Abstract
Background: The significance of ST‐segment depression in acute coronary syndrome has been the subject of debate for many decades. Studies indicate that different manifestations of ST/T changes may have significantly different prognostic implications.
Methods and Results: We studied the correlation of ST/T changes in 12‐lead electrocardiography recorded during pain, to clinical and angiographic findings and in‐hospital prognosis, in patients with non‐ST‐elevation acute coronary syndrome and elevated troponin levels. Fifty consecutive patients could be differentiated into two groups: (1) 25 patients with ST‐segment depression and a negative T wave maximally in leads V4–5, (2) 25 patients with ST‐segment depression and a positive T wave in the precordial lead with maximal ST‐segment depression. Patients in group I had significantly more often left main or left main equivalent coronary artery disease; 76% versus 8% (P < 0.001), heart failure; 40% versus 4% (P = 0.005), and higher in‐hospital mortality; 24% versus 0% (P = 0.02), than patients in group II. The troponin levels did not differ significantly between the groups.
Conclusions: In patients with non‐ST‐elevation acute coronary syndrome and elevated troponin levels two subgroups could be identified. Transient ST‐segment depression and a negative T wave maximally in leads V4–5 during anginal pain predicts left main, left main equivalent, or severe three‐vessel coronary artery disease with high sensitivity and specificity. In patients with ST‐segment depression and a positive T wave, there is a high probability of one‐vessel disease.
Keywords: angina, electrocardiography, prognosis
During the last 60–70 years the significance of ST‐segment depression in acute coronary syndrome has been the subject for debate. In 1950, Levine and Ford described cases with subendocardial circumferential myocardial infarction. 1 They correlated anatomic endocardial lesions to electrocardiographic (ECG) changes in six patients with left main or severe three‐vessel coronary artery disease (CAD). The ECG changes consisted of widespread ST‐segment depression, often associated with widespread inversion of the T wave. These findings have later been confirmed by several authors. 2 , 3 , 4 , 5 Cook, Edwards, and Pruitt stated that ST‐segment depression and T‐wave inversion might occur in transient subendocardial ischemia. 6 They published detailed anatomical studies of large and small subendocardial infarcts, correlating to premortal ECG changes. Despite the studies of these legendary groups of investigators, no new progress in the topic appeared during the following years.
The medical communities did not accept the concepts, probably because it was not possible to reproduce circumferential ischemia in the experimental laboratory.
In the mid 1970s a few groups of investigators in Europe started to investigate the mechanisms of rest angina, spontaneous or induced by ergonovine maleate to provoke ischemia. 7 , 8 They repetitively found that subtotal occlusion of the left anterior descending (LAD) coronary artery, produced ST‐segment depression in leads V2–4. The same leads showed ST‐segment elevation when the artery was totally occluded.
For some reason, the findings of these two groups have not been compared. The different findings of these groups seem to represent two types of ischemia with significantly different clinical and prognostic differences.
The purpose of our study was to investigate the significance of ST‐segment depression and T‐wave changes in acute coronary syndrome, with respect to in‐hospital prognosis, troponin levels, and angiographic findings, in the modern era of cardiology.
METHODS
Subjects
We studied prospectively and consecutively, from November 2000 to March 2002, patients at Tampere University Hospital with acute coronary syndrome and transient ECG changes. Inclusion criteria were symptoms of myocardial ischemia associated with (1) ST‐segment depression (irrespective of orientation of the T wave) or (2) T‐wave inversion, in a 12‐lead ECG recorded during anginal pain, a positive troponin test, and coronary angiography performed during hospital stay.
Exclusion criteria were ST‐segment elevation (apart from leads a VR or V1), heart rate over 100 beats/min during the ECG recording (as tachycardia induces ST/T changes), structural heart disease, or previous bypass surgery.
We also excluded patients with chronic ECG changes: pathological Q waves, left ventricular hypertrophy, bundle branch block, preexcitation, or pacemakers.
All patients in both groups would have been classified into Braunwald class IIIB based on the clinical manifestation. 9 All of them had rest angina within 48 hours without secondary or postinfarction unstable angina. However, based on the newly introduced criteria, they are classified as non‐ST‐elevation MI (myocardial infarction). 10
The study complies with the Declaration of Helsinki. The ethics committee at Tampere University Hospital approved the study protocol. The patients gave their written informed consent for participation.
ECG Analysis
A standard 12‐lead ECG with maximal ST‐segment depression was chosen for measurements. The ECG was recorded at a paper speed of 50 mm/s at a calibration of 1 mV = 10 mm. Three investigators blinded to the angiographic findings analyzed the ECG manually. If the results were not in accord, consensus was found by discussion among the investigators. ST‐segment deviation from the isoelectric line, determined by drawing a line between subsequent PQ segments, was considered elevated or depressed if it was 0.5 mm or more above or below the isoelectric line, respectively. The T wave was considered positive or negative if it was 1 mm or more above or below the isoelectric line, measured more than 120 ms after the J point. The ST‐segment and T‐wave changes were measured separately from all 12 leads with the aid of a hand held magnifying lens. Left ventricular hypertrophy was defined by the Sokolow–Lyon criteria (SV1+ RV5–6≥ 35 mm). Pathological Q waves were defined by standard criteria. 11
Laboratory Analysis
Blood samples for troponin I (cTnI) were collected at baseline and after 6–12 hours. The normal value for cTnI in our hospital is <0.2 μg/L (ACS:180, Bayer Diagnostics, Tarrytown, NY).
Echocardiography
All patients underwent echocardiography by the cardiologist performing the coronary angiography. The examination was not done during chest pain. The ejection fraction (EF) was measured. Significant structural heart disease, for example, valve disease, or cardiomyopathy led to exclusion from the study.
Coronary Angiographic Evaluation
Selective coronary angiography by the femoral or radial route was performed in all patients. The indication for angiography was clinical, not investigational, in all cases. In most patients digital x‐ray equipment was used. The left coronary artery was evaluated from at least four projections (left and right anterior oblique, anteroposterior cranial, and caudal), and the right coronary artery from at least two projections. A significant stenosis was defined as >50% diameter obstruction of the coronary artery lumen diameter (Fig. 1A). Left main equivalent coronary artery disease (LME‐CAD) was defined as a diameter stenosis of >50% in the proximal segment of the left anterior descending and left circumflex artery. Flow in the coronary arteries was graded into four grades (0–3), as described in the Thrombolysis in Myocardial Infarction (TIMI) trial. 12
Figure 1.
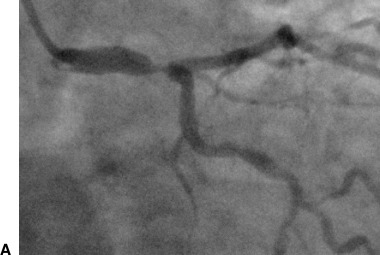
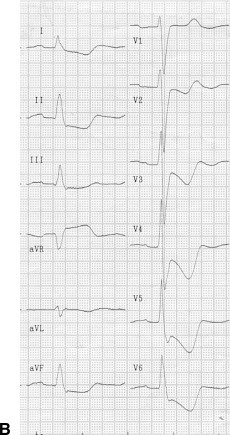
A patient in group I. (A) Coronary angiography shows tight stenosis in the distal left main coronary artery. (B) Electrocardiography (ECG) shows ST‐segment depression and inverted T waves, maximally in leads V4–5, and ST‐segment elevation in lead aVR (circumferential subendocardial ischemia).
Collateral circulation was classed into four grades according to the grading system of Rentrop et al. 13 Briefly, grade 0 was no collateral opacification, grade 1 filling of side branches, grade 2 partial, and grade 3 complete filling of the main branch by collateral vessels.
Severe three‐vessel disease (VD) was defined as significant or total obstruction of the proximal or mid‐segment of all three main epicardial arteries. Other cases with 3‐VD were classified as nonsevere.
The interpreters of the angiography were blinded to the ECG finding.
Statistical Analysis
Proportions were compared with the chi‐square test or Fisher's exact test and quantitative data were compared with the Mann–Whitney test. A probability value of <0.05 was considered statistically significant. All calculations were performed with the SPSS 7.5 statistical package.
RESULTS
We found a total of 52 patients fulfilling the inclusion criteria. Two patients had ST‐segment depression in the precordial leads and marginally significant ST‐segment elevation in lead III. They were excluded from the study after discussion among the investigators. Accordingly, the study group consisted of 50 patients (30 male, 20 female, mean age 69 ± 10 years). We found no patients with isolated T‐wave inversion during rest angina.
Two Patient Groups
According to the results of previous retrospective studies by a study group of one of the authors, 14 , 15 we decided to divide the patients into two groups: (1) group I (T−) consisted of patients with ST‐segment depression and a negative T wave maximally in leads V4–5 (Fig. 1B), (2) group II (T+) consisted of patients with ST‐segment depression and a positive T wave in the precordial lead with maximal ST‐segment depression (Fig. 2B). By chance the number of patients in both groups was 25. Patients in group I were older than those in group II, were smokers less often, had more often signs of universal atherosclerosis and previous angina pectoris, and were more often on aspirin therapy (Table 1). The cTnI levels were slightly higher in group I (median 11.2 μg/L) than in group II (median 3.1 μg/L), P = 0.06. Systolic blood pressure measured during the chest pain episode, when 12‐lead ECG was recorded, did not differ between the groups (147 vs 148 mmHg, P = 0.84). Diastolic pressure was lower in group I than in group II (73 vs. 81 mmHg, P = 0.04).
Figure 2.
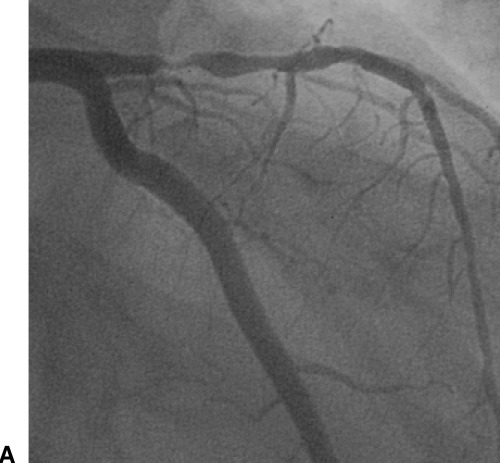
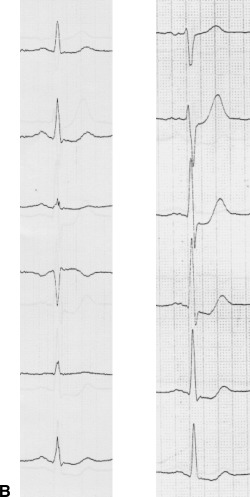
A patient in group II. (A) Angiography shows tight stenosis of the proximal left anterior descending coronary artery. (B) ECG (extremity leads left, precordial leads right) shows ST‐segment depression with positive T wave maximally in leads V3–5 (regional subendocardial ischemia).
Table 1.
Baseline Clinical Characteristics
| Group I (T−) n = 25 (%) | Group II (T+) n = 25 (%) | P Value | |
|---|---|---|---|
| Female gender | 44 | 36 | 0.77 |
| Active smoking | 4 | 32 | 0.03 |
| Hypertension | 60 | 48 | 0.57 |
| Diabetes mellitus | 20 | 12 | 0.70 |
| Universal atherosclerosis | 17 | 0 | 0.05 |
| Previous angina (>2 months) | 68 | 36 | 0.05 |
| Previous PCI | 0 | 4 | 1.00 |
| Previous non‐Q MI | 21 | 4 | 0.19 |
| Previous TIA | 25 | 0 | 0.01 |
| Renal dysfunction | 0 | 5 | 0.37 |
| Current use of medication | |||
| Aspirin | 68 | 24 | 0.004 |
| Beta‐blockers | 72 | 48 | 0.15 |
| Nitrates | 60 | 40 | 0.26 |
| Calcium‐antagonists | 32 | 12 | 0.17 |
| Digitalis | 8 | 8 | 1.00 |
| Statins | 28 | 16 | 0.50 |
| Age years (mean ± SD) | 74 ± 6 | 64 ± 11 | 0.001 |
MI = myocardial infarction; PCI = percutaneous coronary intervention; TIA = transient ischemic attack.
ECG Findings
Three patients in group I had slow atrial fibrillation. All other patients were in sinus rhythm during the ECG recording. In group I 52% of patients had the maximal ST‐segment depression and T‐wave inversion in lead V4 and 48% in lead V5. All patients in group I also had ST‐segment elevation of at least 0.5 mm in lead aVR. In group II all patients had a positive T wave in the lead with maximal ST‐segment depression and the localization of the maximal ST‐segment depression was in the precordial leads.
Angiographic Findings (Table 2)
Table 2.
Coronary Angiography Findings
| Number of Diseased Vessel | Group I (T−) n = 25 (%) | Group II (T+) n = 25 (%) | P Value |
|---|---|---|---|
| 0‐VD | 0 | 8 | 0.49 |
| 1‐VD | 0 | 56 | <0.001 |
| 2‐VD | 0 | 8 | 0.49 |
| Nonsevere 3‐VD | 0 | 20 | 0.05 |
| Severe 3‐VD | 24 | 0 | 0.02 |
| LM‐CAD or LME‐CAD | 76 | 8 | <0.001 |
LM‐CAD = left main coronary artery disease; LME‐CAD = left main equivalent coronary artery disease; VD = vessel disease.
The culprit artery could be defined in only 3 of 25 cases in group I. One patient had an acute plaque rupture of the left main coronary artery. In two cases there was significant stenosis in the left main without any other significant stenoses. In group II the culprit artery could be defined in 76% of cases (of these LAD in 74%, left circumflex in 26%). The mean delay from ECG registration to angiography was 7 days in both groups. Collateral flow grade 0 or 1 on angiography was present in 68% in group I and in 92% in group II, and grade 2 or 3 in 32% and 8%, respectively (P = 0.07). All patients with transient ST‐segment depression and negative T waves, maximally in leads V4–5, had severe 3‐VD, LM‐ or LME‐CAD on angiography (Table 3). All patients with severe 3‐VD presented with this ECG pattern.
Table 3.
The Sensitivity, Specificity, Positive Predictive Value (PPV) and Negative Predictive Value (NPV) for the ECG Pattern with Transient ST‐Segment Depression and Negative T Waves During Pain, Maximally in Leads V4–5, to Predict Different Coronary Artery Disease Severity in Angiography
| Angiography Findings | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|
| Severe 3‐VD | 100 | 57 | 24 | 100 |
| LM‐ or LME‐CAD | 91 | 79 | 76 | 92 |
| Severe 3‐VD or LM‐ or LME‐CAD | 93 | 100 | 100 | 92 |
Abbreviations as in Table 2.
In‐Hospital Follow‐up
The in‐hospital follow‐up event rate was higher in group I than in group II; in‐hospital mortality was 24% versus 0%, respectively (Table 4).
Table 4.
In‐Hospital Follow‐Up
| Group I (T−) n = 25 (%) | Group II (T+) n = 25 (%) | P‐value | |
|---|---|---|---|
| Clinical signs of heart failure | 40 | 4 | 0.005 |
| Ejection fraction | |||
| 30–49% | 42 | 8 | 0.008 |
| ≥50% | 58 | 92 | 0.008 |
| CABG | 76 | 20 | <0.001 |
| PCI | 12 | 52 | 0.005 |
| In‐hospital mortality | 24 | 0 | 0.02 |
CABG = coronary artery bypass grafting; PCI = percutaneous coronary intervention.
DISCUSSION
We set out to prospectively study a well‐defined and homogenous group of patients with acute coronary syndrome and ischemic ST‐segment depression during pain. We included only patients with transient ECG changes and elevated troponin levels. We excluded patients with confounding factors like left ventricular hypertrophy, bundle branch block, and cardiomyopathy. Based on the localization of the maximal ST‐segment depression and the direction of the T wave, we were able to identify two groups of patients, one with severe coronary artery disease and a high in‐hospital mortality, and another with predominantly one‐vessel disease and a good in‐hospital prognosis.
Deviation of the ST segment is a well‐recognized sign of ischemia. 16 ST‐segment elevation caused by sudden occlusion of a coronary artery is a well‐known ECG finding. 17 , 18 In contrast, transient ST‐segment depression in the precordial leads may be caused by different mechanisms like tachycardia, 19 remodeling in chronic Q‐wave anterior MI, 20 inferoposterior MI (reciprocal phenomenon 21 or a sign of multivessel CAD 22 ), and regional ischemia. 8
In 1946 Bayley described the correlation between subendocardial MI and ST‐segment depression. 23 This ECG finding, typical of diffuse injury of the subendocardial layer, was described as “injury‐against‐the‐rule,” that is, ST depression recorded by a precordial lead with the exploring electrode superjacent to an injured region. 24 In the 1940s researchers searched for the electrocardiographic manifestation of subendocardial injury. 25 , 26 It proved difficult, if not impossible, to produce subendocardial damage of sufficient extent and severity to produce measurable electrical effects while sparing a zone of uninjured myocardium between the traumatized tissue and the epicardium in animal models. Levine stated: “Nature, it seems, can fulfill the conditions of this experiment much more readily than can the physiologist.” 1 Still, in the modern era of cardiology, the prognostic implications of different ECG presentations of acute myocardial ischemia without ST‐segment elevation have not been well defined. 27
The Importance of ST‐Segment Depression and Inverted Asymmetric T Waves in Patients without Tachycardia (group I)
We found that the ECG pattern of ST‐segment depression and inverted T waves, maximally in leads V4–5, was strongly associated with LM‐CAD, LME‐CAD, or severe 3‐VD. This ECG pattern has been described by one of the authors as acute circumferential subendocardial ischemia. 28 Forty percent of the patients had clinical signs of heart failure, mostly pulmonary edema. The in‐hospital mortality was high.
Extensive ischemia, for example, caused by sudden obstruction of the left main coronary artery, impairs the relaxation of the left ventricle. 29 The resulting increase in left ventricular end‐diastolic pressure induces severe subendocardial ischemia. Elevation of left ventricular preload explains the high frequency of pulmonary edema in these cases. In animals a constriction of the left main coronary artery, causing global left ventricular ischemia, resulted in a significant decrease in the endocardial‐to‐epicardial flow ratio and a significant increase of end‐diastolic left ventricular transmural pressure. 30
Acute circumferential subendocardial MI is a well‐known clinical, electrocardiographic, and pathologic entity. Many authors have described the pattern of ST‐segment depression, often with negative T waves, as the typical ECG finding in these patients. 2 , 3 , 4 , 5 ST‐segment elevation in leads aVR and V1, reflects cavity potentials from the left ventricle, directed toward the right shoulder, consistent with injury of the subendocardial layer. 5
ST‐Segment Depression with Positive T Wave (group II)
Coronary angiography findings in the patients in group II were significantly less severe than those of the patients in group I. The majority of patients in group II had 1‐VD, about three quarters of these in LAD (Fig. 2A). Fifty‐two percent were treated by coronary angioplasty, and 20% by bypass surgery. In contrast only 12% in group I had angioplasty, and 76% bypass surgery. Only 1 patient in group II had clinical signs of heart failure, and the EF was normal in 92% of patients.
Repolarization of the ventricles generates the T wave. The cellular bases for the repolarization waves in the ECG are not well known. Animal studies have shown that the morphology of the T wave measured across the left ventricular wall appears to be due in large parts to currents flowing down voltage gradient present on the epicardial and endocardial sides of the M‐cell layer, during phases 2 and 3 of the ventricular action potential. 31 Whether the transmural repolarization gradients suggested by in vitro studies are manifest in humans has been the subject of ongoing controversy. 32 In dogs coronary artery ligation resulted in shortening of the action potential in the ischemic epicardial layer generating a tall and peaked T wave. 33 The first electrocardiographic manifestation of a subtotal obstruction of LAD in one‐vessel disease is ST‐segment depression and a positive T wave. 8 The tall T waves are probably caused by high extracellular potassium. 34 This is related to a hyperpolarization of the myocytes due to an opening of the K+‐ATP channel. 35 ST‐segment depression in these patients is most probably caused by a regional, nonextensive, subendocardial ischemia. 7 , 8 , 28 Our finding that no patients in group II had pulmonary edema, and that there was no severe 3‐VD supports this finding. ST‐segment depression with positive T wave has been associated with a high incidence of 1‐VD. 14 The dissociation between ST‐segment and T‐wave orientation is an unusual ECG finding, and represents a challenge for ECG interpretation in acute ischemic syndromes.
Limitations
The number of patients is small. We think, though, that the message from this study has great clinical impact. We did not include patients with normal troponin levels or ST‐segment elevation (apart from leads aVR or V1). There may be some patients with LM‐ or LME‐CAD presenting with this ECG pattern.
Clinical Implications
Our task was to try to prospectively test the relevance of the findings in the literature that different types of ST‐segment depression and T wave changes represent different types of ischemia. Despite the small number of patients the results showed a highly significant statistical difference in disease severity between two groups of patients with distinct prespecified ST/T changes. Our finding should have important clinical impact.
Funding: Medical Research Fund of Tampere University Hospital and The Pirkanmaa Regional Fund of the Finnish Cultural Foundation.
REFERENCES
- 1. Levine HD, Ford RV. Subendocardial infarction: Report of six cases and critical survey of the literature. Circulation 1950;1: 246–263. [DOI] [PubMed] [Google Scholar]
- 2. Myers GB, Sears CH, Hiratzka T. Correlation of electrocardiographic and pathologic findings in ring‐like subendocardial infarction of the left ventricle. Am J Med Sci 1951;222: 417–426. [DOI] [PubMed] [Google Scholar]
- 3. Hackel DB, Wagner GS. Acute circumferential subendocardial infarction. Clin Cardiol 1992;15: 373–376. [DOI] [PubMed] [Google Scholar]
- 4. Raunio H, Rissanen V, Romppanen T, et al Changes in the QRS complex and ST segment in transmural and subendocardial myocardial infarctions. A clinicopathological study. Am Heart J 1979;98: 176–184. [DOI] [PubMed] [Google Scholar]
- 5. Yu PNG, Stewart JM. Subendocardial myocardial infarction with special reference to the electrocardiographic changes. Am Heart J 1950;39: 862–880. [DOI] [PubMed] [Google Scholar]
- 6. Cook RW, Edwards JE, Pruitt RD. Electrocardiographic changes in acute subendocardial infarction. I. Large subendocardial and large nontransmural infarcts. Circulation 1958;18: 603–612. [DOI] [PubMed] [Google Scholar]
- 7. De Servi S, Spechia G, Angoli L. Coronary artery spasm of different degrees as cause of angina at rest with ST segment depression and elevation. Br Heart J 1979;42: 110–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Parodi O, Uthurralt N, Severi S, et al Transient reduction of regional myocardial perfusion during angina at rest with ST‐segment depression or normalization of negative T waves. Circulation 1981;63: 1238–1247. [DOI] [PubMed] [Google Scholar]
- 9. Braunwald E. Unstable angina: A classification. Circulation 1989;80: 410–414. [DOI] [PubMed] [Google Scholar]
- 10. The Joint European Society of Cardiology/American College of Cardiology Committee . Myocardial infarction redefined—a consensus document of the Joint European Society/American College of Cardiology Committee for the Redefinition of Myocardial Infarction. Eur Heart J 2000;21: 1502–1513. [DOI] [PubMed] [Google Scholar]
- 11. ACC Clinical data Standards . American College of Cardiology key data elements and definitions for measuring the clinical management and outcomes of patients with acute coronary syndrome. J Am Coll Cardiol 2001;38: 2114–2130. [DOI] [PubMed] [Google Scholar]
- 12. Chesebro JH, Knatterud G, Roberts R, et al Thrombolysis in Myocardial Infarction (TIMI) trial, Phase I: A comparison between intravenous tissue plasminogen activator and intravenous streptokinase. Clinical findings through hospital discharge. Circulation 1987;76: 142–154. [DOI] [PubMed] [Google Scholar]
- 13. Rentrop KP, Cohen M, Blanke H, et al Changes in collateral channel filling immediately after controlled coronary artery occlusion by an angioplasty balloon in human subjects. J Am Coll Cardiol 1985;5: 587–592. [DOI] [PubMed] [Google Scholar]
- 14. Sclarovsky S, Rechavia E, Strasberg B, et al Unstable angina: ST segment depression with positive versus negative T wave deflections—clinical course, ECG evolution, and angiographic correlation. Am Heart J 1988;116: 933–941. [DOI] [PubMed] [Google Scholar]
- 15. Sclarovsky S, Davidson E, Strasberg B, et al Unstable angina: The significance of ST segment elevation or depression in patients without evidence of increased myocardial oxygen demand. Am Heart J 1986;112: 463–467. [DOI] [PubMed] [Google Scholar]
- 16. Mirvis DV, Goldberger AL. Electrocardiography In Braunwald E, Zipes DP, Libby P. (eds.): Heart Disease. Philadelphia , PA , W.B. Saunders, 2001, pp. 82–128. [Google Scholar]
- 17. Wilson FN, Johnston FD, Hill IGW, et al The electrocardiogram in later stages of experimental myocardial infarction. Proc Assoc Am Physicians 1933;48: 154–163. [Google Scholar]
- 18. Pardee HEB. An electrocardiographic sign of coronary artery obstruction. Arch Intern Med 1920;26: 244–257. [Google Scholar]
- 19. Serizawa T, Carabello B, Grossman W. Effect of pacing inducing ischemia on left ventricular diastolic pressure‐volume relations in dogs with coronary stenoses. Circ Res 1980;46: 430–439. [DOI] [PubMed] [Google Scholar]
- 20. Assali A, Sclarovsky S, Herz I, et al The clinical significance of ST‐segment depression with inverted T wave in V4‐V6 in chronic anterior myocardial infarction. J Am Coll Cardiol 2000;35: 352–357. [DOI] [PubMed] [Google Scholar]
- 21. Sclarovsky Y, Topaz O, Rechavia E. Ischemic ST segment depression in leads V2‐V3 as the presenting electrocardiographic feature of posterolateral wall myocardial infarction. Am Heart J 1987;113: 1085–1090. [DOI] [PubMed] [Google Scholar]
- 22. Hasdai D, Sclarovsky S, Solodky A, et al Prognostic significance of maximal precordial ST‐segment depression in right (V1 to V3) versus left (V4 to V6) leads in patients with inferior wall acute myocardial infarction. Am J Cardiol 1994;74: 1081–1084. [DOI] [PubMed] [Google Scholar]
- 23. Bayley RH. The electrocardiographic effects of injury at the endocardial surface of the left ventricle. Am Heart J 1946;31: 677–684. [DOI] [PubMed] [Google Scholar]
- 24. Bayley RH, LaDue JS. The electrocardiographic evidence of local ventricular ischemia Symposium on heart disease sponsored by Louisiana State University. Baton Rouge , LA , Louisiana State University Press, 1944. [Google Scholar]
- 25. Wolferth CC, Bellet S, Livezey MM, et al Negative displacement of ST segment in the electrocardiogram and its relationship to positive displacement. An experimental study. Am Heart J 1945;29: 220–245. [PubMed] [Google Scholar]
- 26. Sodi‐Pallares D, Vizcaino M, Soberon J, et al Comparative study of the intracavitary potential in man and dog. Am Heart J 1947;33: 819–848. [DOI] [PubMed] [Google Scholar]
- 27. Savonitto S, Ardissino D, Granger CB, et al Prognostic value of the admission electrocardiogram in acute coronary syndromes. JAMA 1999;281: 707–713. [DOI] [PubMed] [Google Scholar]
- 28. Sclarovsky S. Electrocardiography of Acute Myocardial Ischaemic Syndromes. London , UK , Martin Dunitz Ltd, 1999, p. 15. [Google Scholar]
- 29. Baim DS, Grossman W. Grossman's Cardiac Catheterization, Angiography, and Intervention. 6th edition Philadelphia , PA , Lippincott Williams & Wilkins, 2001, p. 382. [Google Scholar]
- 30. Visner MS, Arentzen CE, Parrish DG, et al Effects of global ischemia on the diastolic properties of the left ventricle in the conscious dog. Circulation 1985;71: 610–619. [DOI] [PubMed] [Google Scholar]
- 31. Yan G‐X, Antzelevitch C. Cellular basis for the normal T wave and the electrocardiographic manifestations of the long‐QT syndrome. Circulation 1998;98: 1928–1936. [DOI] [PubMed] [Google Scholar]
- 32. Antzelevitch C. Transmural dispersion of repolarization and the T wave. Cardiovasc Res 2001;50: 426–431. [DOI] [PubMed] [Google Scholar]
- 33. Burgess MJ, Lux RL. Physiologic basis of the T wave In Schlant RS, Hurst JW. (eds.): Advances in Electrocardiography. Vol 2 New York , NY , Grune & Stratton, 1976, pp. 327–337. [Google Scholar]
- 34. Katz AM. Physiology of the Heart. 3rd ed Philadelphia , PA , Lippincott Williams & Wilkins, 2001, p. 644. [Google Scholar]
- 35. Kondo T, Kubota I, Tachibana H, et al Glibenclamide attenuates peaked T wave in early phase of myocardial ischemia. Cardiovasc Res 1996;31: 683–687. [DOI] [PubMed] [Google Scholar]


