Abstract
Background
Although atrial fibrillation (AF) is the most commonly encountered arrhythmia, some of the properties make its detection challenging. In daily practice, underdiagnosis can lead to less effective treatment in prevention of stroke. Based on data from studies on treatment of AF, more intensive follow‐up strategies, including 7‐day Holter recording, 30‐day event recording, and even implantable cardiac monitoring devices, are suggested. The study purpose is to evaluate the performance of a continuous single‐channel loop recorder with automatic AF detection and transtelephonic electrocardiogram (ECG) transmission capabilities.
Methods and Results
A consecutive cohort of 153 patients admitted to the stroke unit with a presumptive diagnosis of ischemic cerebrovascular accident was screened for AF. Twenty‐four‐hour rhythm observation was performed using a single‐channel external loop recorder (ELR) configured for automated AF detection. A total of 45 patients with a known history of AF, AF on the admission ECG, or incomplete registrations were excluded. Extensive additional frequency‐based settings were used to establish a reference registration. In total, 2923 recordings were transmitted. We evaluated all events, of which 1190 were designated by the device as AF. The sensitivity, specificity, PPV, and NPV for identifying AF using the ELR were, respectively, 93%, 51%, 5%, and 99%.
Conclusions
In this ELR validation study, the dedicated AF detection algorithm showed to be highly sensitive but not specific for AF. Applicability of an ELR might be limited for efficacious detection of AF, as manual verification is mandatory for a vast amount of recordings.
Keywords: monitoring, atrial fibrillation, stroke, validation
Atrial fibrillation (AF) is the most common sustained arrhythmia, which affects more than 8 million people in Europe and North America.1, 2 Its incidence and prevalence are increasing in an ageing population.1 AF has an enormous impact in terms of morbidity, mortality, and health care costs.3, 4 The most important complication is an ischemic stroke.5 Effective prevention is possible with the treatment of (new) oral anticoagulants. However, detection can be challenging due to possible short duration, low frequency of paroxysm, and asymptomatic occurrence, which render proper registration difficult.6 There is, therefore, a need for improved monitoring strategies to enhance preventive treatment. Several new modalities have arisen for intensive rhythm observation varying from daily tele‐electrocardiogram (ECG) to implantable cardiac monitoring devices. Devices based on manual triggering will miss asymptomatic or nocturnal episodes. For clinical evaluation, an additional automated algorithm is mandatory. Commercially available devices utilize nondisclosed algorithms or have tested the algorithm in a computer model (in silico). An external loop recorder (ELR) proved feasible in a small study with severe limitations including patients with permanent AF.7 We evaluated the performance of a noninvasive event recorder (Vitaphone, Mannheim, Germany) in daily practice.
METHODS
Study Design and Patient Selection
The study was conducted in Medisch Spectrum Twente (Enschede, The Netherlands). The execution of the study conformed to the principles outlined in the Declaration of Helsinki on research in human subjects and to the procedures of the local Medical Ethics Committee. From July 2011 to October 2011, 169 consecutive patients > 18 years of age were admitted to our hospital with a provisional diagnosis of acute ischemic stroke (Fig. 1). This presumptive diagnosis was made by the on‐call neurologist at the emergency department. The diagnosis was based on the history of the patient, neurologic examination, and computed tomography (CT) scan of the brain. In all patients, ECG, chest x‐ray, and routine laboratory testing were performed. In all patients, a history was taken, a neurologic examination was performed, and a standard examination consisting of ECG, chest x‐ray, routine laboratory tests, and CT scan of the brain was conducted. Patients who were, at that moment, suspected to have an acute ischemic stroke were admitted to the stroke unit and were included in the study. At the stroke unit, all patients were monitored with continuous telemetry by a trained nurse for 24 hours. Patients with a known history of AF or already diagnosed with paroxysmal or persistent AF were excluded (n = 7). A technician connected the ELR at the ward after completion of the continuous telemetry, but the recording was often done before completing the total stroke work‐up to prevent an unnecessary extension of hospital stay. Ancillary testing consists of CT angiography, magnetic resonance angiography, or conventional angiography. Additional evaluation for prothrombotic states was performed in patients <55 years old. Four patients died prior to the start of the ELR monitoring and five patients were discharged before monitoring could be initiated.
Figure 1.
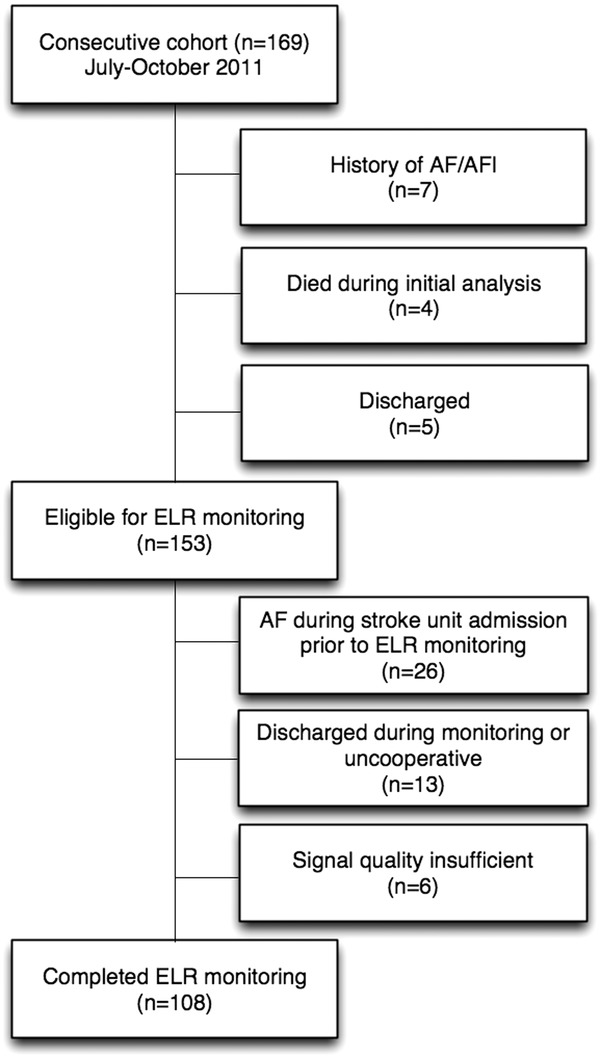
Inclusion flowchart.
ELR
The ELR is a single‐channel device (3100 BT, Vitaphone, Mannheim, Germany) configured using a standardized protocol (Table 1). The AF autodetection is based on a nonadjustable manufacturer‐programmed algorithm. An AF event was triggered based on recognition of R–R interval variability within the last 14 complexes. An episode was classified as AF by the ELR if 6 out of 14 intervals matched RRx – RRy > RRx/8 and RRx – RRy < 2*RRx. We used extensive settings for bradycardia, tachycardia, VT detection (>4 consecutive beats with RR interval <600 ms) pauses, and time‐triggered registration to establish a reference registration for minimizing false negative events (see Table 1). Time‐triggered recordings are not evaluated by the device for rhythm qualification. Recordings fulfilling AF criteria as well as tachycardia or bradycardia were separately coded while maintaining an AF episode code. To prevent continuous recording and transmission of ECGs during longer events, recording was set 30 seconds before and 60 seconds after the triggering event. If an episode exceeded 90 seconds the recording was truncated in two registrations containing start and end. Upon completion of the recording, the registration was automatically transmitted to a preconfigured cell phone by means of a Bluetooth connection. The recording and transmission process was fully automated. To prevent loss of data if the patient is outside cellular network coverage, the device is equipped with a storage capacity of 15 episodes. All registrations were available using a Web‐based management tool. Recordings could be selectively processed based on event trigger or reviewed as full report. Prior to the start of an observation period, a manually triggered recording was performed to visually assess signal quality.
Table 1.
ELR Settings and Transmissions
| Number of | ||
|---|---|---|
| Transmission (Device‐ | ||
| Setting Type | Value | Coded Events) |
| Atrial fibrillation | On | 1190 |
| Bradycardia | 35 | 171 |
| Tachycardia | 140 | 389 |
| Pause | 2.5 seconds | 89 |
| VT detection | On | 279 |
| Time triggera | Every 4 hours | 648 |
| Manual | 157 |
Time trigger performs a snapshot of 90 seconds regardless of the detected rhythm.
Recording Analysis and Definitions
Patients were connected to the devices for 1 day during hospital admittance. An episode of AF was defined as an episode of at least 30 seconds duration.8 Segments of recording with noninterpretable surface ECG due to noise or artifacts were excluded. All ELR registrations were manually reviewed beat‐by‐beat by two qualified analysts blinded to all patient‐related information and compared with ELR‐designated AF episodes. In case of disagreement, a cardiologist–electrophysiologist was consulted. Two physician authors (BOV and JB) had full access to the data and take responsibility for the data and the statistical analyses.
Statistical Considerations
Descriptive statistics are reported as count and percentage for categorical variables, and mean and standard deviation for continuous variables. The overall accuracy of the ELR for AF detection was calculated. Quantification of the performance is represented by the sensitivity, specificity, negative predictive value (NPV), and positive predictive value (PPV) of device‐designated AF episodes. Analyses were performed using statistical software program SPSS 16.0 (SPSS Inc, Chicago, IL, USA).
RESULTS
In total, 153 patients were considered for automated event recording; baseline characteristics during recording are presented in Table 2.
Table 2.
Baseline Characteristics (n = 153)
| Sex (male) | 80 (52.3) |
|---|---|
| Age | 67 ± 13 |
| Medical History | |
| HT | 91 (59.5) |
| DM | 29 (19) |
| COPD | 9 (5.9) |
| iCVA | 12 (7.8) |
| TIA | 16 (10.5) |
| CAD | 10 (6.5) |
| Heart failure | 2 (1.3) |
| Valvular disease | 10 (6.5) |
| Bradytachy syndrome | 1 (0.7) |
| Other arrhythmia | 1 (0.7) |
| Medication | |
| Ca antagonist | 24 (15.7) |
| Beta‐blocker | 36 (23.5) |
| Class 1 AAD | 1 (0.7) |
| Sotalol | 0 (0) |
| Amiodarone | 0 (0) |
| Antiplatelet | 132 (86.3) |
| OAC | 5 (3.3) |
| Statine | 117 (76.5) |
| ACE inhibitor | 42 (27.5) |
| AT2 inhibitor | 25 (16.3) |
| Diuretics | 27 (17.6) |
Data are presented as mean (SD) or number (%). HT = hypertension; DM = diabetes mellitus; COPD = chronic obstructive pulmonary disease; iCVA = ischemic cerebrovascular accident; TIA = transient ischemic attack; CAD = coronary artery disease; AFl = atrial flutter; AAD = antiarrhythmic drugs; OAC = oral anticoagulant; ACE = angiotensin converting enzyme; AT2 = angiotensin II inhibitor.
Twenty‐six patients were excluded since they had AF prior to the start of ELR monitoring. During admission, 13 patients did not complete rhythm monitoring (uncooperativeness or discharge). One hundred fourteen patients received 24‐hour cardiac event monitoring. In six patients, evaluation of additional (time‐ or manual‐triggered) recordings showed R wave undersensing, and recordings of these patients were not processed for analysis. In total, 2923 recordings were transmitted from 108 patients resulting in 73:05 hours of triggered rhythm registration. On average, 25 ± 47 recordings were registered per patient. A total of 157 recordings were manually triggered by a technician for signal verification after electrode placement. No single transmission was received exceeding 14 registrations indicating no buffer overflows had occurred.
Artifacts
A total of 411 (3.8 ± 14.2 median number = 0) event‐triggered recordings were excluded due to artifacts making proper interpretation impossible. The majority of recordings with artifacts was concentrated in patients with severe tremor. Note that, 248 of these events were triggered based on VT detection most susceptible for signal artifacts. A total of 21 (1.7%) registrations flagged as AF could not manually be verified due to artifacts.
Analysis
First, all 1190 events designated by the ELR as AF were evaluated (Table 3). Fifty‐six recordings showed AF according to the HRS/EHRA/ECAS definition;8 in addition, 35 recordings showed an irregular AF pattern consisting of more than 3 complexes not reaching 30 seconds in length. Mean duration of the arrhythmia was 1:12:16 ± 1:24:16 (hh:mm:ss), minimum duration 0:00:38 (hh:mm:ss), maximum 6:03:24 (hh:mm:ss). Subsequently, all remaining recordings were analyzed. Three registrations were categorized as tachycardia, which proved to be AF after manual analysis; the stability criteria of these episodes are presented in Table 4. Two false negative episodes were registered triggered by the extra settings for the reference registrations in patients with multiple false positive registrations. In one patient, nine registrations were false positive qualified as AF, while one episode qualified as tachycardia actually demonstrated AF without being flagged as AF registration (Fig. 2). The three registrations were short in duration ranging from 34 seconds up to 46 seconds. The sensitivity, specificity, PPV, and NPV for identifying AF using the ELR are, respectively, 95%, 51%, 5%, and 100%.
Table 3.
Classification of Registrations
| Atrial Fibrillation | |||
|---|---|---|---|
| after Manual | |||
| Verification | |||
| Yes | No | ||
| Atrial fibrillation according to AF algorithm | Yes | 56 | 1134 |
| No | 3 | 1162 | |
Table 4.
Cycle Length of False Negative Episodes
| Episode | Minimum CL | Maximum CL | Mean CL |
|---|---|---|---|
| 1 | 375 | 1539 | 756 ± 183 |
| 2. | 395 | 469 | 433 ± 14 |
| 3 | 351 | 1053 | 585 ± 158 |
CL = cycle length in milliseconds.
Figure 2.
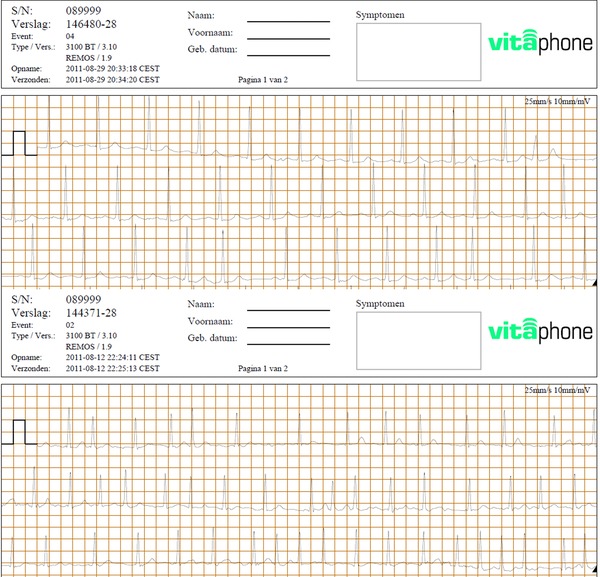
Top electrogram shows a false positive AF registration (event 04) based on premature atrial complexes; bottom electrogram shows a false negative AF registration for AF designated as a tachycardia.
DISCUSSION
In this study, we demonstrated that rhythm observation using an ELR is an acceptable sensitive modality to screen for AF. Several studies suggest prolonged monitoring, however, the standard of care following current guidelines for the detection of AF is 24‐hour Holter monitoring.9, 10 In a previous study, qualitative ELR analysis proved to be less time consuming than quantitative 24 hours Holter analysis.11 For extended periods of time, for example, 7 days monitoring, event recording is suggested to reduce the amount of data. Our data show that event recording using this particular monitor may result in frequent false positive events with a very low proportion of missed episodes of AF. Verification of signal quality is essential for proper signal acquisition. Subsequently, adequate signal processing and event triggering largely depends on the quality of the algorithm.12 The algorithm used in the Vitaphone 3×00 BT is based on irregularity as previous explained. Most devices utilize undisclosed algorithms tested against the MIT‐BIH AF and MIT‐BIH NSR data set containing several episodes of AF and other arrhythmias. Incorporating a high signal quality by means of an implantable loop recorder equipped with a sophisticated algorithm is still no guarantee for success as reported by Eitel et al.13 This suggests that real‐life validation of a specific device is desirable.
Absence of the P wave, as stated in the guideline, could be utilized to detect AF.1 However, even in the presence of good signal transmission, the P‐wave amplitude is very low, making it susceptible to corruption caused by noise.14 Most algorithms used in loop recorders analyze RR interval dynamics to distinguish between AF and other rhythms. RR interval dynamics make the algorithm vulnerable for supraventricular or ventricular extrasystoles, sinus arrhythmias, or SA blocks. As demonstrated in our study, most of the false positives were caused by premature atrial extrasystoles resulting in a low positive predictive value for an AF‐triggered event. More comprehensive algorithms based on the Lorenz distribution of a time series of RR intervals report higher predictive values in silico and in vivo.15 Other statistical processing based on wavelet transform of the RR time series also shows higher predictive values in the presence of ectopic beats or other short rhythm disturbances.16 It should be noted that these predictive values are based on episode durations exceeding the formal 30 seconds as stated in the guideline, which was strictly maintained in our study. One should carefully consider the use of ELR's utilizing publicly available algorithms thereby increasing transparency and efficacy over nondisclosed equipment. Medtronic employs a more comprehensive disclosed algorithm in the implantable loop recorder (Reveal XT) based on the Lorenz distribution, to the best of our knowledge the Dyna‐Vision is the only commercially available ELR with a disclosed algorithm.12, 15 The latter employs a new statistic, the Turning Points Ratio, in combination with the root mean square of successive RR differences and Shannon entropy to characterize this arrhythmia.
The high variability and increased rate of beat intervals in AF ensure that AF detection based on these features is highly sensitive. However, most studies have focused on detection of AF episodes starting from 30 seconds up to >2 minutes from long duration recordings with, for example, the aim of detecting AF episodes in long‐term Holter monitoring. Frequent bursts of high‐rate atrial activity will be detected by a sensitive algorithm as shown in our study. Results of the ASSERT study suggest that these arrhythmias might be of interest in stroke screening although clinical applicability might be limited at present.17
Limitations
This study has several limitations. First of all, validation requires a reference method. Event monitoring without a reference method is prone to underdetection of AF since failure to detect an event will not result in a verifiable registration. Using 24‐hour Holter monitoring would have resulted in continuous registration, which could have been used for verification. Second, if a false positive episode converts into AF no recording will be made and might be missed if the rhythm returns to sinus rhythm before the last 60 seconds of the registered false positive episode. AF burden could be easily underestimated during long periods of AF with relatively stable RR interval dynamics, which will result in an event ending. Time to redetection decreases the reported AF burden. In the absence of a continuous reference registration, no conclusions can be drawn with regards to the burden assessment using an ELR. During the observation at the stroke unit using telemetry short episodes of AF might be missed due to monitor fatigue in the absence of postmonitoring review capabilities. During the rhythm observation patients were allowed to mobilize on the ward introducing potential artifacts and noise. Ambulatory monitoring will introduce a higher risk of the latter potentially decreasing the NPV. To establish an acceptable reference for detection evaluation, we used the recently published SEA‐AF algorithm with minor modifications.18 Extra recordings (i.e., tachycardia, pause, and time triggered) reduce the possibility of missed AF episodes due to underdetection. The high number of registrations and transmission in this study were a result of our best effort to establish a reference data set. The high frequency of false positive AF qualifications might have serious implications if the device is utilized for long‐term monitoring (>7 days) requiring manual verification. We utilize this device in our clinic for follow‐up up to 7 days with an acceptable time consumption for manual validation. Two registrations were classified as false negative measurements, which could not be explained based on the algorithm characteristics (Table 4). The reported incidence of new onset AF is similar to previous reported studies. Employment of ELRs may encounter issues as reduced compliance and cost charges are frequently based on the length of time the device is in use by the patient.19 In vivo validation studies should be performed for ELR with automated event detection to evaluate reliability in a clinical settings.
CONCLUSION
In this study, the AF detection algorithm of this particular monitor proved to be highly sensitive for efficacious AF detection. However, if longer monitoring is required, the high number of false positive registrations will increase the workload of analysts rendering it more useful for research purposes than daily practice. Usability of this ELR for proper quantification of AF burden requires further investigation.
Conflict of interest & funding: nothing to be declared
REFERENCE
- 1. Camm AJ, Kirchhof P, Lip GY, et al. Guidelines for the management of atrial fibrillation: The task force for the management of atrial fibrillation of the society of cardiology (ESC). Eur Heart J 2010;31(19):2369–2429. [DOI] [PubMed] [Google Scholar]
- 2. Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: National implications for rhythm management and stroke prevention: The AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA 2001;285(18):2370–2375. [DOI] [PubMed] [Google Scholar]
- 3. Blomstrom LC, Lip GY, Kirchhof P. What are the costs of atrial fibrillation? Europace 2011;13(Suppl 2):ii9–ii12. [DOI] [PubMed] [Google Scholar]
- 4. Benjamin EJ, Wolf PA, D'Agostino RB, et al. Impact of atrial fibrillation on the risk of death: The Framingham Heart Study. Circulation 1998;98(10):946–952. [DOI] [PubMed] [Google Scholar]
- 5. Lin HJ, Wolf PA, Kelly‐Hayes M, et al. Stroke severity in atrial fibrillation. The Framingham Study. Stroke 1996;27(10):1760–1764. [DOI] [PubMed] [Google Scholar]
- 6. Allessie MA, Boyden PA, Camm AJ, et al. Pathophysiology and prevention of atrial fibrillation. Circulation 2001;103(5):769–777. [DOI] [PubMed] [Google Scholar]
- 7. Muller A, Scharner W, Borchardt T, et al. Reliability of an external loop recorder for automatic recognition and transtelephonic ECG transmission of atrial fibrillation. J Telemed Telecare 2009;15(8):391–396. [DOI] [PubMed] [Google Scholar]
- 8. Calkins H, Kuck KH, Cappato R, et al. 2012 HRS/EHRA/ECAS Expert Consensus Statement on Catheter and Surgical Ablation of Atrial Fibrillation: Recommendations for Patient Selection, Procedural Techniques, Patient Management and Follow‐up, Definitions, Endpoints, and Research Trial Design: A report of the Heart Rhythm Society (HRS) Task Force on Catheter and Surgical Ablation of Atrial Fibrillation. Developed in partnership with the European Heart Rhythm Association (EHRA), a registered branch of the European Society of Cardiology (ESC) and the European Cardiac Arrhythmia Society (ECAS); and in collaboration with the American College of Cardiology (ACC), American Heart Association (AHA), the Asia Pacific Heart Rhythm Society (APHRS), and the Society of Thoracic Surgeons (STS). Endorsed by the governing bodies of the American College of Cardiology Foundation, the American Heart Association, the European Cardiac Arrhythmia Society, the European Heart Rhythm Association, the Society of Thoracic Surgeons, the Asia Pacific Heart Rhythm Society, and the Heart Rhythm Society. Europace 2012. March 1.
- 9. Seet RC, Friedman PA, Rabinstein AA. Prolonged rhythm monitoring for the detection of occult paroxysmal atrial fibrillation in ischemic stroke of unknown cause. Circulation 2011;124(4):477–486. [DOI] [PubMed] [Google Scholar]
- 10. Werkgroep richtlijn beroerte 2000 . Kwaliteitsinstituut voor de Gezondheidszorg in samenwerking met de Nederlandse Vereniging voor Neurologie. Richtlijn beroerte 2013. CBO. Ref Type: Generic
- 11. Roten L, Schilling M, Haberlin A, et al. Is 7‐day event triggered ECG recording equivalent to 7‐day Holter ECG recording for atrial fibrillation screening? Heart 2012;98(8):645–649. [DOI] [PubMed] [Google Scholar]
- 12. Dash S, Chon KH, Lu S, et al. Automatic real time detection of atrial fibrillation. Ann Biomed Eng 2009;37(9):1701–1709. [DOI] [PubMed] [Google Scholar]
- 13. Eitel C, Husser D, Hindricks G, et al. Performance of an implantable automatic atrial fibrillation detection device: Impact of software adjustments and relevance of manual episode analysis. Europace 2011;13(4):480–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. van DP, van GC, Houben RP, et al. Improving sensing and detection performance in subcutaneous monitors. J Electrocardiol 2009;42(6):580–583. [DOI] [PubMed] [Google Scholar]
- 15. Sarkar S, Ritscher D, Mehra R. A detector for a chronic implantable atrial tachyarrhythmia monitor. IEEE Trans Biomed Eng 2008;55(3):1219–1224. [DOI] [PubMed] [Google Scholar]
- 16. Duverney D, Gaspoz JM, Pichot V, et al. High accuracy of automatic detection of atrial fibrillation using wavelet transform of heart rate intervals. Pacing Clin Electrophysiol 2002;25(4 Pt 1):457–462. [DOI] [PubMed] [Google Scholar]
- 17. Kaufman ES, Israel CW, Nair GM, et al. Positive predictive value of device‐detected atrial high‐rate episodes at different rates and durations: An analysis from ASSERT. Heart Rhythm 2012;9:1241–1246. [DOI] [PubMed] [Google Scholar]
- 18. Kallmunzer B, Breuer L, Hering C, et al. A Structured reading algorithm improves telemetric detection of atrial fibrillation after acute ischemic stroke. Stroke 2012;43:994–999. [DOI] [PubMed] [Google Scholar]
- 19. Vasamreddy CR, Dalal D, Dong J, et al. Symptomatic and asymptomatic atrial fibrillation in patients undergoing radiofrequency catheter ablation. J Cardiovasc Electrophysiol 2006;17(2):134–139. [DOI] [PubMed] [Google Scholar]


