Abstract
Background: Ventricular arrhythmias are one of the main causes of sudden death in cardiac sarcoidosis (CS). Little is known about the efficacy of corticosteroid therapy for ventricular arrhythmias in CS.
Methods: Thirty‐one CS patients presenting premature ventricular contractions (PVCs, ≥300/day) were investigated. Fourteen patients had nonsustained ventricular tachycardia (NSVT). All of patients were treated with corticosteroid, and the initial dosage is 30 mg/day of prednisone, which was tapered over a period of 6 months to a maintenance dosage of 10 mg/day. Twenty‐four hour Holter monitoring, signal averaged electrocardiography (SAECG), echocardiography, gallium‐67 scintigraphy, serum angiotensin converting enzyme (ACE) and plasma B‐type natriuretic peptide (BNP) concentrations were assessed before and after corticosteroid therapy.
Results: As a whole, there were no significant differences in the number of PVCs and in the prevalence of NSVT before and after steroid therapy. However, the less advanced LV dysfunction patients (EF ≥ 35%, n = 17) showed significant reduction in the number of PVCs (from 1820 ± 2969 to 742 ± 1425, P = 0.048) and in the prevalence of NSVT (from 41 to 6%, p = 0.039). Late potentials on SAECG were abolished in 3 patients. The less advanced LV dysfunction group showed a significantly higher prevalence of gallium‐67 uptake compared with the advanced LV dysfunction group (EF < 35 %, n = 14). In the advanced LV dysfunction patients, there were no significant differences in these parameters.
Conclusions: Corticosteroid therapy may be effective for ventricular arrhythmias in the early stage, but less effective in the late stage.
Ann Noninvasive Electrocardiol 2011;16(2):140–147
Keywords: cardiac sarcoidosis, corticosteroid, ventricular arrhythmia
INTRODUCTION
Sarcoidosis is a systemic granulomatous disease of unknown etiology involving multiple organs. 1 Cardiac sarcoidosis (CS) is uncommon but has various clinical manifestations and is sometimes fatal. Ventricular arrhythmias are one of the common causes of sudden death in CS patients. In patients with definite diagnosis of CS, corticosteroid therapy should be the absolute indication. Early initiation of corticosteroid therapy is important to preserve LV function and improve outcomes. 2 , 3 Although the early corticosteroid therapy may prevent ventricular arrhythmias, little is known about the effectiveness. Nonsustained ventricular tachycardia (NSVT), defined commonly as 3 or more consecutive beats of a ventricular origin lasting less than 30 seconds, has been reported to have clinical significance for subsequent lethal arrhythmic events and sudden cardiac death in patients with structural heart disease. 4 , 5 , 6 In the present study, to clarify the effect of corticosteroid for ventricular arrhythmias, 24‐h ambulatory Holter monitoring was evaluated in CS patients with ventricular arrhythmias before and after corticosteroid therapy.
METHODS
This study was retrospective with 31 CS patients (6 men and 25 women; mean age 60 ± 9 years) presenting premature ventricular contractions (PVCs, ≥300/day) on 24‐hour ambulatory Holter monitoring. Fourteen patients had NSVT. All patients were referred to our hospital between December 1, 1998 and August 31, 2009. Similar to previous studies, we applied the modified guidelines for the diagnosis of CS based on the study report on diffuse pulmonary diseases from the Japanese Ministry of Health and Welfare (Table 1). 7 , 8 , 9 Significant coronary artery disease was excluded by angiography in all patients. Patients with known other cardiac diseases and were also excluded from the present investigation. All of patients were treated with corticosteroid, and the initial dosage is 30 mg/day of prednisone or its equivalent on alternate days, which was tapered over a period of 6 months to a maintenance dosage of 10 mg/day. Signal averaged elecrocardiography (SAECG), 24‐hour Holter recordings, echocardiography, gallium‐67 scintigraphy, serum angiotensin converting enzyme (ACE) and plasma B‐type natriuretic peptide (BNP) concentrations were assessed before and after corticosteroid therapy. This assessment was carried out within 3 months before corticosteroid therapy, and the mean time‐period from initiation of corticosteroid therapy to the follow up assessment was 7.3 ± 5.9 months. The mean dose of prednisone at the time of assessment was 10 mg in all patients.
Table 1.
Modified Guidelines for the Diagnosis of CS Based on the Study Reporton Diffuse Pulmonary Diseases from the Japanese Ministry of Health and Welfare, 1993 (7)
| 1 Histologic diagnosis group: endomyocardial biopsy demonstrates epithelioid granulomata without caseating granulomata. |
| 2 Clinical diagnosis group: in patients with histologic diagnosis of extracardiac sarcoidosis, cardiac sarcoidosis is suspected when “a” and atleast one of criteria “b” to “d” is present, and other etiologies such as hypertension and coronary artery disea. |
| a. Complete RBBB, left‐axis deviation, AV block, VT, PVC, or pathological Q or ST‐T change on resting or ambulatory electrocardiogram. |
| b. Abnormal wall motion, regional wall thinning, or dilation of the left ventricle. |
| c. Perfusion defect by 201 thallium‐myocardial scintigraphy or abnormal accumulation by 67Ga‐citrate or 99mTc‐PYP myocardial scintigraphy. |
| d. Abnormal intracardiac pressure, low cardiac output, or abnormal wall motion or depressed ejection fraction of the left ventricle. |
AV, atrioventricular; LBBB, left bundle branch block; PVC, premature ventricular contraction; RBBB, right bundle branch block; VT, ventricular tachycardia.
From 24‐hour holter monitoring, the number of PVCs and the presence of NSVT were investigated. NSVT was defined as 3 or more consecutive beats of ventricular origin, with a rate of ≥120 beats/min and lasting less than 30 seconds. LV end‐diastolic and end‐systolic dimensions were determined from B‐mode echocardiography and ejection fraction (EF) was measured by the Modified Simpson's method. SAECG records were obtained from the Frank X, Y, and Z‐leads during sinus rhythm using a Signal Processor DP 1100 (NEC, Tokyo, Japan). Patients with bundle branch block (BBB) and atrioventricular block (AVB) were excluded from SAECG evaluation. A total of 200 cycles were averaged to obtain a noise level of <0.2 μV. The signals were amplified, digitized, averaged and bidirectionally filtered with a band‐pass filter at frequencies between 40 and 250 Hz. The filtered QRS (f‐QRS), the root mean square voltage of the terminal 40 ms (RMS40) in the f‐QRS complex and the duration of low‐amplitude signals < 40 μV (LAS40) in the terminal f‐QRS complex were measured respectively. In the present study, positive Late Potentials (LP) was defined with the modified Gomes’ standard. 10 LP were considered as “positive” if 2 of the following criteria were met: (1) f‐QRS ≥120 ms; (2) RMS40 <20 μV; (3) LAS40 >38 ms.
Twenty‐one patients were already receiving amiodarone or oral beta‐blocker for frequent PVCs, or documented VT. There was no change in anti arrhythmic drug therapy during the entire course of the experiment. Nine patients have complete right bundle branch block (CRBBB). Twelve patients had permanent pacemakers for complete atrio‐ventricular block, and 4 patients had implantable‐cardioverter defibrillators (ICD) for sustained VT. Pulmonary involvement was detected in 21 patients, skin lesions in 17, and eye involvement in 6.
Labolatory Analysis
Serum ACE and plasma BNP concentrations were measured in all sarcoidosis patients. Serum ACE concentrations were measured by a colorimetric method (colorimetric assay kit; Fujirebio, Tokyo, Japan) with p‐hydroxyhippuryl‐ l‐histidyl‐l ‐leucine as the substrate 11 and plasma BNP concentrations were determined with a specific immunoradiometric assay for human BNP with commercial kits (Shionoria kit; Shionogi and Kyowa Medex, Tokyo, Japan).
Statistical Analysis
Measurements are presented as mean value ± SD. Comparisons of measurements between the two groups were analyzed by unpaired t‐test, and comparison of data before and after corticosteroid therapy was analyzed by paired t‐test. Fisher exact test was used for discrete variables. A P‐value <0.05 was considered statistically significant.
RESULTS
There were no significant differences in the number of PVCs before and after steroid therapy in all patients (from 3098 ± 5902 to 3024 ± 8081, P = 0.895). However, when the patients were divided into two groups, with less advanced (EF ≥ 35%) or advanced (EF< 35%) LV dysfunction, they showed different responses (Table 2). In the less advanced LV dysfunction group, the number of PVCs decreased significantly (from 1820 ± 2969 to 742 ± 1425, P = 0.048; Fig. 1) and EF tended to increase (from 52.4 ± 13.2 to 55.1 ± 12.2%, P = 0.060), serum ACE levels and BNP concentrations tended to decrease (ACE; from 17.1 ± 7.1 to 14.4 ± 4.6 IU/l, P = 0.091 BNP; from 123.7 ± 200.1 to 66.3 ± 109.4 pg/ml, P = 0.093) after corticosteroid therapy. The less advanced LV dysfunction group showed a significantly higher prevalence of gallium‐67 uptake compared with the advanced LV dysfunction group (35.3% vs 0.0%, P < 0.05), and a significant reduction in the prevalence of nonsustained ventricular tachycardia after corticosteroid therapy (from 41 to 6%, P = 0.039). In the less advanced LV dysfunction patients without BBB and AVB (n = 5), LP on SAECG were abolished in 3 patients after corticosteroid therapy. F‐QRS and LAS40 were significantly decreased and RMS40 was significantly increased compared with those before corticosteroid therapy (f‐QRS: 125.4 ± 20.7 to 105.6 ± 13.0 msec, P = 0.047 LAS40: 61.8 ± 20.9 to 42.6 ± 18.3 msec, P = 0.040 RMS40: 8.6 ± 1.5 to 16.1 ± 5.1 μV, P = 0.028) (Fig. 2). However, these parameters did not change significantly in the advanced LV dysfunction patients. Representative record of SAECG in the less advanced LV dysfunction group before and after corticosteroid therapy was shown in Figure 3. After corticosteroid therapy, AV conduction improved to normal in 4 of the 8 CAVB patients with the less advanced LV dysfunction group, while none of 4 patients with the advanced LV dysfunction group. Resolution of CRBBB was observed in two patients with the less advanced LV dysfunction group.
Table 2.
Comparison of the Parameters before and after the Administration of Corticosteroid in Both Groups
| Less advanced LV dysfunction group (n = 17) | Advanced LV dysfunction group (n = 14) | |||||
|---|---|---|---|---|---|---|
| Before | After | P‐value | Before | After | P‐value | |
| Age | 57.9 ±10.2 | ‐ | ‐ | 63.6 ±6.4 | ‐ | ‐ |
| Male | 4 | ‐ | ‐ | 2 | ‐ | ‐ |
| Chest radiographic stage (0/I/II/III) | 5/6/6/0 | ‐ | ‐ | 5/3/4/2 | ‐ | ‐ |
| Skin/eye involvement | 8/2 | ‐ | ‐ | 9/4‐ | ‐ | ‐ |
| Disease duration (months) | 12.5 ±11.7 | ‐ | ‐ | 21.8 ±19.0 | ‐ | ‐ |
| PVC (beats/day) | 1820 ±2969 | 742 ±1425 | 0.048 | 4651 ±8050 | 5794 ±11,538 | 0.280 |
| NSVT | 7/17 | 1/17 | 0.039 | 7/14 | 9/14 | 0.704 |
| CAVB | 8/17 | 4/17 | 0.151 | 4/14 | 4/14 | 1.000 |
| EF (%) | 52.4±13.2* | 55.1±12.2 | 0.060 | 26.2±5.5 | 26.5±7.5 | 0.777 |
| ACE (IU/l) | 17.1±7.1 | 14.4±4.6 | 0.091 | 13.6±8.1 | 11.8±4.6 | 0.196 |
| BNP (pg/ml) | 123.7 ±200.1* | 66.3 ±109.4 | 0.093 | 531.7 ±537.6 | 584.6 ±525.0 | 0.136 |
| Ga uptake | 6/17* | 1/17 | 0.085 | 0/14 | 0/14 | 1.000 |
| CRBBB | 4/17 | 2/17 | 0.656 | 5/14 | 5/14 | 1.000 |
| LP | 5/5 | 2/5 | 0.167 | 4/5 | 4/5 | 1.000 |
Data are given as the mean ±SD and number of patients. *P < 0.05 for comparisons of each value between the less advanced and advanced LV dysfunction groups.
Figure 1.
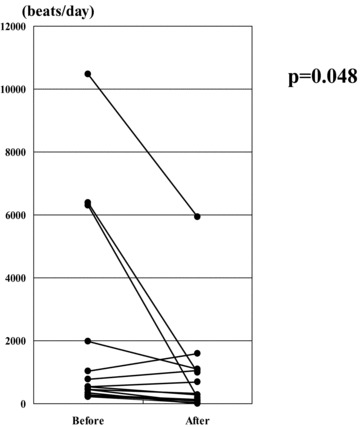
In the less advanced LV dysfunction group, the number of PVCs was significantly decreased after corticosteroid therapy.
Figure 2.
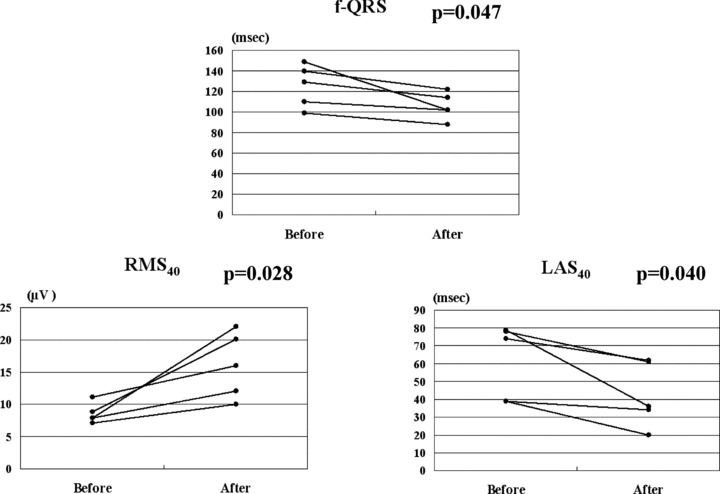
In the less advanced LV dysfunction group, the values of filtered QRS (f‐QRS), low amplitude signals <40 μV (LAS40) were significantly increased and the values of root mean square voltage of the terminal 40 ms (RMS40) was significantly decreased after steroid therapy compared to those at baseline.
Figure 3.
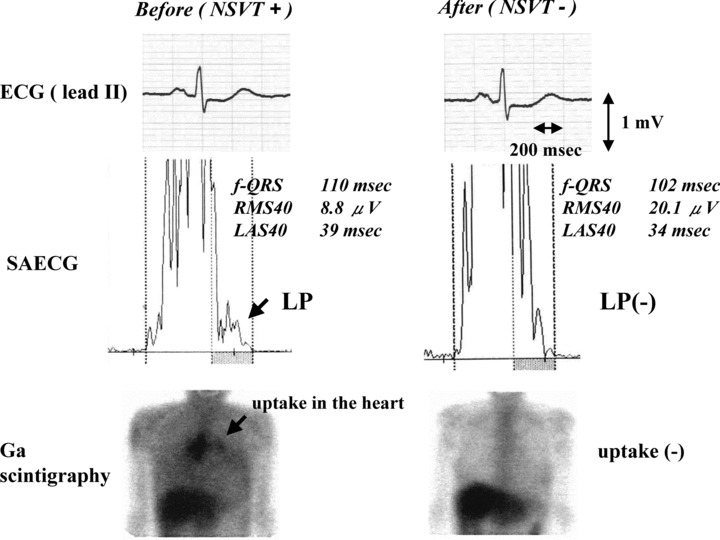
Representative ECG recordings of lead II (top), signal‐averaged ECG (SAECG; middle) and gallium‐67 citrate (Ga) scintigraphy (bottom) before and after corticosteroid therapy in a patient with less advanced LV dysfunction group. After corticosteroid therapy, remission of the LP and Ga uptake at the heart was demonstrated despite the fact that there were no obvious changes in ECG.
DISCUSSION
Major Findings
The present investigation suggested that corticosteroid therapy is effective for ventricular arrhythmias in CS patients with less advanced LV dysfunction. The less advanced LV dysfunction group showed a significant reduction in the number of PVCs and the prevalence of NSVT. F‐QRS and LAS40 were significantly decreased and RMS40 was significantly increased after corticosteroid therapy. However, in the advanced LV dysfunction patients, corticosteroid therapy appeared not to have a significant beneficial effect for ventricular arrhythmias. The different responses to steroid therapy may reflect the degree of irreversible myocardial fibrosis caused by inflammation.
Efficacy of Corticosteroid Therapy for Ventricular Arrhythmias in CS
It is generally accepted that one should start corticosteroid therapy after definite diagnoses of CS. Long‐term benefit of corticosteroid therapy in reducing clinical morbidity and mortality has been demonstrated. 2 Hiramitsu et al. reported national survey on status of steroid therapy for CS in Japan. 12 According the survey, steroid therapy resulted in improvement of New York Heart Association functional class in 54%, no change in 40%, and deterioration in 6% of CS patients. Concerning arrhythmias, Kato et al. described that atrioventricular block resolved in 4 of the 7 CS patients by corticosteroid therapy, but did not resolve or improve in any of the patients without corticosteroid therapy. 13 Although it has been demonstrated that corticosteroid therapy is effective for atrioventricular block in CS, 14 , 15 it remains controversial whether or not corticosteroid therapy is effective for ventricular arrhythmias in CS. Walsh et al. showed that steroid therapy suppressed drug refractory ventricular tachycardia in CS. 16 On the contrary, Winters et al. have reported that steroid therapy did not suppress VT in CS patients. 17 Recently, Uusimaa et al. described outcome of 9 CS patients manifested by VT, all patients required implantable cardioverter defibrillator (ICD) and anti‐arrhythmic medication despite the fact that 8 of them received steroid treatment. 18 In addition, Banba et al demonstrated that corticosteroid treatment did not reduce the total number of PVCs in CS. 19
The present study demonstrated that corticosteroid therapy was effective for ventricular arrhythmias in patients with less advanced LV dysfunction. In similar point of view, steroid therapy was reported to prevent LV remodeling and improve LV function in patients with mildly reduced LV function but not with LVEF < 30%. 3 Therefore earlier initiation of corticosteroid therapy is required in patients CS as soon as the diagnosis was made. BNP is a hormone released mainly from the cardiac ventricles in response to myocardial wall stress, and associated with LV dysfunction in various cardiac conditions. As expected, serum plasma BNP concentrations were significantly higher in the advanced LV dysfunction group than those in the less advanced LV dysfunction group. Accumulation of gallium‐67 and elevation of serum ACE concentrations are often taken as markers of disease activity in CS. gallium‐67 scintigraphy is sensitive for evaluating inflammatory activity, and reported to be useful for predicting the effect of corticosteroid therapy. 20 The less advanced LV dysfunction group had a significantly higher prevalence of gallium‐67 uptake compared with the advanced LV dysfunction group. In addition, gallium‐67 uptake was abolished 5 of 6 patients, and serum ACE levels tended to decrease after corticosteroid therapy. These results suggested that active inflammation might play an important role for the occurrence of ventricular arrhythmias in CS. Thus, CS patients with gallium‐67 uptake and less advanced LV dysfunction are thought to be good candidates for treatment of the steroid therapy. On the contrary, CS patients with advanced LV dysfunction and no gallium‐67 uptake are considered to be nonresponders to corticosteroid therapy, and require earlier initiation of additional therapy such as class III antiarrhythmic agents, catheter ablation, or ICD implantation.
Application of SAECG for Therapeutic Monitoring of CS Patients with VT
Up to the present, magnetic resonance imaging (MRI) and positron emission tomography (PET) scan have renewed the awareness of CS. 21 , 22 We previously reported the early detection of CS using cardiac markers and myocardial integrated backscatter. 23 Although there are many reports regarding the diagnosis of CS, little is known about therapeutic evaluation of the efficacy of corticosteroid in CS. Futamatsu et al. recently reported that gallium‐67 scintigraphy is useful for evaluation of CS with ventricular tachycardia. 24 According to the study, accumulation of gallium‐67 in the heart at the time of diagnosis was detected more frequently in the VT group than in the non‐VT group (14.3 vs 71.4%, P < 0.05). Recently, Casset‐Senon et al. reported the utility of fluorodeoxyglucose PET for adequate management of corticoid therapy in CS complicated by VT. 25 These reports suggested that a decrease in inflammatory activity in the cardiac lesions were associated with the decrease in ventricular arrhythmias.
SAECG is a high‐resolution electrocardiographic technique to reveal conduction abnormalities by detecting very subtle potentials hidden in the terminal portion of QRS complex. We previously reported the possibility of early detection of CS using SAECG, 26 and this time we thought SAECG parameters might be a therapeutic marker for corticosteroid therapy. In the present study, the less advanced LV dysfunction group showed significant improvement of SAECG parameters and significant reduction of the prevalence of NSVT after corticosteroid therapy. It has been suggested that the mechanism of VT in CS is reentry, 18 but abnormal automaticity by sarcoid granulomas might be a cause for the occurrence of VT. The mechanism of VT in CS remains obscure because we did not perform programmed electrical stimulation study. However, reversible conduction abnormality detected by SAECG may reflect arrhythmogenic substrate that might be responsible for abnormal automaticity or reentry.
Study Limitations
There are several limitations in this study. First, our results showed the efficacy of corticosteroid for ventricular arrhythmias in CS in the short term. Long‐term follow‐up is necessary to determine the effectiveness of corticosteroid therapy. Second, corticosteroid may be effective for PVC and NSVT in less advanced LV dysfunction patients, but these ventricular arrhythmias with preserved LV function not always resulted in sudden cardiac death. It is speculative that suppression of PVC and NSVT leads to prevention of sudden cardiac death, therefore the clinical usefulness of the corticosteroid therapy in this study might be limited. Third, the lack of NSVT and decreased PVCs on the 2nd Holter monitoring might be due to spontaneous variability of ventricular arrhythmia or natural disease progression rather than treatment effect. The ESVEM trial suggested that at least a 70% reduction of total number of VPCs is mandatory for efficacy attribution to therapy as beyond the realm of spontaneous variability. 27 Fourth, our sample size was relatively small and there was no control group studied, which might obscure the effectiveness of corticosteroid for VT in CS. A large, multicenter, controlled, randomized trial are needed to assess the true efficacy of corticosteroid administration. Finally, there were 10 (87.1%) patients who were not treated by ICD, because ventricular arrhythmias were clinically suppressed after corticosteroid or antiarrhythmic drug therapy. The 2008 American College of Cardiology/American Heart Association/Heart Rhythm Society guidelines for device based therapy have listed CS as a class IIa recommendation for ICD implantation. 28 Therefore close follow up is needed in these patients.
CONCLUSIONS
Our findings suggest that corticosteroids may be effective for the treatment of CS complicated by ventricular arrhythmias in the early stage, but not be as effective in the late stage. Reversible conduction abnormality detected by SAECG may reflect arrhythmogenic substrate for the occurrence of ventricular arrhythmias, and might be useful for therapeutic evaluation.
Acknowledgments
Acknowledgments The authors thank Michio Ogano, MD and Hiroshige Murata, MD for their technical assistance during the SAECG studies and the physicians in the Departments of Pulmonology, Opthalmology, and Dermatology for referring sarcoidosis patients.
Potential conflicts of interests: none. Financial support: none.
REFERENCES
- 1. Newman LE, Rose CS, Maier LA. Sarcoidosis. N Engl J Med 1997;336:1224–1234. [DOI] [PubMed] [Google Scholar]
- 2. Yazaki Y, Isobe M, Hiroe M, et al Central Japan Heart Study Group. Prognostic determinants of long‐term survival in Japanese patients with cardiac sarcoidosis treated with prednisolone. Am J Cardiol 2001;88:1006–1010. [DOI] [PubMed] [Google Scholar]
- 3. Chiu CZ, Nakatani S, Zhang G, et al Prevention of left ventricular remodeling by long‐term corticosteroid therapy in patients with cardiac sarcoidosis. Am J Cardiol 2005;95:143–146. [DOI] [PubMed] [Google Scholar]
- 4. Doval HC, Nul DR, Grancelli HO, et al Nonsustained ventricular tachycardia in severe heart failure: Independent marker of increased mortality due to sudden death: GESICA‐GEMA Investigators. Circulation 1996;94:3198–3203. [DOI] [PubMed] [Google Scholar]
- 5. Grimm W, Glaveris C, Hoffmann J, et al Arrhythmia risk stratification in idiopathic dilated cardiomyopathy based on echocardiography and 12‐lead, signal‐averaged, and 24‐hour holter electrocardiography. Am Heart J 2000;140:43–51. [DOI] [PubMed] [Google Scholar]
- 6. Monserrat L, Elliott PM, Gimeno JR, et al Non‐sustained ventricular tachycardia in hypertrophic cardiomyopathy: An independent marker of sudden death risk in young patients. J Am Coll Cardiol 2003;42:873–879. [DOI] [PubMed] [Google Scholar]
- 7. Hiraga H, Yuwai K, Hiroe M. Guideline for diagnosis of cardiac sarcoidosis: Study report on diffuse pulmonary disease from the Japanese Ministry of Health and Welfare (in Japanese). 1993;6:23–24. [Google Scholar]
- 8. Smedema JP, Snoep G, van Kroonenburgh MP, et al Evaluation of the accuracy of gadolinium‐enhanced cardiovascular magnetic resonance in the diagnosis of cardiac sarcoidosis. J Am Coll Cardiol 2005;45:1683–1690. [DOI] [PubMed] [Google Scholar]
- 9. Patel MR, Cawley PJ, Heitner JF, et al Detection of myocardial damage in patients with sarcoidosis. Circulation. 2009;120:1969–1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gomes JA, Winters SL, Martinson M, et al The prognostic significance of quantitative signal‐averaged variables relative to clinical variables, site of myocardial infarction, ejection fraction and ventricular premature beats: A prospective study. J Am Coll Cardiol 1989;13:377–384. [DOI] [PubMed] [Google Scholar]
- 11. Kasahara Y, Ashihara Y. Colorimetry of angiotensin‐I converting enzyme activity in serum. Clin Chem 1981;27:1922–1925. [PubMed] [Google Scholar]
- 12. Hiramitsu S, Morimoto S, Uemura A, et al National survey on status of steroid therapy for cardiac sarcoidosis in Japan. Sarcoidosis Vasc Diffuse Lung Dis 2005;22:210–213. [PubMed] [Google Scholar]
- 13. Kato Y, Morimoto S, Uemura A, et al Efficacy of corticosteroids in sarcoidosis presenting with atrioventricular block. Sarcoidosis Vasc Diffuse Lung Dis. 2003;20:133–137. [PubMed] [Google Scholar]
- 14. Umetani K, Ishihara T, Yamamoto K, et al Successfully treated complete atrioventricular block with corticosteroid in a patient with cardiac sarcoidosis: Usefulness of Gallium‐67 and Thallium‐201 scintigraphy. Int Med 2000;39:245–248. [DOI] [PubMed] [Google Scholar]
- 15. Sugishita K, Togashi Y, Aizawa A, et al Postpartum complete atrioventricular block due to cardiac sarcoidosis. Steroid therapy without permanent pacemaker. Int Heart J 2008;49:377–384. [DOI] [PubMed] [Google Scholar]
- 16. Walsh MJ. Systemic sarcoidosis with refractory ventricular tachycardia and heart failure. Br Heart J 1978;40:931–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Winters SL, Cohen M, Greenberg S, et al Sustained ventricular tachycardia associated with sarcoidosis: Assessment of the underlying cardiac anatomy and the prospective utility of programmed ventricular stimulation, drug therapy and an implantable antitachycardia device. J Am Coll Cardiol 1991;18:937–943. [DOI] [PubMed] [Google Scholar]
- 18. Uusimaa P, Ylitalo, K Anttonen O, et al Ventricular tachyarrhythmia as a primary presentation of sarcoidosis. Europace 2008;10:760–766. [DOI] [PubMed] [Google Scholar]
- 19. Banba K, Kusano KF, Nakamura K, et al Relationship between arrhythmogenesis and disease activity in cardiac sarcoidosis. Heart Rhythm 2007;4:1292–1299. [DOI] [PubMed] [Google Scholar]
- 20. Okayama K, Kurata C, Tawarahara K, et al Diagnostic and prognostic value of myocardial scintigraphy with thallium‐201 and gallium‐67 in cardiac sarcoidosis. Chest 1995;107:330–334. [DOI] [PubMed] [Google Scholar]
- 21. Shimada T, Shimada K, Sakane T, et al Diagnosis of cardiac sarcoidosis and evaluation of the effects of steroid therapy by gadolinium‐DTPA‐enhanced magnetic resonance imaging. Am J Med 2001;110:520–527. [DOI] [PubMed] [Google Scholar]
- 22. Okumura W, Iwasaki T, Toyama T, et al Usefulness of fasting 18F‐FDG PET in identification of cardiac sarcoidosis. J Nucl Med 2004;45:1989–1998. [PubMed] [Google Scholar]
- 23. Yasutake H, Seino Y, Kashiwagi M, et al Detection of cardiac sarcoidosis using cardiac markers and myocardial integrated backscatter. Int J Cardiol 2005;102:259–268.2288;2288;2288. [DOI] [PubMed] [Google Scholar]
- 24. Futamatsu H, Suzuki J, Adachi S, et al Utility of gallium‐67 scintigraphy for evaluation of cardiac sarcoidosis with ventricular tachycardia. Int J Cardiovasc Imaging 2006;22:443–448. [DOI] [PubMed] [Google Scholar]
- 25. Casset‐Senon D, Philippe L, Renard JP, et al Recurrent ventricular tachycardia in cardiac sarcoidosis: Usefulness of fluorodeoxyglucose positron emission tomography for adequate management of corticoid therapy after placement of an implantable cardioverter defibrillator. J Nucl Cardiol 2008;15:282–285. [DOI] [PubMed] [Google Scholar]
- 26. Yodogawa K, Seino Y, Ohara T, et al Non‐invasive detection of latent cardiac conduction abnormalities in patients with pulmonary sarcoidosis. Circ J. 2007;71:540–545. [DOI] [PubMed] [Google Scholar]
- 27. The ESVEM Investigators . The ESVEM trial: Electrophysiologic study versus electrocardiographic monitoring for selection of antiarrhythmic therapy of ventricular tachyarrhythmias. Circulation 1989;79:1354–1360. [DOI] [PubMed] [Google Scholar]
- 28. Epstein AE, DiMarco JP, Ellenbogen KA, et al ACC/AHA/HRS 2008 Guidelines for Device‐Based Therapy of Cardiac Rhythm Abnormalities: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices): developed in collaboration with the American Association for Thoracic Surgery and Society of Thoracic Surgeons. Circulation 2008;117:350–408. [DOI] [PubMed] [Google Scholar]


