Abstract
Background: Previous studies have described the clinical usefulness of the presence of nonsustained ventricular tachycardia (NSVT) and defined heart rate turbulence (HRT) in stratifying patients at risk. We prospectively assessed whether HRT can facilitate the predictive accuracy of NSVT for identifying patients at risk for serious arrhythmic events in patients with left ventricular (LV) dysfunction.
Methods: We enrolled 299 consecutive patients with LV dysfunction (ejection fraction ≤ 40%) including ischemic (n = 184) and nonischemic causes (n = 115). The presence of NSVT was assessed on Holter electrocardiograms (ECGs). HRT was simultaneously measured from Holter ECGs, assessing two parameters: turbulence onset (TO) and turbulence slope (TS). HRT was considered positive when both TO and TS were abnormal. The end point was defined as of sudden cardiac death (SCD) and sustained ventricular tachyarrhythmias (VTs).
Results: NSVT was documented in 93 patients (32.7%). For HRT assessment, 17 patients (5.6%) were not utilized. Of 282 patients, 68 (24.1%) were HRT positive. During follow‐up of 960 ± 444 days, 14 patients (5.0%) reached the end point. NSVT, HRT, and diabetes were significantly associated with the end point. On multivariate analysis, NSVT had the strongest value for the end point, with an HR of 4.4 (95%CI, 1.4–14.3; P = 0.0138). When NSVT combined with HRT, the predictive accuracy is more increased, with an HR of 8.2 (95%CI, 2.9–23.3; P < 0.0001). The predictive values of the combination were higher than single use of NSVT or HRT.
Conclusions: HRT can facilitate the predictive accuracy of NSVT for identifying patients at risk for serious arrhythmic events in patients with LV dysfunction.
Keywords: heart rate turbulence; ventricular tachyarrhythmias; left ventricular dysfunction; risk stratification; sudden cardiac death, Holter electrocardiogram
Previous clinical studies have described that prophylactic implantation of implantable cardioverter defibrillator (ICD) contributes a significant risk reduction in patients at risk for sudden cardiac death (SCD). 1 At present, a reduced left ventricular ejection fraction (LVEF), 2 , 3 the presence of nonsustained ventricular tachycardia (NSVT) on Holter electrocardiogram (ECG), 4 , 5 microvolt T‐wave alternans, 6 and autonomic markers such as heart rate variability, 7 and induction of sustained ventricular tachyarrhythmias (VTs) by electrophysiologic testing 8 , 9 have been proposed as predictors of cardiac mortality in patients with structural heart disease. These risk‐stratifiers can detect low‐risk patients but cannot select high‐risk patients. Therefore, an alternative, more accurate method of risk prediction might be needed. One approach is to assess some markers, which are affected by different factor, and to combine each marker.
Heart rate turbulence (HRT) 10 measured from Holter ECG is a marker of the autonomic response to perturbations of arterial blood pressure after single ventricular premature complexes (VPCs) and has been introduced as an autonomic predictor for cardiac mortality in various clinical settings such as post‐myocardial infarction (MI), dilated cardiomyopathy (DCM), and heart failure. 10 , 11 , 12 , 13 , 14 A previous report 15 showed that combined assessment of HRT and NSVT can predict SCD in patients after acute MI, whereas in subgroup analysis, none of the Holter variables including NSVT predicted the event in those with a low LVEF. However, in that study, arrhythmic events such as ventricular tachycardias were excluded from the end point.
In the present study, we prospectively assessed whether HRT can facilitate the predictive accuracy of NSVT 4 , 5 , 16 , 17 , 18 detected on 24‐hour Holter ECGs for identifying patients at risk of SCD and serious VTs in patients with LV dysfunction including ischemic and nonischemic etiologies.
METHODS
Patient Population
This prospective study included 299 consecutive patients (218 men and 81 women; mean age 67 ± 15 years) with LV systolic dysfunction (LVEF ≤ 40%), undergoing 24‐hour Holter ECG at our institutes between June 2006 and December 2009. The study was approved by the Ethics Committee of our institutes. The clinical characteristics of the patient population are listed in Table 1 . LVEF were measured by echocardiography (modified Simpson method), radionuclide angiography, or magnetic resonance imaging. Patients were excluded at HRT assessment if they had chronic or persistent atrial fibrillation, an implanted permanent pacemaker, cardiac resynchronization therapy (biventricular pacing), or if they were in the acute phase of congestive heart failure or MI. These exclusion criteria were based on the inability to perform a HRT determination or reliable data analysis. Informed consent was obtained from each patient.
Table 1.
Baseline Characteristics of the Study Patients
| Clinical Features | n = 299 |
|---|---|
| Age (years) | 67 ± 15 |
| Gender (male/female) | 218 /81 |
| LVEF (%) | 34 ± 7 |
| Ischemic cardiomyopathy | 184 (62%) |
| NYHA I/II/III | 261/30/8 |
| Diabetes | 102 (34%) |
| Renal dysfunction | 57 (19%) |
| Hypertension | 183 (61%) |
| Hypercholesterolemia | 128 (43%) |
| Medications | |
| Aspirin | 223 (75%) |
| Beta‐blockers | 236 (79%) |
| Statins | 139 (47%) |
| ACE‐inhibitors/ARB | 242 (81%) |
| Class III antiarrhythmic drugs | 59 (20%) |
| ICD implantation | 18 (6%) |
LVEF, left ventricular ejection fraction; NYHA, New York Heart Association;
ACE, angiotensin‐converting enzyme; ARB, angiotensin receptor blocker;
ICD, implantable cardioverter defibrillator
All patients had chronic, stable heart failure, New York Heart Association (NYHA) class I–III and we excluded patients in NYHA class IV from this study. The average LVEF and was 34 ± 7%. Adjuvant medication consisted of aspirin in 223 patients (75%), beta‐blockers in 236 patients (79%), angiotensin‐converting enzyme (ACE) inhibitors, or angiotensin receptor blockers (ARBs) in 242 patients (81%), and class III antiarrhythmic drugs in 59 patients (20%). This study included 18 patients (6%) with an implantable cardioverter‐defibrillator (ICD) as a means of primary prevention of SCD.
Cause of LV dysfunction was classified into two categories: ischemic causes (n = 184) and nonischemic causes (n = 115). Ischemia was determined to be the cause of LV dysfunction if any of the following criteria were met: (1) stenosis of a major coronary artery of ≥75% on angiography; (2) a history of percutaneous coronary revascularization; (3) a history of coronary artery bypass graft surgery; or (4) a documented history of MI. All other etiologies were considered to be nonischemic. Nonischemic causes consisted of idiopathic dilated cardiomyopathy (DCM) (90.4%), the dilated phase of hypertrophic cardiomyopathy (8.7%), and other causes (0.9%). In the ischemic group, 166 patients (90.2%) underwent percutaneous coronary intervention and 18 patients (9.8%) underwent coronary artery bypass graft surgery in the acute phase of MI. One hundred forty‐five patients (78.3%) had anterior wall infarction and 26 patients (14.1%) had inferior wall infarction.
Measurement of HRT and Documentation of NSVT
For the measurement of HRT and the documentation of NSVT, all patients underwent 24‐hour Holter ECG recordings. HRT was automatically measured using an algorithm based on 24‐hour Holter ECGs obtained using the MARS Holter system (GE Healthcare Inc., Milwaukee, WI, USA). HRT parameters included turbulence onset (TO) and turbulence slope (TS) which were determined according to a previously published method. 10 The TO and TS were dichotomized at predefined cut points (TO = 0%, TS = 2.5 ms/RR interval). In this study, patients were defined as HRT positive when both TO and TS were abnormal (TO ≥ 0% and TS ≤ 2.5 ms/RR interval), and as HRT negative when TO and/or TS were normal (TO < 0% and/or TS > 2.5 ms/RR interval) or when HRT cannot be calculated because of no or too few suitable VPCs. 19 , 20 , 21
The presence or absence of NSVT was simultaneously determined with the Holter recordings of daily activities. According to previous reports, 4 NSVT was identified as three or more consecutive ventricular extrasystoles at a rate ≥100 bpm.
Assessment of Other Risk Variables
In this study, the following risk predictors were also prospectively assessed: age and gender of the patients, causes of LV dysfunction, a history of diabetes mellitus, renal dysfunction, hypertension, and hypercholesterolemia, use of medical treatm‐ent such as aspirin, β‐blockers, statins, angiotensin‐converting enzyme (ACE) inhibitors/angiotensin receptor blockers (ARBs), and class III antiarrhythmic drugs, and implantation of an ICD.
Follow‐Up and Study End Point
All patients were followed as outpatients at our institutes. Regular follow‐up contact was obtained at a hospital visit every 2 or 4 weeks. When patients did not visit for more than 8 weeks, our medical staffs conducted telephonic interviews.
The end point was prospectively defined as SCD and the documentation of sustained VTs. In patients who died, the causes were verified from the hospital and autopsy records, and from either the primary physicians or those who had witnessed the death. Patients who died of nonarrhythmic causes such as pomp failure, ischemic event, cancer, and stroke were not included in the end point. In patients with an ICD, an appropriate episode of defibrillation therapy and antitachycardia pacing were included in the end point. The records were verified from monitoring ECGs taken in an emergency room, ambulatory ECGs captured out‐of‐hospital, or by checking the ICD memory.
Statistical Analysis
Numeric data are expressed as mean ± standard deviation. Kappa test was used to assess the relation between NSVT and HRT outcomes. For analysis of the association between the end points and the clinical factors, univariate and multivariate Cox regression analyses were performed. Results of event‐free analyses were presented with hazard ratios (HRs) and 95% confidence intervals (CI). Sensitivity, specificity, positive and negative predictive values, and the predictive accuracy of an event‐free prediction were evaluated and also assessed by 2‐sample test for equality of proportions. A difference in event‐free rates was shown using the Kaplan–Meier method and the log rank test. A value for P < 0.05 was considered statistically significant.
RESULTS
Outcomes of HRT and NSVT
Although HRT measurements were performed on 24‐hour Holter ECGs in all 299 patients, values obtained from 17 patients (5.7%) were not utilized for the assessment because there were frequent VPCs with bigeminy or trigeminy (3 patients), and the occurrence of paroxysmal atrial fibrillation (14 patients). Therefore, clinical data from 282 patients were assessed. Average values for TO and TS were −0.08 ± 2.22% (abnormal in 110 patients [39.0%]) and 3.65 ± 5.05 msec/RR interval (abnormal in 124 patients [44.0%]), respectively. According to the data, HRT was determined as positive in 68 patients (24.1%) and negative in 214 patients (75.9%).
NSVT was documented on Holter ECGs in 93 patients (32.7%). The mean number of VPCs was 1992 ± 5371 beats per 24 hour.
Arrhythmic Events during Follow‐Up
Follow up was completed in all patients and no patient was lost to follow up. During a mean follow‐up of 960 ± 444 days, the end point occurred in 14 patients (5.0%) including 9 patients (3.1%) suffering from SCD, 2 patients (0.7%) suffering arrhythmogenic death, and 3 patients (1.0%) suffering from defibrillation therapy for ventricular fibrillation in patients with an ICD.
Eight patients died of heart failure progression and three patients suffered noncardiac death (lung cancer, septic shock, and intestinal pneumonia); these patients were therefore excluded from the study end point.
Association between Risk Variables and the End Point
On univariate analysis, diabetes, documentation of NSVT, and a HRT positive outcome were significantly associated with the end point (Table 2). Other risk variables did not attain statistical significance. To test which factor was the most significant, we performed multivariate analysis. Documentation of NSVT was the most significant predictor for the end point (an HR of 4.4 95%CI, 1.4–14.3; P = 0.0138). In addition, a HRT positive outcome was also significant variable on multivariate analysis (an HR of 3.2; 95%CI, 1.1–9.4; P = 0.0349).
Table 2.
Comparison of Risk Variables in Patients Who Did or Did Not Meet the End Point
| Variables | End Point (n = 14) | Event ‐Free Survival (n = 268) | Univariate Analysis HR (95%CI) | P Value | Multivariate Analysis HR (95%CI) | P Value |
|---|---|---|---|---|---|---|
| Median age (years) | 70 ± 11 | 66 ± 15 | 0.4091 | |||
| Gender (male) | 11/3 | 195/73 | 0.7089 | |||
| Ischemic cardiomyopathy | 9 (64%) | 168 (63%) | 0.8590 | |||
| Diabetes | 9 (64%) | 86 (32%) | 3.2 (1.1–8.9) | 0.0285 | 3.6 (1.1–10.6) | 0.0229 |
| Renal dysfunction | 5 (36%) | 46 (17%) | 0.0859 | |||
| Hypertension | 12 (86%) | 161 (60%) | 0.1316 | |||
| Hypercholesterolemia | 8 (57%) | 117 (44%) | 0.4271 | |||
| Medications | ||||||
| Aspirin | 12 (86%) | 211 (79%) | 0.3629 | |||
| Beta‐blockers | 14 (100%) | 222 (83%) | ||||
| Statins | 6 (43%) | 133 (50%) | 0.7328 | |||
| ACE‐inhibitors/ARB | 11 (79%) | 231 (86%) | 0.7184 | |||
| Class III antiarrhythmic drugs | 4 (29%) | 54 (20%) | 0.3638 | |||
| ICD implantation | 2 (14%) | 12 ( 4%) | 0.1693 | |||
| Holter ECG findings | ||||||
| NSVT | 10 (71%) | 83 (31%) | 4.1 (1.5–11.4) | 0.0065 | 4.4 (1.4–14.3) | 0.0138 |
| HRT | 8 (57%) | 60 (22%) | 4.3 (1.5–12.5) | 0.0079 | 3.2 (1.1–9.4) | 0.0349 |
HR = hazard ratio; CI = confidence interval, ECG = electrocardiogram; NSVT = nonsustained ventricular tachycardia; HRT = heart rate turbulence.
Of 93 patients with NSVT documentation, 33 (11.6%) had a positive HRT outcome. In 191 patients with no NSVT documentation, 35 (12.3%) had a negative HRT outcome (Table 3). Conversely, of 68 patients with a positive HRT outcome, 33 (11.6%) had NSVT documentation. In negative HRT patients (216 patients), 156 (54.9%) had no NSVT documentation. Therefore, 95 patients (33.5%) had discordant outcomes with respect to NSVT and HRT. In a bivariate analysis, there was no concordance between NSVT and HRT outcomes (kappa value of –0.77). When looking at the concordance in two subgroups (i.e., ischemic and nonischemic LV dysfunction patients), it remained not. Kappa values of both the ischemic and nonischemic population were negative (kappa value of −0.92 and –0.52, respectively), as shown in Table 3.
Table 3.
Concordance between NSVT and HRT Outcomes
| HRT Positive | HRT Negative | ||
|---|---|---|---|
| All patients | (kappa score –0.77) | ||
| Total | (n = 284) | 68 | 216 |
| Documented NSVT | (n = 93) | 33 | 60 |
| No documented NSVT | (n = 191) | 35 | 156 |
| Ischemic patients | (kappa score –0.92) | ||
| Total | (n=177) | 46 | 131 |
| Documented NSVT | (n=44) | 20 | 24 |
| No documented NSVT | (n=133) | 26 | 107 |
| Nonischemic patients | (kappa score –0.52) | ||
| Total | (n=107) | 22 | 85 |
| Documented NSVT | (n=49) | 13 | 36 |
| No documented NSVT | (n=58) | 9 | 49 |
When NSVT and HRT were combined, the predictive value for the end point was more increased, with an HR of 8.2 (95%CI, 2.9–23.3; P < 0.0001) on multivariate analysis. Figure 1 shows Kaplan‐Meier event‐free curves for the end point using NSVT, HRT and the combination of two markers in the study population. The predictive values are shown in Table 4. Although the negative predictive value for the end point did not differ among them, the specificity, positive predictive value, and predictive accuracy of the combination were higher than single use of these markers.
Figure 1.
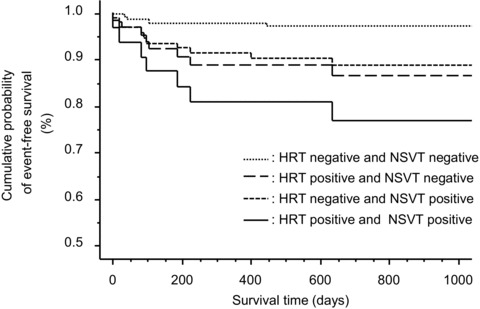
Kaplan‐Meier event‐free curves for serious arrhythmic events based on HRT and NSVT alone and in combination in the study population
Table 4.
Predictive Values of HRT and/or NSVT for the End Point
| Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | PA (%) | |
|---|---|---|---|---|---|
| NSVT | 10/14 (71) | 185/268 (69)* | 10/93 (11) | 185/189 (98) | 195/282 (69)‡ |
| HRT | 8/14 (57) | 208/268 (78)† | 8/68 (12) | 208/214 (97) | 216/282 (77)** |
| NSVT + HRT | 7/14 (50) | 242/268 (90)*† | 7/33 (21) | 242/249 (97) | 249/282 (88)‡** |
Values are expressed as number of patients (%). PPV = positive predictive value; NPV = negative predictive value; PA = predictive accuracy.
*P < 0.0001; †P = 0.0001; ‡P = 0.0004; **P < 0.0001.
DISCUSSION
This study revealed that HRT and NSVT are more powerful predictors for SCD and life‐threatening arrhythmias than other risk variables in patients with LV dysfunction. Serious VTs are the most common mechanisms responsible for SCD in patients in NYHA class I to III with LV dysfunction. 22 In the era of treatment for fatal VTs with an ICD, it is mostly possible to prevent SCD. Cost‐efficient primary prevention of SCD in patients with LV dysfunction requires risk stratification and identification of high‐risk subgroups. Combined assessment of HRT and NSVT was more associated with higher predictive values for SCD and VTs than single use of two markers.
Noninvasive factors that reflect the severity of disease, such as a reduced LVEF and high NYHA functional class, are associated with all‐cause mortality in patients with LV dysfunction, 1 , 23 but do not have a high positive predictive value for cardiac mortality. 24 Microvolt T‐wave alternans measured during exercise testing has been suggested to predict SCD in patients with LV dysfunction, 6 , 25 but such testing often resulted in indeterminate test outcomes due to the occurrence of VPCs during exercise testing or some physical problems. It is well known that heart rate measurements such as heart rate variability have been introduced as a useful technique in identifying patients at risk of cardiac mortality, particularly in post–myocardial infarction setting. 26 Unfortunately, this marker is not utilized in patients with frequent extrasystoles because data are derived from normal‐to‐normal beat analyses. 26 As is well known, most patients with LV dysfunction have sporadic or frequent VPCs. 23 In LV dysfunction patients with frequent VPCs, HRT may be more useful than heart rate variability.
There is extensive clinical evidence about HRT as a predictor of serious cardiac events in various clinical settings. Schmidt et al. 10 and ATRAMI substudy 11 revealed that HRT is an independent predictor of cardiac mortality in patients following MI. EPHESUS study showed that HRT can predict cardiovascular mortality in post‐MI patients with LV dysfunction. 27 In post MI setting with preserved LV function, ISAR‐Risk study showed that the predictive value of autonomic dysfunction including HRT was similar to that of reduced LVEF. 28 In patients with nonischemic cardiomyopathies, Grimm et al. 29 investigated the clinical value of noninvasive risk markers including HRT by analyzing data from patients with nonischemic DCM, using a retrospective study design. They concluded that an abnormal HRT was not significantly associated with heart transplantation but there was significant association between reduced LVEF and heart transplantation in patients with idiopathic DCM on multivariate analysis. Our previous study 13 showed that the value of HRT in patients with DCM, stratified by risk, and HRT is a more powerful predictor than other markers, including a reduced LVEF, in predicting cardiac mortality and arrhythmic events.
Mäkikallio et al. 15 reported that all Holter variables predicted the occurrence of SCD in patients after acute MI, but only reduced TS and NSVT remained as marked SCD predictors after adjustment for age, diabetes, and ejection fraction, whereas in a subgroup analysis, none of the Holter variables predicted SCD among those with low LVEF, but many variables predicted SCD among those with preserved LVEF, particularly TS. On the other hand, Cygankiewicz et al. 12 showed that abnormal HRT was independently associated with increased all‐cause mortality, sudden death and death due to heart failure progression in patients with congestive heart failure including ischemic and nonischemic causes. The results of the present study were not only similar to previous studies but also provided novel additional information as one of the risk‐stratification strategies. Our results also highlight the fact that combined assessment of HRT and NSVT can be used to potentially risk‐stratify patients for SCD and arrhythmic events preventable by an ICD. Almost one third of the present patients had discordant outcomes with respect to HRT and NSVT. However, it is important to highlight the fact that having both HRT positive and NSVT documentation was associated with a favorable positive predictive value for SCD and serious VTs, which was better than those of single use.
Although the ACC/AHA/ESC 2006 guidelines for management of patients with ventricular arrhythmias and prevention of SCD 30 state that a reduced LVEF is the most useful risk predictor for overall‐cause mortality, this is not the case for arrhythmogenic mortality. In predicting SCD due to serious VTs, combined assessment of HRT and NSVT appears to be a better methodology in patients with LV dysfunction.
Study Limitations
Because of the limited scope of our study, we did not include microvolt T‐wave alternans, 6 , 25 heart rate variability, 26 baroreflex sensitivity, 26 and other electrocardiographic markers, which would be significant predictors. We do not know the impact of these markers on cardiac death or arrhythmic events in this study population.
This manuscript was supported in part by a Grant‐in‐Aid (21590909, 21500420, 22136011, and 24591074) for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan and by the Research Promotion Grant from Toho University Graduate School of Medicine (No. 12‐01 to T.I.). There were no potential conflicts of interest for all authors.
REFERENCES
- 1. Bardy GH, Lee KL, Mark DB, et al Amiodarone or an implantable cardioverter‐defibrillator for congestive heart failure. N Engl J Med 2005;352:225–237. [DOI] [PubMed] [Google Scholar]
- 2. Moss AJ, Zareba W, Hall WJ, et al Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med 2002;346:877–883. [DOI] [PubMed] [Google Scholar]
- 3. Grimm W, Christ M, Bach J, et al Noninvasive arrhythmia risk stratification in idiopathic dilated cardiomyopathy: Results of the Marburg Cardiomyopathy Study. Circulation. 2003;108:2883–2891. [DOI] [PubMed] [Google Scholar]
- 4. de Sousa MR, Morillo CA, Robelo FT, et al Non‐sustained ventricular tachycardia as a predictor of sudden cardiac death in patients with left ventricular dysfunction: A meta‐analysis. Eur J Heart Fail 2008;10:1007–1014. [DOI] [PubMed] [Google Scholar]
- 5. Scirica BM, Braunwald E, Belardinelli L, et al Relationship between nonsustained ventricular tachycardia after non‐ST‐elevation acute coronary syndrome and sudden cardiac death: Observations from the metabolic efficiency with ranolazine for less ischemia in non‐ST‐elevation acute coronary syndrome‐thrombolysis in myocardial infarction 36 (MERLIN‐TIMI 36) randomized controlled trial. Circulation 2010;122:455–462. [DOI] [PubMed] [Google Scholar]
- 6. Verrier, RL , Klingenheben T, Malik M, et al Microvolt T‐wave alternans: Physiologic basis, methods of measurement, and clinical utility: Consensus statement by the International Society for Holter and Noninvasive Electrocardiology. J Am Coll Cardiol 2011;58:1309–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Huikuri HV, Mäkikallio TH, Peng CK, et al Fractal correlation properties of R‐R interval dynamics and mortality in patients with depressed left ventricular function after acute myocardial infarction. Circulation 2000;101:629–635. [DOI] [PubMed] [Google Scholar]
- 8. Buxton A, Lee KL, DiCarlo L, et al Electrophysiologic testing to identify patients with coronary artery disease who are at high risk for sudden death. N Engl J Med 2000;342:1937–1945. [DOI] [PubMed] [Google Scholar]
- 9. Milner PG, Dimarco JP, Lerman BB. Electrophysiological evaluation of sustained ventricular tachyarrhythmias in idiopathic dilated cardiomyopathy. Pacing Clin Electrophysiol 1988;11:562–568. [DOI] [PubMed] [Google Scholar]
- 10. Schmidt G, Malik M, Barthel P, et al Heart‐rate turbulence after ventricular premature beats as a predictor of mortality after acute myocardial infarction. Lancet 1999;353:1390–1396. [DOI] [PubMed] [Google Scholar]
- 11. Ghuran A, Reid F, La Rovere MT, et al ATRAMI Investigators: Heart rate turbulence based predictors of fatal and nonfatal cardiac arrest (the Autonomic Tone and Reflexes After Myocardial Infarction substudy). Am J Cardiol 2002;89:184–190. [DOI] [PubMed] [Google Scholar]
- 12. Cygankiewicz I, Zareba W, Vazquez R, et al Muerte Subita en Insuficiencia Cardiaca Investigators: Heart rate turbulence predicts all‐cause mortality and sudden death in congestive heart failure patients. Heart Rhythm 2008;5:1095–1102. [DOI] [PubMed] [Google Scholar]
- 13. Miwa Y, Ikeda T, Sakaki K, et al Heart rate turbulence as a predictor of cardiac mortality and arrhythmic events in patients with dilated cardiomyopathy: A prospective study. J Cardiovasc Electrophysiol 2009;20:788–795. [DOI] [PubMed] [Google Scholar]
- 14. Ikeda T, Miwa Y, Abe A Nakazawa K. Usefulness of heart rate turbulence for predicting cardiac events in patients with nonischemic dilated cardiomyopathy. J Electrocardiol 2011;44:669–672. [DOI] [PubMed] [Google Scholar]
- 15. Mäkikallio TH, Barthel P, Schneider R, et al Prediction of sudden cardiac death after acute myocardial infarction: Role of Holter monitoring in the modern treatment era. European Heart Journal 2005;26:762–769. [DOI] [PubMed] [Google Scholar]
- 16. Denes P, Gillis AM, Pawitan Y, et al Prevalence, characteristics and significance of ventricular premature complexes and ventricular tachycardia detected by 24‐hour continuous electrocardiographic recording in the Cardiac Arrhythmia Suppression trial. Am J Cardiol 1991;68:887–896. [DOI] [PubMed] [Google Scholar]
- 17. Pires LA, Wagshal AB, Huang SKS. Nonsustained ventricular tachycardia: Identification and management of high‐risk patients. Am Heart J 1993;126:189–200. [DOI] [PubMed] [Google Scholar]
- 18. Pires LA, Lehmann MH, Buxton AE, et al Differences in inducibility and prognosis of in‐hospital versus out‐of‐hospital identified nonsustained ventricular tachycardia in patients with coronary artery disease: Clinical and trial design implications. J Am Coll Cardiol 2001;38:1156–1162. [DOI] [PubMed] [Google Scholar]
- 19. Bauer A, Malik M, Schmidt G, et al Heart rate turbulence: Standards of measurement, physiological interpretation, and clinical use: International Society for Holter and Noninvasive Electrophysiology Consensus. J Am Coll Cardiol 2008;52:1353–65. [DOI] [PubMed] [Google Scholar]
- 20. Barthel P, Schneider R, Bauer A, et al Risk stratification after acute myocardial infarction by heart rate turbulence. Circulation 2003;108:1221–1226. [DOI] [PubMed] [Google Scholar]
- 21. Miwa Y, Miyakoshi M, Hoshida K, et al Heart rate turbulence can predict cardiac mortality following myocardial infarction in patients with diabetes mellitus. J Cardiovasc Electrophysiol 2011;22:1135–1140 [DOI] [PubMed] [Google Scholar]
- 22. Effect of metoprolol CR/XL in chronic heart failure . Metoprolol CR/XL Randomized Intervention Trial in Congestive Heart Failure (MERITHF). Lancet 1999;353:2001–2007. [PubMed] [Google Scholar]
- 23. Keogh AM, Baron DW, Hickie JB. Prognostic guides in patients with idiopathic or ischemic dilated cardiomyopathy assessed for cardiac transplantation. Am J Cardiol 1990;65:903–908. [DOI] [PubMed] [Google Scholar]
- 24. Dec GW, Fuster V. Idiopathic dilated cardiomyopathy. N Engl J Med 1994;331:1564–1575. [DOI] [PubMed] [Google Scholar]
- 25. Cantillon DJ, Stein KM, Markowitz SM, et al Predictive value of microvolt T‐wave alternans in patients with left ventricular dysfunction. J Am Coll Cardiol 2007;50:166–173. [DOI] [PubMed] [Google Scholar]
- 26. La Rovere MT, Bigger JT Jr, Marcus FI, et al Baroreflex sensitivity and heart‐rate variability in prediction of total cardiac mortality after myocardial infarction. ATRAMI (Autonomic Tone and Reflexes After Myocardial Infarction) Investigators. Lancet 1998;351:478–484. [DOI] [PubMed] [Google Scholar]
- 27. Stein PK, Deedwania P. Usefulness of abnormal heart rate turbulence to predict cardiovascular mortality in high‐risk patients with acute myocardial infarction and left ventricular dysfunction (from the EPHESUS study). Am J Cardiol 2009;103:1495–1499. [DOI] [PubMed] [Google Scholar]
- 28. Bauer A, Barthel P, Schneider R, et al Improved Stratification of Autonomic Regulation for risk prediction in post‐infarction patients with preserved left ventricular function (ISAR‐Risk). Eur Heart J 2009;30:576–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Grimm W, Sharkova J, Christ M, et al Prognostic Significance of Heart Rate Turbulence Following Ventricular Premature Beats in Patients with Idiopathic Dilated Cardiomyopathy. J Cardiovasc Electrophysiol 2003;14:819–824. [DOI] [PubMed] [Google Scholar]
- 30. Zipes DP, Camm AJ, Borggrefe M, et al ACC/AHA/ESC 2006 Guidelines for Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death: A Report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines. J Am Coll Cardiol 2006;48(Issue 5): e247–e346. [DOI] [PubMed] [Google Scholar]


