Abstract
Brugada phenocopies (BrP) have emerged as new clinical entities that are etiologically distinct from true Brugada syndrome (BrS). BrP are characterized by an ECG pattern that is phenotypically identical to true BrS (type 1 or type 2); however, BrP are caused by various other factors such as mechanical mediastinal compression, myocardial ischemia, pericarditis, myocarditis, pulmonary embolism, and metabolic disturbances. We report a case of an electrocardiographic BrP in a patient with pectus excavatum deformity in the absence of true BrS using currently defined BrP diagnostic criteria. A systematic review of ECG manifestations associated with pectus excavatum is also discussed.
Keywords: Brugada phenocopy, Brugada syndrome, pectus excavatum
INTRODUCTION
Brugada syndrome (BrS) is an inherited cardiac sodium channelopathy that predisposes individuals to malignant ventricular arrhythmias and sudden cardiac death (SCD). BrS occurs in patients with an apparently normal structural heart and is characterized by two ECG patterns observed primarily in the right precordial leads. The type 1 Brugada pattern is classically described as a “coved” ST‐segment elevation (≥0.2 mV) with T‐wave inversion, while type 2 Brugada pattern has a “saddle‐back” ST‐segment appearance. Patients with BrS are typically survivors of cardiac arrest; present with polymorphic ventricular tachycardia; have a history of nonvasovagal syncope; family history may be positive for SCD in members younger than 45 years old in the absence of an acute coronary syndrome; or a family member has a type 1 Brugada pattern.1
Brugada phenocopy (BrP) is an interesting clinical phenomenon that has recently been established.2, 3 BrP are defined as type 1 or type 2 Brugada patterns in the absence of true congenital BrS. BrP are caused by a myriad of clinical circumstances including electrolyte abnormalities, mechanical mediastinal compression, myocardial ischemia, pericarditis, myocarditis, and pulmonary embolism (Table 1).4 In order to differentiate BrP from BrS, systematic diagnostic criteria have recently been established3, 5 and applied6 (Table 2).
Table 1.
Brugada Phenocopy Etiological Categoriesa
| Category | |
|---|---|
| i. Metabolic conditions | |
| ii. Mechanical compression | |
| iii. Ischemia & pulmonary embolism | |
| iv. Myocardial & pericardial disease | |
| v. ECG modulation | |
| vi. Miscellaneous |
Adapted from Anselm and Baranchuk.4
Table 2.
Brugada Phenocopy Diagnostic Criteriaa
| i. | The ECG pattern has a type 1 or type 2 Brugada morphology. |
| ii. | The patient has an underlying condition that is identifiable. |
| iii. | The ECG pattern resolves after resolution of the underlying condition. |
| iv. | There is a low clinical pretest probability of true Brugada syndrome determined by lack of symptoms, medical history, and family history. |
| v. | Negative provocative testing with sodium channel blockers such as ajmaline, flecainide, or procainamide. |
| vi. | Provocative testing not mandatory if surgical RVOT manipulation has occurred within the last 96 hours. |
| vii. | The results of genetic testing are negative (desirable but not mandatory because the SCN5A mutation is identified in only 20% to 30% of probands affected by true Brugada syndrome). |
Adapted from Anselm et al.3,6
Pectus excavatum accounts for 90% of all anterior chest wall deformities and is characterized by a sternal depression beginning from the manubrium and progressing to the xiphoid process. It occurs in 1/400 to 1/1000 live births, being three to five times more prevalent in males than females. The deformity is considered cosmetic; however, depending on its severity, it may impair cardiorespiratory function and predispose individuals to chest and back pain. The diagnosis is clinical and its severity can be calculated using the Haller index from CT scan that measures the maximal internal transverse diameter of the thorax, divided by the shortest anteroposterior depth as measured from the internal aspect of the sternum to the anterior cortex of the vertebral body. Normal index is 2.5. An index >3.25 indicate significant pectus excavatum. When clinically indicated, pectus excavatum can be treated with surgical correction.7
Brugada ECG patterns have been previously reported in two young patients with pectus excavatum.8 Those patients did not undergo provocative testing with sodium channel blockers; however, the author believed that the ECG patterns were benign based on the negative prior history of syncope or familial SCD. He based his arguments on the previously reported disappearance of these ECG patterns after surgical correction of pectus excavatum.8, 9 This was presumed to be the consequence of chronic mechanical injury to the right ventricle caused by compression of the anterior chest wall.7, 8 The first patient from Katoka's series8 depicts some similarities with a type 1 Brugada pattern, while patient #2 resembles a type 2 Brugada pattern, as the case that we are presenting here.
In this report, we present a case of a healthy asymptomatic young male patient with pectus excavatum found to have a Brugada ECG pattern in the absence of true BrS. We also performed a systematic review of all the possible ECG manifestations seen in patients with pectus excavatum.
METHODS AND SEARCH STRATEGY
A literature review was performed on the following databases: Ovid MEDLINE from 1946 to November Week 3, 2012 (30 articles), Embase from 1947 to 2012 Week 48 (42 articles), and Web of Knowledge (2 articles). The search was limited to human studies published in English. All relevant articles were retrieved and reviewed by two investigators (SFMA, AB). The reference list of all the articles was also carefully searched for additional articles. We used the following MeSH terms: ECG, electrocardiogram, electrocardiography, funnel chest, pectus excavatum, Brugada, Brugada phenocopy.
Case Report and ECG Description
A 24‐year‐old male presented with chest pain. He was otherwise healthy and not receiving any medications. He had no history of syncope and denied any family history of SCD. Physical examination revealed malformation of the anterior chest wall consistent with pectus excavatum (Fig. 1A). The cardiovascular examination was normal. A chest x‐ray showed narrowing of the anteroposterior distance between the sternum and the spine with relative compression of the heart (Fig. 1B). Echocardiography showed no structural or wall motion abnormalities.
Figure 1.
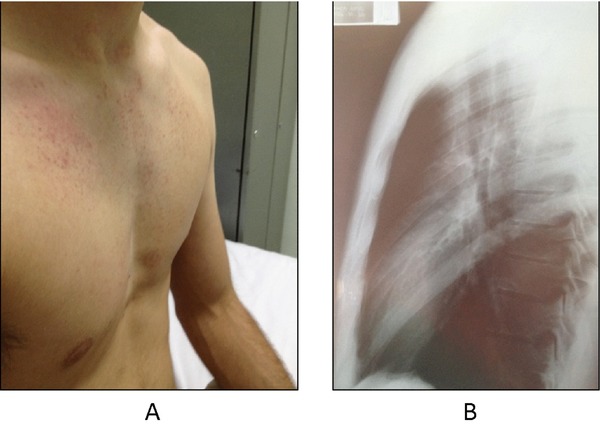
Patient with pectus excavatum A photograph demonstrating the patient's pectus excavatum deformity on physical examination (A). A lateral chest x‐ray of the patient confirming the defect and also revealing a narrowing of the anteroposterior distance between the sternum and the spine with relative compression of the heart (B).
The initial standard 12‐lead ECG with the precordial electrodes placed in the 4th intercostal space (ICS) showed sinus rhythm, heart rate 65 bpm, P‐wave duration 80 ms, PR interval 160 ms, QRS duration 80 ms and normal axis. A “saddle‐back” ST‐segment elevation in lead V2 was consistent with a type 2 Brugada pattern (Fig. 2A). The 12‐lead ECG with high‐precordial lead placement in the 2nd ICS (Fig. 2B) showed sinus rhythm, heart rate 75 bpm, P‐wave duration 80 ms, PR interval 120 ms, QRS duration 100 ms, and normal axis. The ECG was consistent with an incomplete right bundle branch block (iRBBB) pattern with rsR’ complex in leads V1–V2 and a “saddle‐back” ST‐segment elevation in lead V3. When the ECG was obtained in the 5th ICS (Fig. 2C), away from the right ventricular outflow tract (RVOT), it showed disappearance of both the iRBBB and the “saddle‐back” ST‐segment elevation.
Figure 2.
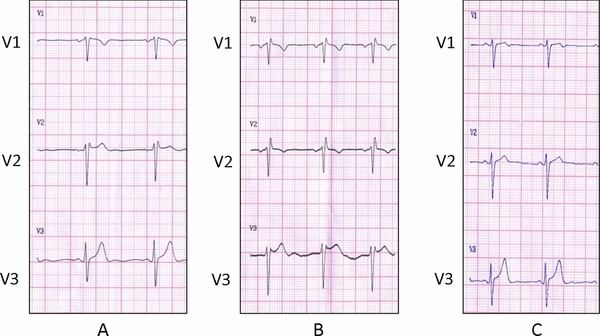
(A) Standard 12‐lead ECG (4th ICS, Leads V1–V3). Sinus rhythm, heart rate 65 bpm, P‐wave duration is 80 ms, PR interval is 160 ms, QRS duration is 80 ms, and a normal axis. The ECG is consistent with a “saddle‐back” ST‐segment elevation in lead V2 (type 2 Brugada pattern). (B) High precordial 12‐lead ECG (2nd ICS, Leads V1–V3). Sinus rhythm, heart rate 75 bpm, P‐wave duration is 80 ms, PR interval is 120 ms, QRS duration is 100 ms, and a normal axis. The ECG is consistent with iRBBB pattern with rsR’ complex in V1–V2 and a “saddle‐back” ST segment elevation in lead V3. (C) 12‐lead ECG (5th ICS, Leads V1–V3). Sinus rhythm, heart rate 60 bpm, P‐wave duration is 80 ms, PR interval is 120 ms, QRS duration is 100 ms, and a normal axis. Disappearance of both the iRBBB and the “saddle‐back” ST‐segment elevation.
A subsequent pharmacological challenge test with Ajmaline (Class Ia antiarrhythmic with potent sodium channel blocking effects) did not induce a type 1 Brugada pattern (Fig. 3A). The ECG pattern described above was present in several ECGs prior to the current visit and persisted in ECGs during and after the drug challenging test.
Figure 3.
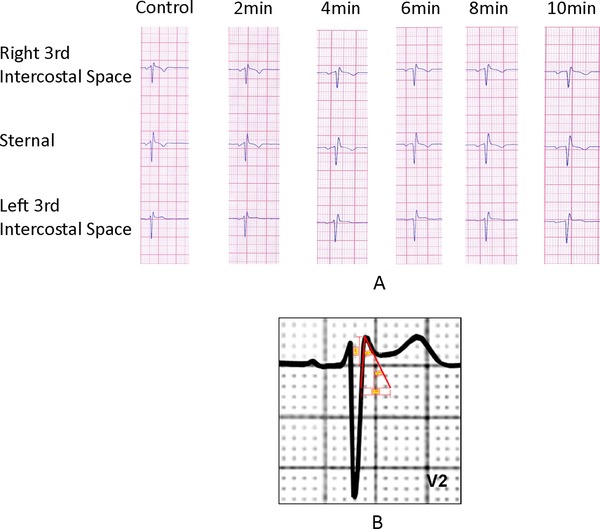
(A) Negative pharmacological provocative test. Negative ajmaline (Class Ia antiarrhythmic with potent sodium channel blocking effects) provocative test showing no ST‐segment elevation in the parasternal leads. (B) The characteristics of the triangle formed by the r’ The β angle formed by the ascending S and descending r’ is 34°. The duration of the base of the triangle of r’ at 5 mm from the high takeoff is 3.29 mm. Maximum R height: 2.86 mm.
ECG Manifestations of Pectus Excavatum
Normal electrocardiograms have been described in patients with pectus excavatum. However, abnormal electrocardiograms have also been described in the absence of an underlying cardiac pathology. Commonly encountered ECG findings include iRBBB or complete right bundle branch block (RBBB);8, 9, 10, 11, 12, 13, 14, 15, 16 right axis deviation;9, 15, 17, 18, 19 T‐wave inversion mainly in the right precordial leads;9, 10, 20 acute myocardial ischemia patterns including ST‐segment elevation;21 ST‐segment depression;22 poor R‐wave progression in the right precordial leads;23 and abnormal Q waves.10, 24 Also, P‐wave changes in the right precordial leads have been reported including negative P waves9 and prominent P waves.22 There are also reports of supraventricular arrhythmia (atrial flutter or fibrillation) caused by cardiac compression and irritation of the atrium.21 Overall, the most frequently cited ECG pattern was iRBBB or RBBB.
All of the aforementioned changes were most commonly observed in the right precordial leads, suggesting that the right ventricle is directly affected by the thoracic deformity.20 The cause of these ECG manifestations is thought to be due to cardiac anatomical displacement, rotation of the heart, and mechanical mediastinal compression mainly on the right ventricle.
DISCUSSION
This is the first case report to systematically prove BrP in the context of pectus excavatum by using currently established BrP diagnostic criteria3, 6 and excluding sodium channel dysfunction with provocative testing (Table 2). We have also shown that in patients with pectus excavatum, the right precordial leads elicit the type 2 Brugada pattern. This pattern is dependent on anatomical lead position (Fig. 2) and suggests that the ECG pattern is associated with the right ventricle and the RVOT.
In addition, according to the new ECG criteria described in the literature,1 in type 2 Brugada pattern, the characteristics of the triangle formed by the r’ in leads V1–V2 are useful for the diagnosis of type 2 Brugada pattern. The β angle formed by the ascending S and descending r’ should be >58° and the duration of the base of the triangle of r’ at 5 mm from the high takeoff should be >3.5 mm to be considered as a Brugada pattern.25 In our case, the β angle is 34° and the duration of the base of the triangle is 3.29 cm (Fig. 3B) which argues against a congenital Brugada pattern. Moreover, in our patient, the duration of the QRS is constant from leads V1–V6, as opposed to the type 2 Brugada pattern where there is a mismatch between leads V1 and V6, with a longer QRS duration in V1 than in V6.1 Additionally, the high takeoff of r’ coincides with the J‐point elevation (Fig. 4) as opposed to what is found in a congenital Brugada pattern where the J‐point is after the high takeoff.1
Figure 4.
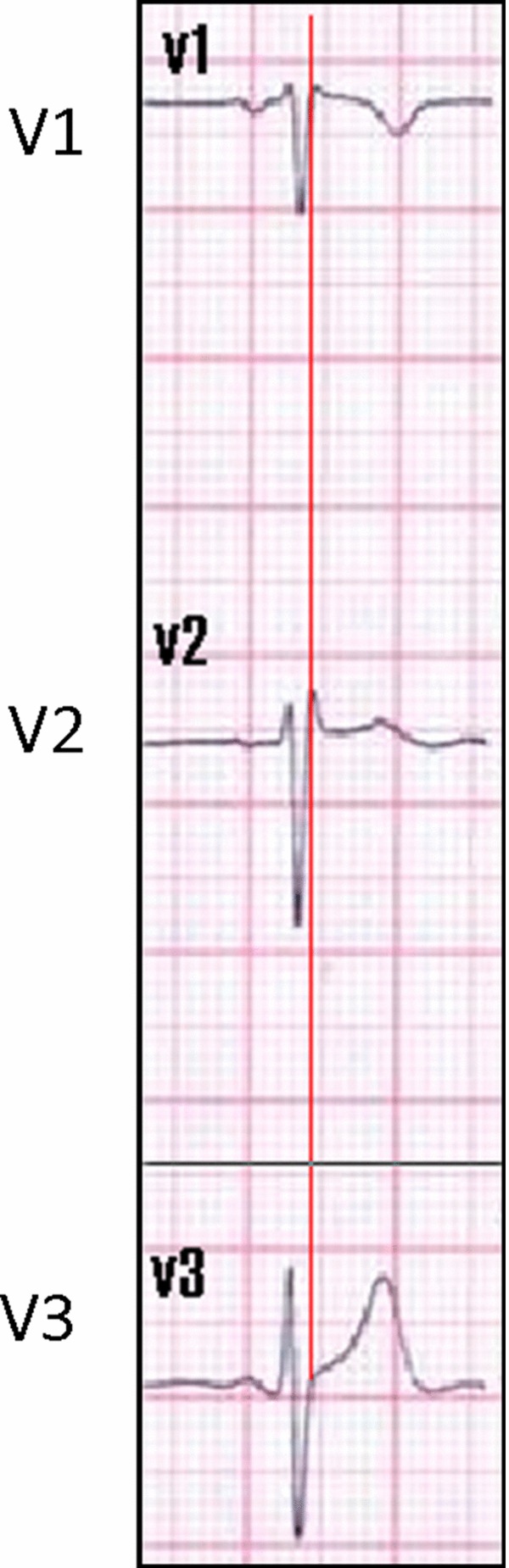
J‐point analysis. The high takeoff of the r’ coincides with the J‐point elevation in this patient as opposed to Brugada pattern where the J‐point is after the High takeoff of r’.
All of these ECG characteristics, along with a negative provocative test to rule out sodium channel dysfunction, confirm that the ECG findings in this case report are not due to congenital BrS; rather they are likely secondary to the presence of pectus excavatum. We hypothesize that mechanical mediastinal compression from pectus excavatum onto the right ventricle causes changes in the RVOT resulting in BrP.
CONCLUSION
The pectus excavatum deformity is another cause of Brugada phenocopy. Several other ECG abnormalities can be seen in patients with pectus excavatum without underlying cardiac pathology. The mechanism causing BrP is not fully understood but it is most likely due to cardiac anatomical rotation and displacement, along with mechanical compression of the RVOT.
Statement: This manuscript is original; it has not and will not be submitted for publication elsewhere. Each author has contributed, read, and approved it for submission.
Disclosures: None
REFERENCES
- 1. Bayés de Luna A, Brugada J, Baranchuk A, et al. Current electrocardiographic criteria for diagnosis of Brugada pattern: a consensus report. J Electrocardiol 2012;45:433–442. [DOI] [PubMed] [Google Scholar]
- 2. Baranchuk A, Nguyen T, Ryu MH, et al. Brugada phenocopy: New terminology and proposed classification. Ann Noninv Electrocardiol 2012;17:299–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Anselm DD, Baranchuk A. Brugada phenocopy: Redefinition and updated classification. Am J Cardiol 2013;111:453. [DOI] [PubMed] [Google Scholar]
- 4. Anselm DD, Baranchuk A. Brugada phenocopy in the context of pulmonary embolism. Int J Cardiol 2013. Feb 25 Epub Ahead of Print. [DOI] [PubMed] [Google Scholar]
- 5. Anselm DD, Pérez‐Riera AR, Femenía F, et al. Brugada phenocopy in a patient with surgically repaired pentalogy of Fallot. Revista Iberoamericana de Arritmologia 2012;3:20–24. [Google Scholar]
- 6. Anselm DD, Barbosa‐Barros R, de Sousa Belém L, et al. Brugada phenocopy induced by acute inferior ST‐segment elevation myocardial infarction with right ventricular involvement. Inn Card Rhythm Manag 2013;4:1092–1094. [Google Scholar]
- 7. Fokin AA, Steuerwald NM, Ahrens WA, et al. Anatomical, histologic, and genetic characteristics of congenital chest wall deformities. Semin Thorac Cardiovasc Surg 2009;21:44–57. [DOI] [PubMed] [Google Scholar]
- 8. Kataoka H. Electrocardiographic patterns of the Brugada syndrome in 2 young patients with pectus excavatum. J Electrocardiol 2002;35:169–171. [DOI] [PubMed] [Google Scholar]
- 9. Gahrton G. ECG changes in pectus excavatum (funnel chest). A pre‐ and postoperative study. Acta Med Scand 1961;170:431–438. [PubMed] [Google Scholar]
- 10. Elisberg EI. Electrocardiographic changes associated with pectus excavatum. Ann Intern Med 1958;49:130–141. [DOI] [PubMed] [Google Scholar]
- 11. Yokoyama M, Wada J. Yanagisawa M, et al. Characteristic findings of electrocardiogram in patients with funnel chest. Jpn Circ J 1981;45:895. [Google Scholar]
- 12. Goertzen M, Baltzer A, Schulitz KP. Long‐term results after operation for funnel chest. Arch Orthop Trauma Surg 1993;112:289–291. [DOI] [PubMed] [Google Scholar]
- 13. Grigorov M. About the significance of right bundle branch block in chest wall deformity patients. Adv Cardiol 1977;19:268–269. [DOI] [PubMed] [Google Scholar]
- 14. Vega Diaz F, Navarrete Pelous A, Gonzalez Valdes F, et al. Pectus excavatum. Hemodynamic and electrocardiographic considerations. Am J Cardiol 1962;10:272–277. [DOI] [PubMed] [Google Scholar]
- 15. Landtman B. The heart in funnel chest; pre‐and postoperative studies of seventy cases. Ann Paediat Fenn 1958;4:181–190. [PubMed] [Google Scholar]
- 16. Van Buchem FSP, Nieveen J. Pectus excavatum. Acta Med Scand 1963;174:657–663. [DOI] [PubMed] [Google Scholar]
- 17. Martins De Oliveira J, Sambhi Mp, Zimmerman Ha. The electrocardiogram in pectus excavatum. Br Heart J 1958;20:495–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Abstracts of the 46th Annual Meeting of the Association for European Paediatric and Congenital Cardiology with joint sessions with the Japanese Society of Pediatric Cardiology and Cardiac Surgery . Istanbul, Turkey. May 23–26, 2012. Cardiol Young 2012;22 (Suppl. 1): Abstract P‐127. [PubMed] [Google Scholar]
- 19. Teplick JG, Drake EH. The roentgen and cardiac manifestations of funnel chest. Am J Roentgenol Radium Ther 1946;56:721–735. [PubMed] [Google Scholar]
- 20. Dressler W, Rosesler H. Electrocardiographic changes in funnel chest. Am Heart J 1950;40:877–883. [DOI] [PubMed] [Google Scholar]
- 21. Wachtel FW, Ravitch MM, Grishman A. The relationship of pectus excavatum to heart disease. Am Heart J 1956;52:121–137. [DOI] [PubMed] [Google Scholar]
- 22. Vogelsang A. cardiac compression from funnel chest. CMAJ 1953;68:356–363. [PMC free article] [PubMed] [Google Scholar]
- 23. Ruskin J, Whalen RE, Orgain ES. Electrocardiogram and vectorcardiogram simulating myocardial infarction in patient with pectus excavatum and straight back. Am J Cardiol 1968;21:446–449. [DOI] [PubMed] [Google Scholar]
- 24. Penchas S, Keynan A. Acute myocardial infarction pattern in the ECG of a patient with funnel‐chest. J Electrocardiol 1969;2:285–288. [DOI] [PubMed] [Google Scholar]
- 25. Chevallier S, Forclaz A, Tenkorang J, et al. New electrocardiographic criteria for discriminating between Brugada types 2 and 3 patterns and incomplete right bundle branch block. J Am Coll Cardiol 2011;58:2290–2298. [DOI] [PubMed] [Google Scholar]


