOI] [PubMed] [Google Scholar]
- 47.Di Stefano A, Roinel N, de Rouffignac C, Wittner M. Transepithelial Ca2+ and Mg2+ transport in the cortical thick ascending limb of Henle's loop of the mouse is a voltage-dependent process. Ren Physiol Biochem. 1993;16(4):157–66. PubMed PMID: 7689239. [DOI] [PubMed] [Google Scholar]
- 48.Simon DB, Lu Y, Choate KA, Velazquez H, Al-Sabban E, Praga M, Casari G, Bettinelli A, Colussi G, Rodriguez-Soriano J, McCredie D, Milford D, Sanjad S, Lifton RP. Paracellin-1, a renal tight junction protein required for paracellular Mg2+ resorption. Science. 1999;285(5424):103–6. PubMed PMID: 10390358. [DOI] [PubMed] [Google Scholar]
- 49.Hou J, Paul DL, Goodenough DA. Paracellin-1 and the modulation of ion selectivity of tight junctions. J Cell Sci. 2005;118(Pt 21):5109–18. doi: 10.1242/jcs.02631. PubMed PMID: 16234325. [DOI] [PubMed] [Google Scholar]
- 50.Gunzel D, Amasheh S, Pfaffenbach S, Richter JF, Kausalya PJ, Hunziker W, Fromm M. Claudin-16 affects transcellular Cl- secretion in MDCK cells. J Physiol. 2009;587(Pt 15):3777–93. doi: 10.1113/jphysiol.2009.173401. PubMed PMID: 19528248; PMCID: PMC2746610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hou J, Shan Q, Wang T, Gomes AS, Yan Q, Paul DL, Bleich M, Goodenough DA. Transgenic RNAi depletion of claudin-16 and the renal handling of magnesium. J Biol Chem. 2007;282(23):17114–22. doi: 10.1074/jbc.M700632200. PubMed PMID: 17442678. [DOI] [PubMed] [Google Scholar]
- 52.Konrad M, Schaller A, Seelow D, Pandey AV, Waldegger S, Lesslauer A, Vitzthum H, Suzuki Y, Luk JM, Becker C, Schlingmann KP, Schmid M, Rodriguez-Soriano J, Ariceta G, Cano F, Enriquez R, Juppner H, Bakkaloglu SA, Hediger MA, Gallati S, Neuhauss SC, Nurnberg P, Weber S. Mutations in the tight-junction gene claudin 19 (CLDN19) are associated with renal magnesium wasting, renal failure, and severe ocular involvement. Am J Hum Genet. 2006;79(5):949–57. doi: 10.1086/508617. PubMed PMID: 17033971; PMCID: PMC1698561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hou J, Renigunta A, Konrad M, Gomes AS, Schneeberger EE, Paul DL, Waldegger S, Goodenough DA. Claudin-16 and claudin-19 interact and form a cation-selective tight junction complex. J Clin Invest. 2008;118(2):619–28. doi: 10.1172/JCI33970. PubMed PMID: 18188451; PMCID: PMC2176193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hou J, Renigunta A, Gomes AS, Hou M, Paul DL, Waldegger S, Goodenough DA. Claudin-16 and claudin-19 interaction is required for their assembly into tight junctions and for renal reabsorption of magnesium. Proc Natl Acad Sci U S A. 2009;106(36):15350–5. doi: 10.1073/pnas.0907724106. PubMed PMID: 19706394; PMCID: 2741254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wilcox ER, Burton QL, Naz S, Riazuddin S, Smith TN, Ploplis B, Belyantseva I, Ben-Yosef T, Liburd NA, Morell RJ, Kachar B, Wu DK, Griffith AJ, Riazuddin S, Friedman TB. Mutations in the gene encoding tight junction claudin-14 cause autosomal recessive deafness DFNB29. Cell. 2001;104(1):165–72. PubMed PMID: 11163249. [DOI] [PubMed] [Google Scholar]
- 56.Ben-Yosef T, Belyantseva IA, Saunders TL, Hughes ED, Kawamoto K, Van Itallie CM, Beyer LA, Halsey K, Gardner DJ, Wilcox ER, Rasmussen J, Anderson JM, Dolan DF, Forge A, Raphael Y, Camper SA, Friedman TB. Claudin 14 knockout mice, a model for autosomal recessive deafness DFNB29, are deaf due to cochlear hair cell degeneration. Hum Mol Genet. 2003;12(16):2049–61. PubMed PMID: 12913076. [DOI] [PubMed] [Google Scholar]
- 57.Thorleifsson G, Holm H, Edvardsson V, Walters GB, Styrkarsdottir U, Gudbjartsson DF, Sulem P, Halldorsson BV, de Vegt F, d'Ancona FC, den Heijer M, Franzson L, Christiansen C, Alexandersen P, Rafnar T, Kristjansson K, Sigurdsson G, Kiemeney LA, Bodvarsson M, Indridason OS, Palsson R, Kong A, Thorsteinsdottir U, Stefansson K. Sequence variants in the CLDN14 gene associate with kidney stones and bone mineral density. Nat Genet. 2009;41 (8):926–30. doi: 10.1038/ng.404. PubMed PMID: 19561606. [DOI] [PubMed] [Google Scholar]
- 58.Gong Y, Renigunta V, Himmerkus N, Zhang J, Renigunta A, Bleich M, Hou J. Claudin-14 regulates renal Ca(+)(+) transport in response to CaSR signalling via a novel microRNA pathway. EMBO J. 2012;31(8):1999–2012. doi: 10.1038/emboj.2012.49. PubMed PMID: 22373575; PMCID: 3343334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Corre T, Olinger E, Harris SE, Traglia M, Ulivi S, Lenarduzzi S, Belge H, Youhanna S, Tokonami N, Bonny O, Houillier P, Polasek O, Deary IJ, Starr JM, Toniolo D, Gasparini P, Vollenweider P, Hayward C, Bochud M, Devuyst O. Common variants in CLDN14 are associated with differential excretion of magnesium over calcium in urine. Pflugers Arch. 2017;469(1):91–103. doi: 10.1007/s00424-016-1913-7. PubMed PMID: 27915449. [DOI] [PubMed] [Google Scholar]
- 60.Gong Y, Hou J. Claudin-14 underlies Ca(+)(+)-sensing receptor-mediated Ca(+)(+) metabolism via NFAT-microRNA-based mechanisms. J Am Soc Nephrol. 2014;25(4):745–60. doi: 10.1681/ASN.2013050553. PubMed PMID: 24335970; PMCID: 3968499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gong Y, Himmerkus N, Plain A, Bleich M, Hou J. Epigenetic regulation of microRNAs controlling CLDN14 expression as a mechanism for renal calcium handling. J Am Soc Nephrol. 2015;26(3):663–76. doi: 10.1681/ASN.2014020129. PubMed PMID: 25071082; PMCID: 4341477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Oddsson A, Sulem P, Helgason H, Edvardsson VO, Thorleifsson G, Sveinbjornsson G, Haraldsdottir E, Eyjolfsson GI, Sigurdardottir O, Olafsson I, Masson G, Holm H, Gudbjartsson DF, Thorsteinsdottir U, Indridason OS, Palsson R, Stefansson K. Common and rare variants associated with kidney stones and biochemical traits. Nat Commun. 2015;6:7975. doi: 10.1038/ncomms8975. PubMed PMID: 26272126; PMCID: PMC4557269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Milatz S, Himmerkus N, Wulfmeyer VC, Drewell H, Mutig K, Hou J, Breiderhoff T, Muller D, Fromm M, Bleich M, Gunzel D. Mosaic expression of claudins in thick ascending limbs of Henle results in spatial separation of paracellular Na+ and Mg2+ transport. Proc Natl Acad Sci U S A. 2017;114(2):E219–E27. doi: 10.1073/pnas.1611684114. PubMed PMID: 28028216; PMCID: PMC5240732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Breiderhoff T, Himmerkus N, Stuiver M, Mutig K, Will C, Meij IC, Bachmann S, Bleich M, Willnow TE, Muller D. Deletion of claudin-10 (Cldn10) in the thick ascending limb impairs paracellular sodium permeability and leads to hypermagnesemia and nephrocalcinosis. Proc Natl Acad Sci U S A. 2012;109(35):14241–6. doi: 10.1073/pnas.1203834109. PubMed PMID: 22891322; PMCID: 3435183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bongers E, Shelton LM, Milatz S, Verkaart S, Bech AP, Schoots J, Cornelissen EAM, Bleich M, Hoenderop JGJ, Wetzels JFM, Lugtenberg D, Nijenhuis T. A Novel Hypokalemic-Alkalotic Salt-Losing Tubulopathy in Patients with CLDN10 Mutations. J Am Soc Nephrol. 2017;28(10):3118–28. doi: 10.1681/ASN.2016080881. PubMed PMID: 28674042; PMCID: PMC5619954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Klar J, Piontek J, Milatz S, Tariq M, Jameel M, Breiderhoff T, Schuster J, Fatima A, Asif M, Sher M, Mabert K, Fromm A, Baig SM, Gunzel D, Dahl N. Altered paracellular cation permeability due to a rare CLDN10B variant causes anhidrosis and kidney damage. PLoS Genet. 2017;13(7):e1006897. doi: 10.1371/journal.pgen.1006897. PubMed PMID: 28686597; PMCID: PMC5521874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hadj-Rabia S, Brideau G, Al-Sarraj Y, Maroun RC, Figueres ML, Leclerc-Mercier S, Olinger E, Baron S, Chaussain C, Nochy D, Taha RZ, Knebelmann B, Joshi V, Curmi PA, Kambouris M, Vargas-Poussou R, Bodemer C, Devuyst O, Houillier P, El-Shanti H. Multiplex epithelium dysfunction due to CLDN10 mutation: the HELIX syndrome. Genet Med. 2017. doi: 10.1038/gim.2017.71. PubMed PMID: 28771254. [DOI] [PubMed] [Google Scholar]
- 68.Voets T, Nilius B, Hoefs S, van der Kemp AW, Droogmans G, Bindels RJ, Hoenderop JG. TRPM6 forms the Mg2+ influx channel involved in intestinal and renal Mg2+ absorption. J Biol Chem. 2004;279(1):19–25. doi: 10.1074/jbc.M311201200. PubMed PMID: 14576148. [DOI] [PubMed] [Google Scholar]
- 69.Loffing J, Vallon V, Loffing-Cueni D, Aregger F, Richter K, Pietri L, Bloch-Faure M, Hoenderop JG, Shull GE, Meneton P, Kaissling B. Altered renal distal tubule structure and renal Na(+) and Ca(2+) handling in a mouse model for Gitelman's syndrome. J Am Soc Nephrol. 2004;15(9):2276–88. doi: 10.1097/01.ASN.0000138234.18569.63. PubMed PMID: 15339977. [DOI] [PubMed] [Google Scholar]
- 70.Nijenhuis T, Vallon V, van der Kemp AW, Loffing J, Hoenderop JG, Bindels RJ. Enhanced passive Ca2+ reabsorption and reduced Mg2+ channel abundance explains thiazide-induced hypocalciuria and hypomagnesemia. J Clin Invest. 2005;115(6):1651–8. doi: 10.1172/JCI24134. PubMed PMID: 15902302; PMCID: 1090474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Greger R, Velazquez H. The cortical thick ascending limb and early distal convoluted tubule in the urinary concentrating mechanism. Kidney Int. 1987;31(2):590–6. PubMed PMID: 3550228. [DOI] [PubMed] [Google Scholar]
- 72.Glaudemans B, van der Wijst J, Scola RH, Lorenzoni PJ, Heister A, van der Kemp AW, Knoers NV, Hoenderop JG, Bindels RJ. A missense mutation in the Kv1.1 voltage-gated potassium channel-encoding gene KCNA1 is linked to human autosomal dominant hypomagnesemia. J Clin Invest. 2009;119(4):936–42. doi: 10.1172/JCI36948. PubMed PMID: 19307729; PMCID: PMC2662556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.de Baaij JH, Groot Koerkamp MJ, Lavrijsen M, van Zeeland F, Meijer H, Holstege FC, Bindels RJ, Hoenderop JG. Elucidation of the distal convoluted tubule transcriptome identifies new candidate genes involved in renal Mg(2+) handling. Am J Physiol Renal Physiol. 2013;305(11):F1563–73. doi: 10.1152/ajprenal.00322.2013. PubMed PMID: 24089412. [DOI] [PubMed] [Google Scholar]
- 74.Belge H, Gailly P, Schwaller B, Loffing J, Debaix H, Riveira-Munoz E, Beauwens R, Devogelaer JP, Hoenderop JG, Bindels RJ, Devuyst O. Renal expression of parvalbumin is critical for NaCl handling and response to diuretics. Proc Natl Acad Sci U S A. 2007;104(37):14849–54. doi: 10.1073/pnas.0702810104. PubMed PMID: 17804801; PMCID: 1976223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lee CT, Ng HY, Lee YT, Lai LW, Lien YH. The role of calbindin-D28k on renal calcium and magnesium handling during treatment with loop and thiazide diuretics. Am J Physiol Renal Physiol. 2016;310(3):F230–6. doi: 10.1152/ajprenal.00057.2015. PubMed PMID: 26582761; PMCID: PMC4888563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Stuiver M, Lainez S, Will C, Terryn S, Gunzel D, Debaix H, Sommer K, Kopplin K, Thumfart J, Kampik NB, Querfeld U, Willnow TE, Nemec V, Wagner CA, Hoenderop JG, Devuyst O, Knoers NV, Bindels RJ, Meij IC, Muller D. CNNM2, encoding a basolateral protein required for renal Mg2+ handling, is mutated in dominant hypomagnesemia. Am J Hum Genet. 2011;88(3):333–43. doi: 10.1016/j.ajhg.2011.02.005. PubMed PMID: 21397062; PMCID: PMC3059432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Meyer TE, Verwoert GC, Hwang SJ, Glazer NL, Smith AV, van Rooij FJ, Ehret GB, Boerwinkle E, Felix JF, Leak TS, Harris TB, Yang Q, Dehghan A, Aspelund T, Katz R, Homuth G, Kocher T, Rettig R, Ried JS, Gieger C, Prucha H, Pfeufer A, Meitinger T, Coresh J, Hofman A, Sarnak MJ, Chen YD, Uitterlinden AG, Chakravarti A, Psaty BM, van Duijn CM, Kao WH, Witteman JC, Gudnason V, Siscovick DS, Fox CS, Kottgen A, Genetic Factors for Osteoporosis C, Meta Analysis of G, Insulin Related Traits C. Genome-wide association studies of serum magnesium, potassium, and sodium concentrations identify six Loci influencing serum magnesium levels. PLoS Genet. 2010;6(8). doi: 10.1371/journal.pgen.1001045. PubMed PMID: 20700443; PMCID: PMC2916845 Affymetrix, a role that is managed by the Committee on Conflict of Interest of the Johns Hopkins University School of Medicine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Goytain A, Quamme GA. Functional characterization of ACDP2 (ancient conserved domain protein), a divalent metal transporter. Physiol Genomics. 2005;22(3):382–9. doi: 10.1152/physiolgenomics.00058.2005. PubMed PMID: 15899945. [DOI] [PubMed] [Google Scholar]
- 79.Bockenhauer D, Feather S, Stanescu HC, Bandulik S, Zdebik AA, Reichold M, Tobin J, Lieberer E, Sterner C, Landoure G, Arora R, Sirimanna T, Thompson D, Cross JH, van't Hoff W, Al Masri O, Tullus K, Yeung S, Anikster Y, Klootwijk E, Hubank M, Dillon MJ, Heitzmann D, Arcos-Burgos M, Knepper MA, Dobbie A, Gahl WA, Warth R, Sheridan E, Kleta R. Epilepsy, ataxia, sensorineural deafness, tubulopathy, and KCNJ10 mutations. N Engl J Med. 2009;360(19):1960–70. doi: 10.1056/NEJMoa0810276. PubMed PMID: 19420365; PMCID: PMC3398803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dimke H, Desai P, Borovac J, Lau A, Pan W, Alexander RT. Activation of the Ca(2+)-sensing receptor increases renal claudin-14 expression and urinary Ca(2+) excretion. Am J Physiol Renal Physiol. 2013;304(6):F761–9. doi: 10.1152/ajprenal.00263.2012. PubMed PMID: 23283989; PMCID: PMC4959880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Loupy A, Ramakrishnan SK, Wootla B, Chambrey R, de la Faille R, Bourgeois S, Bruneval P, Mandet C, Christensen EI, Faure H, Cheval L, Laghmani K, Collet C, Eladari D, Dodd RH, Ruat M, Houillier P. PTH-independent regulation of blood calcium concentration by the calcium-sensing receptor. J Clin Invest. 2012;122(9):3355–67. doi: 10.1172/JCI57407. PubMed PMID: 22886306; PMCID: PMC3428075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shareghi GR, Agus ZS. Magnesium transport in the cortical thick ascending limb of Henle's loop of the rabbit. J Clin Invest. 1982;69(4):759–69. PubMed PMID: 7076846; PMCID: PMC370129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sato T, Courbebaisse M, Ide N, Fan Y, Hanai JI, Kaludjerovic J, Densmore MJ, Yuan Q, Toka HR, Pollak MR, Hou J, Lanske B. Parathyroid hormone controls paracellular Ca2+ transport in the thick ascending limb by regulating the tight-junction protein Claudin14. Proc Natl Acad Sci U S A. 2017;114(16):E3344–E53. doi: 10.1073/pnas.1616733114. PubMed PMID: 28373577; PMCID: PMC5402431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Groenestege WM, Hoenderop JG, van den Heuvel L, Knoers N, Bindels RJ. The epithelial Mg2+ channel transient receptor potential melastatin 6 is regulated by dietary Mg2+ content and estrogens. J Am Soc Nephrol. 2006;17(4):1035–43. doi: 10.1681/ASN.2005070700. PubMed PMID: 16524949. [DOI] [PubMed] [Google Scholar]
- 85.Ferre S, Veenstra GJ, Bouwmeester R, Hoenderop JG, Bindels RJ. HNF-1B specifically regulates the transcription of the gammaa-subunit of the Na+/K+-ATPase. Biochem Biophys Res Commun. 2011;404(1):284–90. doi: 10.1016/j.bbrc.2010.11.108. PubMed PMID: 21130072. [DOI] [PubMed] [Google Scholar]
- 86.Thebault S, Alexander RT, Tiel Groenestege WM, Hoenderop JG, Bindels RJ. EGF increases TRPM6 activity and surface expression. J Am Soc Nephrol. 2009;20(1):78–85. doi: 10.1681/ASN.2008030327. PubMed PMID: 19073827; PMCID: PMC2615736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Petrelli F, Borgonovo K, Cabiddu M, Ghilardi M, Barni S. Risk of anti-EGFR monoclonal antibody-related hypomagnesemia: systematic review and pooled analysis of randomized studies. Expert Opin Drug Saf. 2012;11 Suppl 1:S9–19. doi: 10.1517/14740338.2011.606213. PubMed PMID: 21843103. [DOI] [PubMed] [Google Scholar]
- 88.Hardwick LL, Jones MR, Brautbar N, Lee DB. Magnesium absorption: mechanisms and the influence of vitamin D, calcium and phosphate. J Nutr. 1991;121(1):13–23. PubMed PMID: 1992050. [DOI] [PubMed] [Google Scholar]
- 89.Carney SL, Wong NL, Quamme GA, Dirks JH. Effect of magnesium deficiency on renal magnesium and calcium transport in the rat. J Clin Invest. 1980;65(1):180–8. doi: 10.1172/JCI109649. PubMed PMID: 7350197; PMCID: PMC371353. [DOI] [PMC free article] [PubMed] [Google Scholar]