Abstract
Background: Nonsustained ventricular tachycardia (nVT) may have ominous implications for patients with hypertrophic cardiomyopathy (HCM). The microvolt T‐wave alternans (TWA) has been proposed as a noninvasive tool‐identifying patients at risk of sudden cardiac death and ventricular tachycardia/fibrillation (VT/VF). The aim of the study was to determine the significance of TWA in predicting nVT episodes and compare how other electrocardiographic parameters can predict the occurrence of nVT.
Methods: The study group consisted of 88 patients with HCM. TWA was assessed during exercise test using the CH2000 system. All patients underwent Holter monitoring (HM) within 2–4 weeks before TWA test (preexercise HM1) and immediately after (postexercise HM2). During HM, we analyzed: arrhythmias, QT intervals, the presence of late ventricular potentials (LP), heart rate variability, heart rate turbulence.
Results: Depending on TWA results, the patients were divided into two groups: TWA+; 46 patients (52.3%) with positive/indeterminate results, and TWA–; 42 patients (47.7%) with negative results. The nVT episodes were more frequent among TWA(+) both in HM1 and HM2. The presence of TWA increases the risk of postexercise nVT over twenty times (OR = 21.03).
Moreover, in HM1, QTc and LP, and in HM2, again QTc and N‐terminal precursor of brain natriuretic peptide proved to be significant predictors of nVT. The addition of TWA to the models did not improve the arrhythmia risk assessment.
Conclusions: Repolarization abnormality plays an important role in generating nVT in patients with HCM, but TWA does not specifically predict the risk of arrhythmic end point.
Ann Noninvasive Electrocardiol 2011;16(3):276–286
Keywords: hypertrophic cardiomyopathy, T‐wave alternans, risk of nVT episodes
Hypertrophic cardiomyopathy (HCM) is a genetically determined heart muscle disorder, occurring with the frequency of 1:500, similar for men and women. The most devastating consequence of this disease is sudden cardiac death (SCD), occurring with the frequency up to 2% annually among adults and up to 6% annually among children, regardless of the intensity of HCM symptoms. 1 Therefore, in accordance with the guidelines of the American College of Cardiology and the European Society of Cardiology, all HCM patients should undergo sudden death risk assessment. 2 , 3
The highest risk of SCD has been associated with: (1) prior cardiac arrest or spontaneously occurring sustained ventricular tachycardia (VT), (2) the family history of premature SCD, (3) unexplained syncope particularly in young patients, (4) nonsustained ventricular tachycardia (nVT), (5) extreme left ventricular (LV) hypertrophy with wall thickness >30 mm, and (6) abnormal blood pressure response during upright exercise.
However, most of the clinical markers of SCD risk in HCM are limited by a relatively low positive predictive value due to low event rates. 4 , 5 , 6 On the other hand, the most important aspect of the management of patients with HCM is to assess SCD risk. Preventive strategies may then be considered, including implantable cardioverter defibrillator (ICD) implantation. Ventricular arrhythmias may have ominous implications in HCM patients. 7 , 8 , 9 Sudden death is primarily related to hemodynamically unstable VT or ventricular fibrillation (VF). A substrate for dangerous arrhythmias in HCM is structurally abnormal myocardium. Myocardial disarray, ischemia, and fibrosis modify the electrophysiological properties of cardiomyocytes, which leads to dispersion and diversification of the impulse conduction, and which promotes the reentry phenomenon. 10 Although sustained VT is very dangerous, especially if it is hemodynamically unstable, other ventricular arrhythmias may also carry unfavorable prognosis in HCM. Numerous studies indicated that nVT in Holter monitoring (HM) was associated with 2‐ to 2.5‐fold increase in SCD risk. 4 , 5 , 7 , 8 A very interesting but less well‐studied aspect in HCM group is the prevalence of VT during exercise. Gimeno et al. showed that exercise‐induced nVT was associated with an increased risk of SCD. 11
Early determination of arrhythmia risk profile is of crucial importance in ICD era. Numerous noninvasive electrocardiographic parameters are used for the assessment of risk of dangerous arrhythmias. However, in HCM they have been rarely used and were found to be inadequate. 12 The recently introduced microvolt T‐wave alternans (TWA) seems to be another promising diagnostic method and its significance in HCM patients has not been established.
This study sought (1) to evaluate the usefulness of TWA for the prediction of VT episodes and (2) to determine the most useful noninvasive model of VT risk prediction in patients with HCM.
METHODS
The study group consisted of patients diagnosed with HCM, remaining in the care of the 2nd Chair and Department of Cardiology of the Medical University of Lodz and of the Outpatient Cardiology Clinic.
The diagnosis of HCM was defined as an otherwise unexplained increase in the end‐diastolic wall thickness >15 mm in at least one myocardial segment. 3
Standard transthoracic echocardiograms were performed with ultrasound system Vivid 7 (GE Healthcare, Waukesha, WI, USA). The following variables were taken into consideration:
-
1
LV end‐diastolic diameter,
-
2
left atrial diameter,
-
3
interventricular septum thickness,
-
4
LV muscle mass,
-
5
maximum LV outflow gradient (a resting gradient higher than 30 mmHg was defined as significant),
-
6
the presence of mitral valve systolic anterior motion toward the septum and
-
7
LV ejection fraction (LVEF).
Pulsed Doppler recordings at the tip of the mitral leaflets were used to determine peak early filling (E‐wave) velocity, peak atrial filling (A‐wave) velocity, and E/A ratio. Family history of SCD, the frequency of syncopal episodes and the New York Heart Association (NYHA) class were analyzed for all the patients.
The value of serum N‐terminal precursor of brain natriuretic peptide (NT‐proBNP) was measured by means of electrochemiluminescence immunoassay on an Elecsys 1010 analyzer (Roche Diagnostics GmbH, Mannheim, Germany).
Holter Monitoring
All study subjects underwent two sessions of 24‐hour electrocardiogram (ECG) HM:
-
1
The first one was performed during routine daily activities within 2–4 weeks before the TWA exercise test (preexercise HM1).
-
2
The second Holter session was started immediately after the TWA exercise test (postexercise HM2).
Both Holter sessions were performed by means of the CardioScan 12 system (DMSoftware, Stateline, NV, USA). Three‐channel recorders of the type 300–7 were used. The sampling rate was 1024 Hz for the first 10 minutes and 512 Hz for the rest of the recording. The electrodes were placed on the chest using Frank's orthogonal X, Y, Z lead system.
Arrhythmia Analysis
The analysis was carried out in a typical manner, evaluating the average, the maximal and the minimal heart rate, the occurrence of premature beats, and of episodes of nVT defined as three or more consecutive ventricular beats at a rate >100 per minute lasting less than 30 seconds.
Heart Rate Variability (HRV) Analysis
HRV analysis was performed according to Standards of the Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. 13 Time‐domain parameters: standard deviation (SDNN) (ms), RMSSD (ms), pNN50, were determined for each 24‐hour period. Spectral analysis was made using the Fast Fourier transform (FFT, CardioScan 12.0 Version 12.4 software package). Five‐minute fragments were repeatedly transformed and averaged throughout the 24‐hour period. The following frequency‐domain parameters were assessed: total power spectrum; power spectrum within low‐frequency range (LF) 0.04–0.15 Hz; power spectrum within high‐frequency range (HF) 0.15–0.40 Hz. The LF and HF values were expressed as ms2. The LF/HF ratio was also calculated for each subject.
Heart Rate Turbulence (HRT) Analysis
The analysis was done using an algorithm adapted from the Web page for noncommercial use of HRT (http://www.h-r-t.org). Two parameters describing HRT were calculated. Turbulence onset (TO) was defined as the difference between the mean of the first two sinus RR intervals after ventricular premature beat (VPB), and of the last two sinus RR intervals before VBP, divided by the mean value of the last two sinus RR intervals before VPB. TO (expressed in percent) was calculated for each VPB and then averaged. Turbulence slope (TS) was defined as the maximum positive value of the slope of a regression line assessed over any sequence of five subsequent sinus RR intervals within the first 20 sinus intervals after VPB. TS was calculated on the basis of an averaged local tachogram. The TS value was expressed in ms/RR interval. The HRT was defined as abnormal if the TO was >0% or/and the TS was <2.5 ms/RR. 14
QT Interval Analysis
The CardioScan 12 system has the advanced QT validation program. QT was analyzed on a “beat‐by‐beat” basis separately for each channel. The system regarded as the end of the T wave the point determined by the intersection of a line tangential to the downslope of the T wave with the isoelectric baseline. The statistical analysis considered the longest QT interval. The corrected QT interval (QTc) was calculated according to Bazett's formula: QTc = QT/√RR(s).
The Analysis of the Presence of Ventricular Late Potentials (LP)
The analysis was carried out from the first 10 minutes of the ECG monitoring, while the patient remained in the recumbent position. For the analysis, 250–300 QRS complexes of the best technical quality were chosen. Time‐domain analysis was then performed on filtered QRS complexes, obtained after the signal had been amplified and averaged. LP were recognized when at least two of the following criteria were met. 15
-
1
The duration of a filtered QRS complex >114 ms.
-
2
The root mean square of the voltage of the final 40 ms of the filtered QRS (RMS40) < 20 μV.
-
3
The duration of low‐amplitude signals of the filtered QRS < 40 μV (LAS) > 38 ms.
Measurement of TWA
TWA was measured at rest and during exercise using a CH2000 system (Cambridge Heart Inc., Bedford, MA, USA) based on the spectral method of analysis described by Smith et al. 16 After careful skin preparation, seven standard electrodes were placed in standard lead positions and seven multisegment special (high resolution) electrodes were arranged in Frank's orthogonal (XYZ) configuration. The manual treadmill exercise test to achieve the heart rate of 120 bpm for at least 2 minutes was performed. However, the occurrence of alarming symptoms (e.g., chest pain, dyspnea, dizziness, arrhythmia, or ischemia in ECG) was the reason for the premature termination of the test. The maximal achieved work load was expressed in metabolic equivalents of task (METs). Blood pressure response during exercise was considered abnormal when the systolic blood pressure did not increase by 10 mmHg or more during exercise. 17 The alternans was measured at a heart rate of 100–110 and 110–120 beats/min. The test was considered positive if the alternans voltage was ≥1.9 μV and the alternans ratio was >3.0 with an onset heart rate ≤110 beats/min. The test was considered negative if the alternans was absent during a sustained interval of exercise at a heart rate >105 beats/min. If the result did not meet the criteria described above, the test was considered indeterminate.
All the patients underwent the examination while taking their regular medications, including beta‐blockers.
Statistical Analysis
Calculations were made using STATISTICA PL 7.1 and SPSS 14.0 (SPSS Inc., Chicago, IL, USA).
Continuous variables were presented as mean ± SD and range (min‐max); frequencies and percents were presented for categorical variables. Differences between groups for continuous data were tested using an independent samples t‐test (for normally distributed variables) or by means of the Mann‐Whitney U tests (if the distribution of variables was different from normal). For categorical variables, the statistical analysis was based on the chi‐square test for independence or Fisher's exact test.
To assess the connection between the selected variables and the risk of the occurrence of nVT episodes, the univariate Cox regression analysis was used. Odds ratios (OR) were presented with 95% CI. Backward stepwise logistic regression was applied to create two optimizing models that were used to evaluate the significance of TWA in the prediction of nVT episodes. Sensitivity and specificity analyses were performed on both of these models and receiver operating characteristic (ROC) curves were generated. The results of the analyses were considered significant at a level of P < 0.05.
RESULTS
Our study comprised 88 patients. They had NYHA functional class I (43 patients) or II (45 patients). All of them performed a treadmill exercise test and the presence of TWA was assessed. No patient had nVT episodes or other alarming symptoms during the exercise test. The mean maximal heart rate was 120 beats/min, and the mean maximal work load was 5.4 METs. Abnormal blood pressure response during exercise was observed in 39 (44.3%) patients.
The TWA test was positive in 44 (50%), negative in 42 (47.7%) patients, and indeterminate in 2 (2.3%). The indeterminate tests were completed but not classifiable as positive or negative because of noise. On the grounds of the test result, the patients were divided into two groups: group 1—TWA(+): 46 (52.3%) patients with positive or indeterminate test result and group 2—TWA(–): 42 (47.7%) patients with negative test result.
A comparison of demographic and clinical characteristics between TWA(+) and TWA(–) patients is presented in Table 1. The positive result of the TWA test was observed more often among men (76.1% vs 42.9%; P < 0.001). TWA(+) patients were significantly more frequently in NYHA class II and had a higher serum NT‐proBNP value than those from the TWA(–) group (445.7 vs 165.2 pg/mL; P < 0.001). The episodes of unexplained syncope in the past were more often reported by patients from the TWA(+) group (67.4% vs 14.3%; P < 0.0001), but the frequency of sudden death among family members was similar in both groups (26.1% vs 14.3%; P = 0.15). From among all the analyzed echocardiographic parameters, only the left ventricular outflow tract gradient was more common in the TWA(+) group (67.4% vs 19%; P < 0.001).
Table 1.
Demographic and Clinical Profile of HCM Patients with TWA(+) and TWA(–)
| Parameter | TWA(–) n = 42 | TWA(+) n = 46 | P‐Value |
|---|---|---|---|
| Age (years) | 49.52 ± 16.36 | 53.98 ± 10.84 | 0.160 |
| Gender (F/M) | 24/18 | 11/35 | 0.001 |
| Family history of SCD | 6 (14.3%) | 12 (26.1%) | 0.06 |
| Syncope | 6 (14.3%) | 31 (67.4%) | <0.001 |
| NYHA class II | 17 (40.5%) | 28 (60.9%) | 0.04 |
| NT‐proBNP (pg/mL) | 165.2 ± 59.2 | 445.7 ± 223.9 | <0.001 |
| IVS (mm) | 21.7 ± 3.9 | 22.02 ± 4.19 | 0.7 |
| LV mass (g) | 319.6 ± 103.87 | 329.4 ± 83.7 | 0.5 |
| LVEF (%) | 57.4 ± 7.9 | 56.3 ± 7.3 | 0.9 |
| Patients with LVOT gradient >30 mmHg | 8 (19%) | 31 (67.4%) | <0.001 |
| Patients with abnormal BP response | 19 (45.2%) | 20 (43.4%) | 0.9 |
BP= blood pressure; IVS = interventricular septum thickness; LVEF = left ventricular ejection fraction; LVOT = left ventricular outflow tract; NT‐proBNP = N‐terminal precursor of brain natriuretic peptide; SCD = sudden cardiac death.
The results of both HM sessions: preexercise (HM1) and postexercise (HM2), are presented in Tables 2 and 3. The nVT episodes were more frequent among TWA(+) patients in both HM1 (54.3%) and HM2 (67.4%). LP were more often found in the TWA(+) group (41.3% vs 21.4%) (Table 2). Those patients had also longer QTc in both Holter sessions. The HRV parameters, both time domain and frequency domain, as well as the HRT parameters, did not differ significantly in both the studied groups.
Table 2.
Comparison of the Results of the Preexercise Holter Monitoring (HM1) in HCM Patients with TWA(+) and TWA(–)
| Variable | TWA(–) N = 42 | TWA(+) N = 46 | P‐Value |
|---|---|---|---|
| Heart rate (beats/min) | |||
| Maximal | 120 ± 15.2 | 115.6 ± 15.6 | 0.5 |
| Minimal | 46.4 ± 7.3 | 45.5 ± 5.8 | 0.7 |
| Mean | 67.4 ± 8.5 | 65.5 ± 6.7 | 0.3 |
| The number of PVB | 1048.5 ± 116.5 | 1491.8 ± 127.5 | 0.09 |
| Patients with nVT episodes | 5 (11.9%) | 25 (54.3%) | <0.001 |
| SD (ms) | 124.4 ± 25.7 | 126.3 ± 30 | 0.8 |
| LF (ms2) | 441.1 ± 120.7 | 462.8 ± 125.1 | 0.4 |
| HF (ms2) | 215.3 ± 93.4 | 203.5 ± 94.3 | 0.8 |
| TS ms/RR | 3.5 ± 1.4 | 3.2 ± 1.1 | 0.6 |
| TO (%) | −0.8 ± 1.7 | −0.7 ± 1.4 | 0.9 |
| QTc (ms) | 432 ± 20.1 | 475.4 ± 36.8 | <0.001 |
| Patients with LP | 9 (21.4%) | 19 (41.3%) | 0.05 |
HF = high‐frequency power spectrum; LF = low‐frequency power spectrum; LP = ventricular late potentials; NVT = nonsustained ventricular tachycardia (preexercise); PVB = premature ventricular beat; QTc = corrected QT interval; SD = standard deviation; TO = turbulence onset; TS = turbulence slope.
Table 3.
Comparison of the Results of the Postexercise Holter Monitoring (HM2) in HCM Patients with TWA(+) and TWA(–)
| Variable | TWA(–) | TWA(+) | P‐Value |
|---|---|---|---|
| The number of PVB | 908.6 ± 217.3 | 1597.2 ± 532.4 | 0.07 |
| Patients with nVT episodes | 3 (7.1%) | 31 (67.4%) | <0.001 |
| SD (ms) | 121.8 ± 31.4 | 128.1 ± 27.3 | 0.7 |
| LF (ms2) | 468.1 ± 147.2 | 484.3 ± 156.3 | 0.5 |
| HF (ms2) | 221.3 ± 88.4 | 236.2 ± 87.2 | 0.8 |
| TS ms/RR | 3.8 ± 1.6 | 4.1 ± 2.0 | 0.7 |
| TO (%) | −0.9 ± 2.2 | −0.8 ± 1.8 | 0.8 |
| QTc (ms) | 440.9 ± 32.2 | 470.5 ± 46.2 | <0.001 |
| Patients with LP | 8 (19.0%) | 17 (37%) | 0.05 |
HF = high‐frequency power spectrum; LF = low‐frequency power spectrum; LP = ventricular late potentials; NVT = nonsustained ventricular tachycardia (postexercise); PVB = premature ventricular beat; QTc = corrected QT interval; SD = standard deviation; TO = turbulence onset; TS = turbulence slope.
To assess the influence of clinical, echocardiographic, and electrocardiographic variables on the risk of the appearance of nVT, the univariate regression analysis was performed. Although the patients with a positive result of the TWA test had a markedly increased risk of nVT occurrence in standard HM (OR = 9.25), the highest risk of this arrhythmia was connected with the presence of LP (Table 4).
Table 4.
Parameters Showing an Independent Relationship with Runs of nVT in Pre‐ and Postexercise Monitoring (HM1 and HM2); Univariate Regression Analysis
| Parameters (Variables) | Preexercise Monitoring: HM1 OR (95% CI) | Postexercise Monitoring: HM2 OR (95% CI) |
|---|---|---|
| Family history of SCD | 3.5 | 3.01 |
| (1.35–9.41) | (1.34–9.05) | |
| Syncope in the past | 3.06 | 4.28 |
| (1.3–7.24) | (1.3–7.8) | |
| NT‐proBNP | 1.05 | 1.07 |
| (1.00–1.32) | (1.01–1.41) | |
| TWA(+) | 9.25 | 21.03 |
| (3.07–27.9) | (6.28–57.4) | |
| QTc | 1.04 | 1.05 |
| (1.02–1.41) | (1.02–1.5) | |
| LP | 10.8 | 11.4 |
| (3.5–33.28) | (3.5–33.3) |
LP = ventricular late potentials; NT‐proBNP = N‐terminal precursor of brain natriuretic peptide; QTc‐ =corrected QT interval.
However, when the parameters obtained from the postexercise HM were analyzed, TWA turned out to be the most important predictor of arrhythmia. Interestingly enough, the presence of TWA was related to a strong increase in the risk of postexercise nVT (OR = 21) (Table 4).
To create the models useful to assess the risk of the occurrence nVT episodes and determine the role of TWA in the prediction of risk, backward stepwise logistic regression was applied. Two models of variables were created to evaluate the significance of TWA in the prediction of nVT episodes: the first one with TWA test results and the second one without TWA.
The independent predictors of nVT were identified separately for two Holter sessions: preexercise (HM1) and postexercise (HM2).
Predictors of nVT Risk in Preexercise HM (HM1)
Only QTc and LP were independent predictors of nVT episodes recorded in preexercise Holter recording. A diagnostic model including QTc and LP measurement made it possible to predict the risk of nVT with high specificity (89.8%) and with reasonable sensitivity (63.9%). The inclusion of TWA in this model did not improve the arrhythmia risk assessment (Tables 5 and 6).
Table 5.
Optimized Statistical Models Used for the Assessment of the Potential Risk of the Occurrence of nVT Episodes in Holter Monitoring
| (a) Preexercise/(HM1): Assessment of the Potential Risk of the Occurrence of Spontaneous nVT Episodes (nVT1) | ||
|---|---|---|
| Variables | Model without TWA OR (95% CI) | Model with TWA OR (95% CI) |
| LP1 | 3.70 (1.02–13.51) | 2.16 (0.49–9.51) |
| QTc 1 | 1.03 (1.01–1.42) | 1.02 (1.00–1.42) |
| TWA+ | 2.76 (0.72–10.50) | |
| (b) Postexercise (HM2): Assessment of the Potential Risk of the Occurrence of Postexercise nVT Episodes | ||
| Variables | Model without TWA OR (95% CI) | Model with TWA OR (95% CI) |
| NT‐proBNP | 1.06 (1.00–1.41) | 1.01 (1.00–1.40) |
| QTc 2 | 1.04 (1.00–1.52) | 1.02 (0.96–1.51) |
| TWA+ | 4.04 (0.88–18.5) | |
LP = ventricular late potentials; NT‐proBNP = N‐terminal precursor of brain natriuretic peptide; QTc = corrected QT interval.
Table 6.
The Usefulness of the Two Diagnostic Models (with TWA and without TWA) in the Prediction of nVT Episodes
| Diagnostic models | Prediction of Preexercise nVT | Prediction of Postexercise nVT | ||
|---|---|---|---|---|
| LP(1) + QTc (1) | LP(1) + QTc (1) + TWA | NT‐proBNP + QTc (2) | NT‐proBNP + QTc (2) + TWA | |
| Sensitivity | 63.9% | 66.7% | 80.0% | 80.0% |
| Specificity | 89.8% | 85.9% | 94.3% | 92.2% |
| PPV | 79.3% | 71.4% | 90.2% | 88.8% |
| NPV | 80.3% | 83.0% | 87.3% | 89.5% |
LP = ventricular late potentials; NPV = negative predictive value; PPV = positive predictive value; QTc = corrected QT interval.
Additionally, to assess the discrimination ability of the models in the prediction of preexercise nVT episodes, the areas under the ROC curve were compared and no significant differences were observed (Fig. 1).
Figure 1.
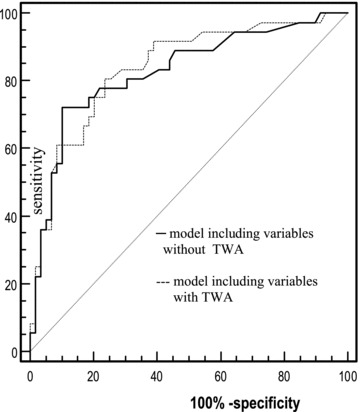
ROC curves of multivariate logistic regression. Sensitivity and specificity of the models in the prediction of preexercise nVT episodes. No significant differences between the models with and without TWA. Area under curve (AUC) = 0.011; asymptotic 95%; confidence interval (–0.045–0.067); P = 0.692.
Assessment of nVT Risk in Immediate Postexercise Monitoring (HM2)
A diagnostic model including one clinical variable (NT‐proBNP) and one electrocardiographic parameter (QTc) made it possible to predict the risk of postexercise nVT episodes with the specificity of 94.3% and sensitivity of 80% (Tables 5 and 6). The addition of the TWA test did not improve the predictive value, although nVT was more common among patients in whom the TWA test was positive. As is shown in Figure 2, the discrimination ability of the two models in the prediction of postexercise nVT episodes was comparable.
Figure 2.
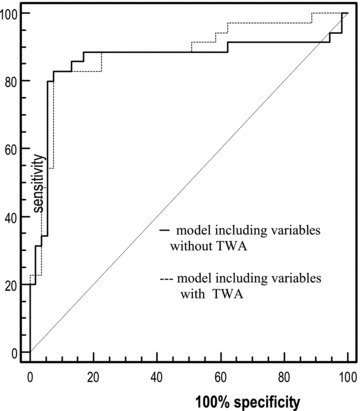
ROC curves of multivariate logistic regression. Sensitivity and specificity of the models in the prediction of postexercise nVT episodes. No significant differences between the models with and without TWA. Area under curve (AUC) = 0.019; asymptotic 95%; confidence interval (–0.042–0.080); P = 0.545.
DISCUSSION
The novel findings of our study demonstrate the feasibility of the TWA test and its strong relationship with the presence of VT in HM. Microvolt TWA was found in more than half of our study patients with HCM. The presence of TWA was connected with an over 9‐fold higher risk of spontaneous nVT, and an over 20‐fold higher risk of postexercise nVT. However, the addition of the TWA test result to the prognostic models incorporating electrocardiographic and clinical variables did not improve the predictive value of the model for nVT occurrence. A model including two electrocardiographic parameters: QTc + LP turned out to be optimal for the prediction of spontaneous nVT episodes. As regards the risk of postexercise nVT, the best predictive model in our study was based on two parameters: electrocardiographic—QTc, and clinical—serum NT‐proBNP values.
SCD is undoubtedly the most dangerous complication of HCM and is preventable with ICD therapy. The most common cause of SCD in HCM is VT and/or VF. Therefore, there is a need for noninvasive diagnostic methods that can help to identify the HCM patients with a high risk of serious ventricular arrhythmia.
Microvolt TWA is a noninvasive test of arrhythmia vulnerability. TWA reflects spatial or temporal dispersion of repolarization, both of which may precede VT/VF. 18 , 19 From many of the studies conducted so far, it can be inferred that the prognostic value of TWA is comparable with the prognostic significance of VT induced during electrophysiological examination. 20 Momiyama et al. first reported TWA in patients with HCM, although the study population was extremely small (12 patients) and the correlation between TWA and the clinical variables of family history, cardiac function, and histopathological changes was not demonstrated. 21 Another Japanese study proved that TWA could be registered in up to 50% of patients with HCM. 22 In our study, TWA was positive in 52% of Caucasian patients. At present, there is a considerable controversy regarding the usefulness of TWA in the prediction of SCD in cardiovascular diseases 23 , 24 and data on the predictive value of TWA in HCM remain limited. Kuroda et al. showed that the sensitivity of TWA for nVT was quite high (90%) in the HCM group. 25 In their study, beta‐blockers, calcium antagonists, and antiarrhythmic agents were discontinued before the examination. We performed the TWA test in patients who still took the medications; however, our findings were similar to those obtained by Kuroda et al. The results of HM (pre‐ and postexercise) confirmed the high percentage of nVT episodes in patients with a positive TWA test. Interestingly enough, the presence of TWA was connected with an over 20‐fold higher risk of postexercise nVT. Different results were recently reported by Fuchs et al. 26 In a group of 21 patients with HCM they did not find any significant correlation between the positive TWA and the presence of ventricular arrhythmias on HM. Meanwhile, Kim et al. reported that TWA measured from ICD electrograms was significantly greater before spontaneous VT. 27 In those two studies, beta‐blockers and amiodarone were not discontinued. In a very interesting study, Cuoco et al. showed that TWA did not predict the results of electrophysiology studies in the HCM group, and no relationship between TWA and traditional risk factors was observed. 28
Small study groups, different methods of TWA test, discontinuation of beta‐blocker therapy, or lack thereof—all those factors make the interpretation and comparison of available studies extremely difficult.
Undoubtedly, the data on predictive value of TWA in HCM are conflicting. It must be stressed that other electrocardiographic risk factors of nVT episodes, such as QT interval prolongation, QT dispersion, and ventricular late potentials, have been rarely evaluated in patients with HCM. We have included all those parameters into statistical models with and without TWA. It turned out that models including (QTc + LP) and (QTc + NT‐proBNP) were sufficient to predict the risk of spontaneous and postexercise nVT with high specificity and sensitivity. The addition of TWA to those models did not improve their predictive value.
The QTc has been considered to be a risk factor in the prediction of ventricular arrhythmia for years. Similarly to TWA, those parameters characterize the repolarization period. The connection between QT indices and TWA remains undetermined. Shimizu et al. suggested that intracellular calcium handling is linked to the mechanism of TWA in a long QT model. 29 Janusek et al. demonstrated the relationship between the duration of repolarization and TWA amplitude. 30 However, the presence of TWA is mainly associated with temporal ventricular repolarization inhomogeneity. In HCM, myocardial abnormalities are often localized, and interventricular septum is most affected. This irregularity may increase the clinical significance of QT indices reflecting rather a spatial repolarization inhomogeneity. Kuroda et al. reported that the measurement of QT interval (especially during exercise) might be valuable for the prediction of SCD in HCM. 25 In their study, the sensitivity of QTc with more than 500 ms for VT was 90%. We found the QTc to be (together with LP and NT‐proBNP) the most important prognostic determinants of nVT risk stratification. However, some authors claimed that prolongation of the QT interval was common in patients with HCM and might not be of prognostic significance in the absence of syncope or cardiac arrest. 31
In the majority of previous studies, the prevalence of LP was approximately 10%, with a higher incidence in patients with VT. 32 , 33 In our study, the percentage of patients with LP was 26% and the presence of LP was an independent predictor of an increased risk of nVT. Our results corroborate recent findings by Limongelli et al. 34 The authors described a strong correlation between LP and LV thickness and their relationship with nVT in patients with HCM. It must be stressed that some authors suggested the inability of signal‐averaged electrocardiogram to risk‐stratify patients with HCM, probably because of the prominent LV hypertrophy, which masked the LP. 35 However, our results as well as reports of other authors indicate that LP are useful for the assessment of arrhythmia risk in patients with HCM.
Among all independent nVT predictors found in our study, NT‐proBNP was the only one nonelectrocardiographic. The data on the relationship between BNP and ventricular arrhythmias in HCM are limited. The Japanese authors found the correlation between plasma BNP levels and symptomatic VT in patients with HCM. 36 Recently, it was suggested that plasma BNP levels relate to the functional status in HCM. 37 Most of our patients have only slight symptoms of heart failure (NYHA II—51%, NYHA I—49%) with preserved LVEF. However, in patients with LV diastolic dysfunction and increased LV thickness, the histological substrate may become more important than the purely electrical one for the occurrence of postexercise arrhythmia. This may explain why NT‐proBNP replaced the LP in our prognostic model of postexercise nVT risk stratification.
Evaluation of postexercise arrhythmia represents a very important part of our study. Only a few studies reported the prevalence of VT during exercise in HCM. 10 , 38 In our study, all the patients performed a treadmill exercise test according to the accepted protocol for TWA measurement. No patients developed nVT episodes during the exercise test, but the frequency of occurrence of nVT episodes after exercise testing was higher in the TWA(+) group. It must be stressed that the prognostic models used in our study had higher sensitivity, specificity, and predictive values for risk stratification of postexercise nVT than for risk stratification of spontaneous nVT. Therefore, the exercise test, which is safe in HCM, facilitates the identification of patients at risk of nVT. The remaining question is whether those patients are at risk of SCD as well. A lot of studies reported that nVT was one of the major risk factors of sudden death. 4 , 5 , 7 , 8 , 39 Adabag et al. showed that nVT is found during ambulatory ECG monitoring in about 20%–30% of adult HCM patients, and that it is usually asymptomatic. 40 The authors also documented low positive and high negative predictive values of ventricular arrhythmia for sudden death.
To resolve the problem of what kind of risk we stratify using our prognostic models (risk of nVT or risk of sudden death), long‐term observation is needed. However, our results support the following conclusions:
-
1
Microvolt TWA is common in HCM patients.
-
2
In HCM patients, TWA is a useful predictor of nVT but it does not improve the arrhythmia prediction beyond that obtained by means of QT indices, ventricular late potentials, and NT‐proBNP.
-
3
The sensitivity, specificity, and the predictive values of electrocardiographic prognostic tests are higher for postexercise nVT episodes than for spontaneous nVT episodes in HCM.
The study was supported by research grant no. 502–11–587 from the Medical University of Lodz.
REFERENCES
- 1. Elliott P, McKenna WJ. Hypertrophic cardiomyopathy. Lancet 2004;363:1881–1891. [DOI] [PubMed] [Google Scholar]
- 2. Maron BJ, McKenna WJ, Danielson GK, et al American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents; European Society of Cardiology Committee for Practice Guidelines, American College of Cardiology/European Society of Cardiology Clinical Expert Consensus Document on Hypertrophic Cardiomyopathy. A report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents and the European Society of Cardiology Committee for Practice Guidelines. Eur Heart J 2003;24:1965–1991. [DOI] [PubMed] [Google Scholar]
- 3. Maron BJ, Towbin JA, Thiene G, et al American Heart Association; Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; Council on Epidemiology and Prevention. Contemporary definitions and classification of cardiomyopathies: An American Heart Association scientific statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention. Circulation 2006;113:1807–1816. [DOI] [PubMed] [Google Scholar]
- 4. McKenna WJ, Behr ER. Hypertrophic cardiomyopathy: Management, risk stratification, and prevention of sudden death. Heart 2002;87:169–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Behr ER, McKenna WJ. Hypertrophic cardiomyopathy. Curr Treat Options Cardiovasc Med 2002;4:443–453. [DOI] [PubMed] [Google Scholar]
- 6. Keren A, Syrris P, McKenna WJ. Hypertrophic cardiomyopathy: The genetic determinants of clinical disease expression. Nat Clin Pract Cardiovasc Med 2008;5:747–752. [DOI] [PubMed] [Google Scholar]
- 7. Spirito P, Rapezzi C, Autore C, et al Prognosis of asymptomatic patients with hypertrophic cardiomyopathy and non‐sustained ventricular tachycardia. Circulation 1994;90:2743–2747. [DOI] [PubMed] [Google Scholar]
- 8. Monserrat L, Elliott PM, Gimeno JR, et al Non‐sustained ventricular tachycardia in hypertrophic cardiomyopathy: An independent marker of sudden death risk in young patients. J Am Coll Cardiol 2003;42:873–879. [DOI] [PubMed] [Google Scholar]
- 9. Maron BJ, Spirito P. Implantable defibrillators and prevention of sudden death in hypertrophic cardiomyopathy. J Cardiovasc Electrophysiol 2008;19:1118–1126. [DOI] [PubMed] [Google Scholar]
- 10. Gimeno JR, Tome‐Esteban M, Lofiego C, et al Exercise‐induced ventricular arrhythmias and risk of sudden cardiac death in patients with hypertrophic cardiomyopathy. Eur Heart J 2009;30(21):2599–2605. [DOI] [PubMed] [Google Scholar]
- 11. Varnava AM, Elliott PM, Sharma S, et al Hypertrophic cardiomyopathy: The interrelation of disarray, fibrosis and small vessel disease. Heart 2000;84:476–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Maron BJ, Leyhe MJ 3rd, Casey SA, et al Assessment of QT dispersion as a prognostic marker for sudden death in a regional nonreferred, hypertrophic cardiomyopathy cohort. Am J Cardiol 2001;87:114–115. [DOI] [PubMed] [Google Scholar]
- 13. Task Force of The European Society of Cardiology and The North American Society of Pacing and Electrophysiology. Heart rate variability. Standards of measurement, physiological interpretation and clinical use. Eur Heart J 1996;17:354–387. [PubMed] [Google Scholar]
- 14. Schmidt G, Malik M, Barthel P, et al Heart‐rate turbulence after ventricular premature beats as a predictor of mortality after acute myocardial infarction. Lancet 1999;353:1390–1396. [DOI] [PubMed] [Google Scholar]
- 15. Breithardt G, Cain ME, El‐Sherif N, et al Standards for analysis of ventricular late potentials using high resolution electrocardiography: A statement by a task force committee of the European Society of Cardiology, the American Heart Association and the American College of Cardiology. J Am Coll Cardiol 1991;17:999–1006. [DOI] [PubMed] [Google Scholar]
- 16. Smith JM, Clancy EA, Valeri CR, et al Electrical alternans and cardiac electrical instability. Circulation 1988;77:110–121. [DOI] [PubMed] [Google Scholar]
- 17. Sadoul N, Prasad K, Elliott PM, et al Prospective prognostic assessment of blood pressure response during exercise in patients with hypertrophic cardiomyopathy. Circulation 1997;96:2987–2991. [DOI] [PubMed] [Google Scholar]
- 18. Narayan SM. T‐wave alternans and the susceptibility to ventricular arrhythmias. J Am Coll Cardiol 2006;47:269–281. [DOI] [PubMed] [Google Scholar]
- 19. Gold MR, Bloomfield DM, Anderson KP, et al A comparison of T‐wave alternans, signal averaged electrocardiography and programmed ventricular stimulation for arrhythmia risk stratification. J Am Coll Cardiol 2000;36:2247–2253. [DOI] [PubMed] [Google Scholar]
- 20. Costantini O, Hohnloser SH, Kirk MM, et al The ABCD (alternans before cardioverter defibrillator) trial: Strategies using T‐wave alternans to improve efficiency of sudden cardiac death prevention. J Am Coll Cardiol 2009;53(6):471–479. [DOI] [PubMed] [Google Scholar]
- 21. Momiyama Y, Hartikainen J, Nagayoshi H, et al Exercise‐induced T‐wave alternans as a marker of high risk in patients with hypertrophic cardiomyopathy. Jpn Circ J 1997;61:650–656. [DOI] [PubMed] [Google Scholar]
- 22. Kuroda N, Ohnishi Y, Yoshida A, et al Clinical significance of T‐wave alternans in hypertrophic cardiomyopathy. Circ J 2002;66:457–462. [DOI] [PubMed] [Google Scholar]
- 23. Shusterman V, Lampert R, London B. The many faces of repolarization instability: Which one is prognostic? J Electrocardiol 2009;42:511–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cutler MJ, Rosenbaum DS. Risk stratification for sudden cardiac death: Is there a clinical role for T‐wave alternans? Heart Rhythm 2009;6:S56–S61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kuroda N, Ohnishi Y, Adachi K, et al Relationship between the QT indices and the microvolt‐level T wave alternans in cardiomyopathy. Jpn Circ J 2001;65:974–978. [DOI] [PubMed] [Google Scholar]
- 26. Fuchs T, Torjman A. The usefulness of microvolt T‐wave alternans in the risk stratification of patients with hypertrophic cardiomyopathy. Isr Med Assoc J 2009;11:606–610. [PubMed] [Google Scholar]
- 27. Kim JW, Pak HN, Park JH, et al Defibrillator electrogram T‐wave alternans as a predictor of spontaneous ventricular tachyarrhythmias in defibrillator recipients. Circ J 2009;73:55–62. [DOI] [PubMed] [Google Scholar]
- 28. Cuoco F, Spencer WH 3rd, Fernandes V, et al Implantable cardioverter‐defibrillator therapy for primary prevention of sudden death after alcohol septal ablation of hypertrophic cardiomyopathy. J Am Coll Cardiol 2008;52:1718–1723. [DOI] [PubMed] [Google Scholar]
- 29. Shimizu W, Antzelevitch C. Cellular and ionic basis for T‐wave alternans under long QT conditions. Circulation 1999;99:1499–1507. [DOI] [PubMed] [Google Scholar]
- 30. Janusek D, Karczmarewicz S, Pawłowski Z, et al Relationship between duration of repolarisation and T wave amplitude in patients with positive or negative T wave alternans. Kardiol Pol 2005;62:517–525. [PubMed] [Google Scholar]
- 31. Fananapazir L, Chang AC, Epstein SE, et al Prognostic determinants in hypertrophic cardiomyopathy. Prospective evaluation of a therapeutic strategy based on clinical, Holter hemodynamic and electrophysiological findings. Circulation 1992;86:730–740. [DOI] [PubMed] [Google Scholar]
- 32. Kulakowski P, Counihan PJ, Camm AJ, et al The value of time and frequency domain, and spectral temporal mapping analysis of the signal‐averaged electrocardiogram in identification of patients with hypertrophic cardiomyopathy at increased risk of sudden death. E Heart J 1993;14:941–950. [DOI] [PubMed] [Google Scholar]
- 33. Vester EG, Emschermann C, Stobbe U, et al Late potentials and heart rate variability in heart muscle disease. E Heart J 1994;15:C25–C33. [DOI] [PubMed] [Google Scholar]
- 34. Limongelli G, Pacileo G, Cerrato F, et al Myocardial ultrasound tissue characterization in patients with hypertrophic cardiomyopathy: Noninvasive evidence of electrical and textural substrate for ventricular arrhythmias. J Am Soc Echocardiogr 2003;16:803–807. [DOI] [PubMed] [Google Scholar]
- 35. Chang AC, McAreavey D, Fananapazir L. Identification of patients with hypertrophic cardiomyopathy at high risk for sudden death. Curr Opin Cardiol 1995;10:9–15. [DOI] [PubMed] [Google Scholar]
- 36. Oka K, Tsujino T, Nakao S, et al Symptomatic ventricular tachyarrhythmia is associated with delayed gadolinium enhancement in cardiac magnetic resonance imaging and with elevated plasma brain natriuretic peptide level in hypertrophic cardiomyopathy. J Cardiol 2008;52:146–153. [DOI] [PubMed] [Google Scholar]
- 37. Kitaoka H, Kubo T, Okawa M, et al Utility of tissue Doppler imaging to predict exercise capacity in hypertrophic cardiomyopathy: Comparison with B‐type natriuretic peptide. J Cardiol 2009;53:361–367. [DOI] [PubMed] [Google Scholar]
- 38. Bunch TJ, Chandrasekaran K, Ehrsam JE, et al Prognostic significance of exercise induced arrhythmias and echocardiographic variables in hypertrophic cardiomyopathy. Am J Cardiol 2007;99:835–838. [DOI] [PubMed] [Google Scholar]
- 39. Maron BJ, Savage DD, Wolfson JK, et al Prognostic significance of 24 hour ambulatory electrocardiographic monitoring in patients with hypertrophic cardiomyopathy: A prospective study. Am J Cardiol 1981;48:252–257. [DOI] [PubMed] [Google Scholar]
- 40. Adabag AS, Casey SA, Kuskowski MA, et al Spectrum and prognostic significance of arrhythmias on ambulatory Holter electrocardiogram in hypertrophic cardiomyopathy. J Am Coll Cardiol 2005;45:697–704. [DOI] [PubMed] [Google Scholar]


